Multiple Xenosteroid Pollutants Biomarker Changes in Cultured Nile Tilapia Using Wastewater Effluents as Their Primary Water Source
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Samples Collection and Processing
2.3. Extraction and Quantification of Estrogenic Endocrine Disrupting Chemicals (E-EDCs) in Fish Tissues and Water Samples
2.4. Gene Expression Analysis
2.5. Hormones Analysis
2.6. Tissue Histopathology
- TI <10: normal/healthy structure; tissue architecture and histology were well developed and showed no impairments or pathological changes.
- TI 11–20: slight modifications of normal tissue architecture and morphology (e.g., change in cell size) were present.
- TI 21–30: moderate modifications of normal tissue architecture and morphology were present.
- TI 31–36: pronounced modifications of normal tissue architecture and morphology were present.
2.7. Statistical Analysis
3. Results
3.1. Estrogenic Endocrine Disrupting Chemicals (E-EDCs) Analysis in Fish Tissues and Water
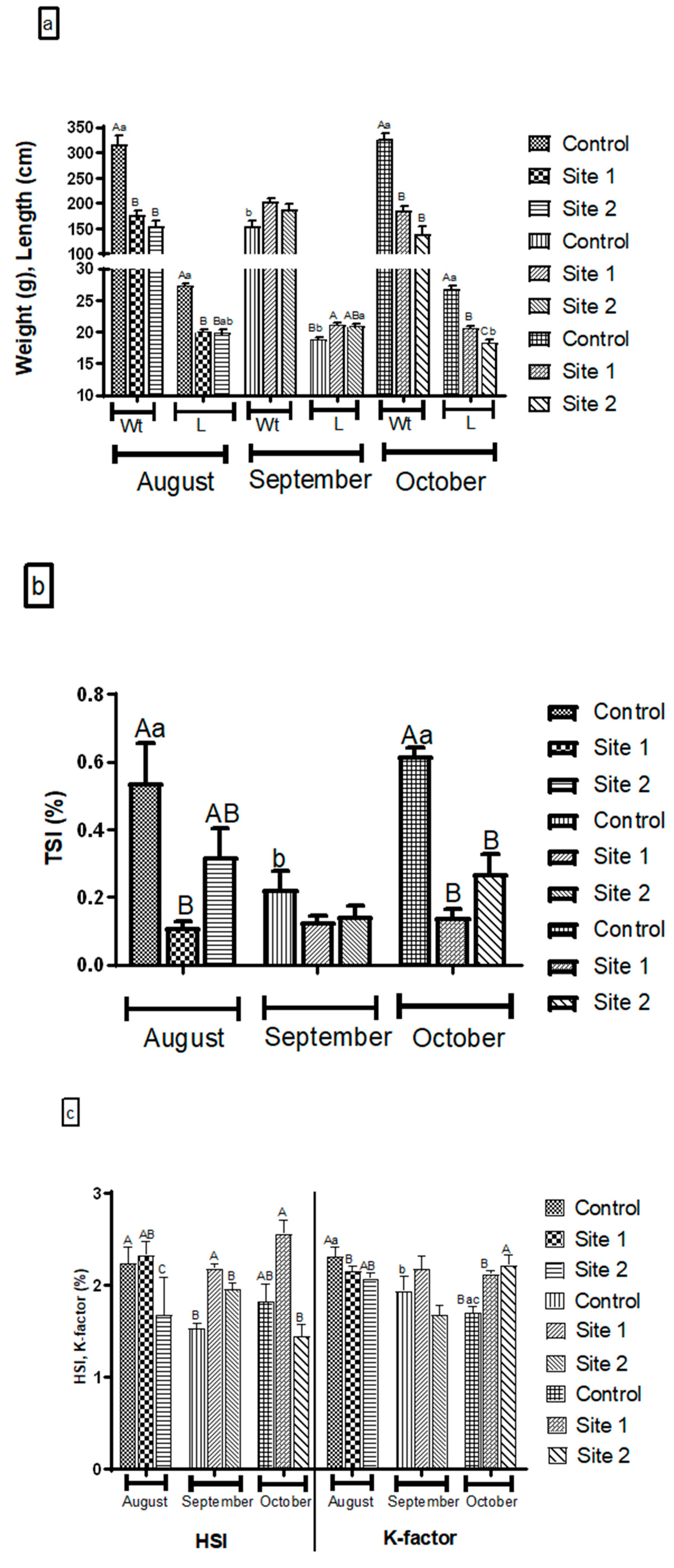
3.2. Fish Biometric and Organosomatic Measurements
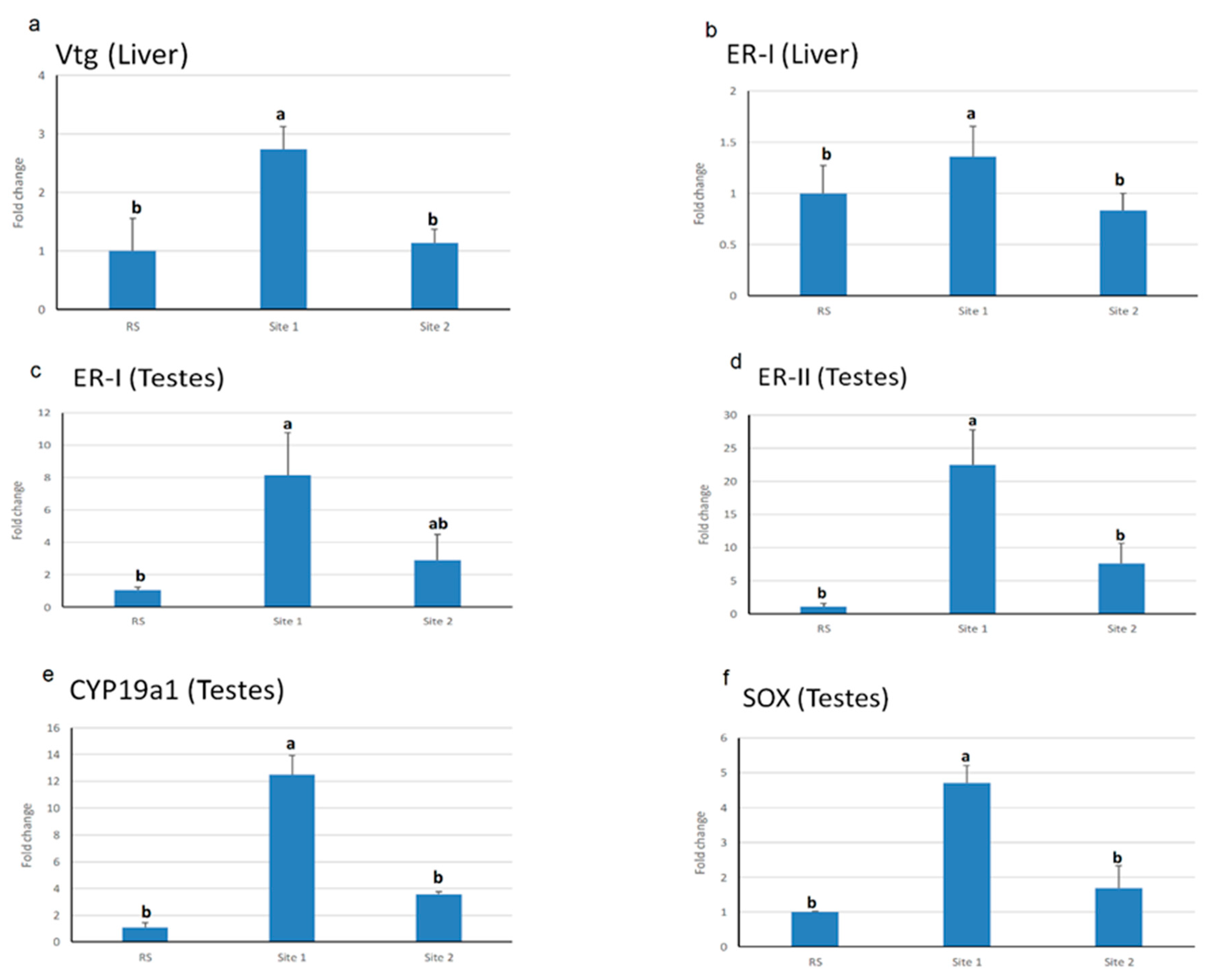
3.3. Endocrine Gene Expression Analysis in Testes and Liver Tissues
3.4. Hormones Analysis
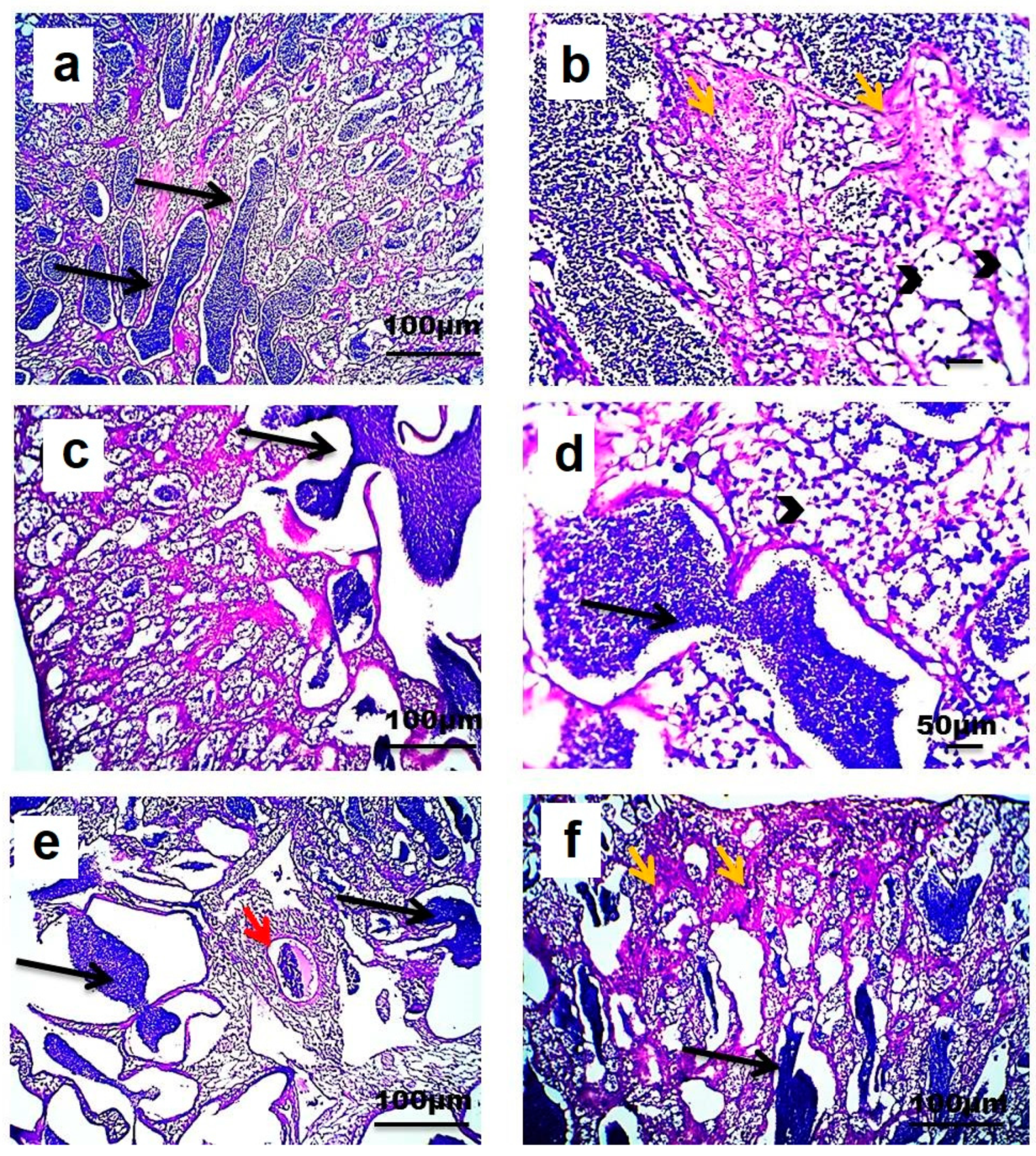
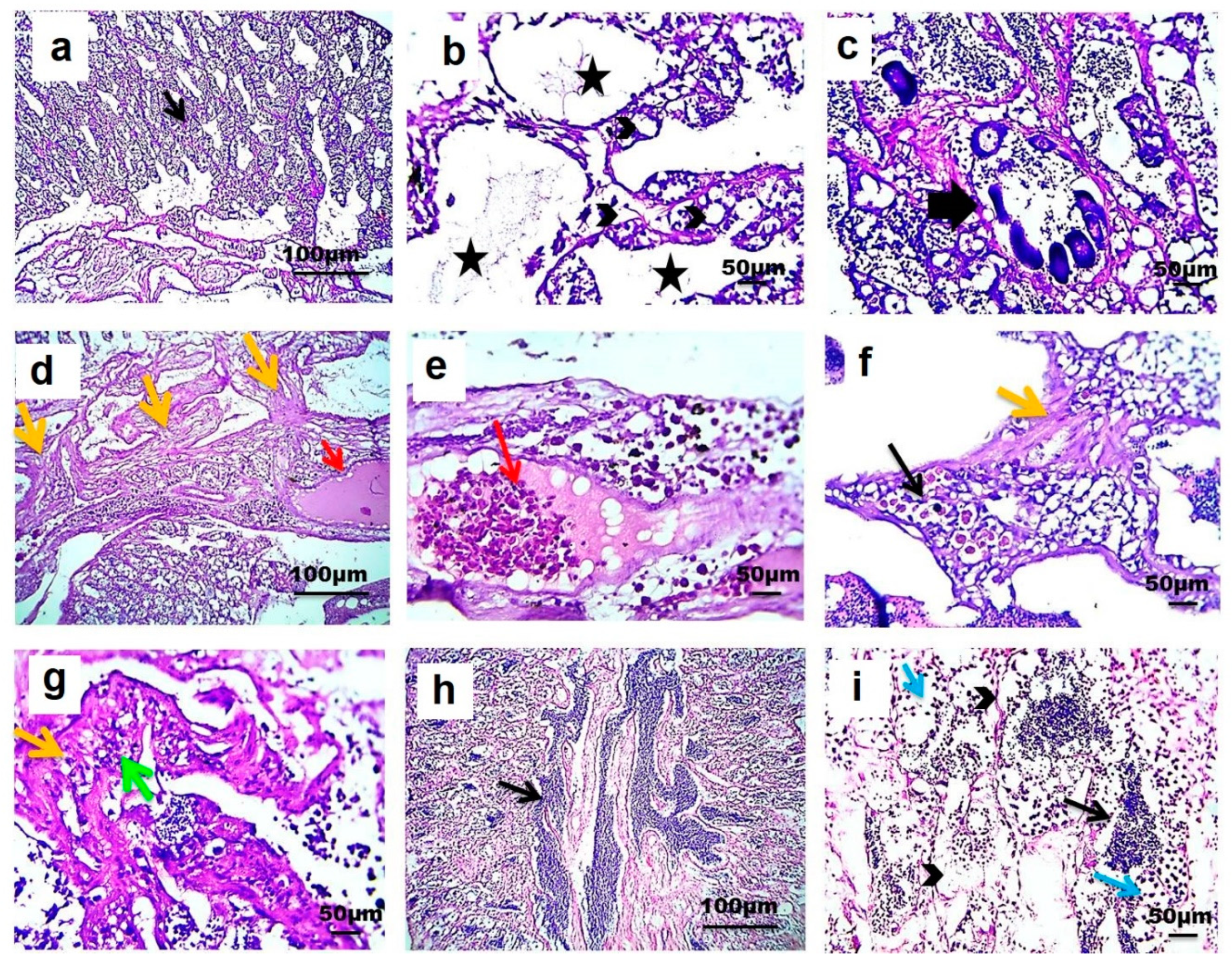
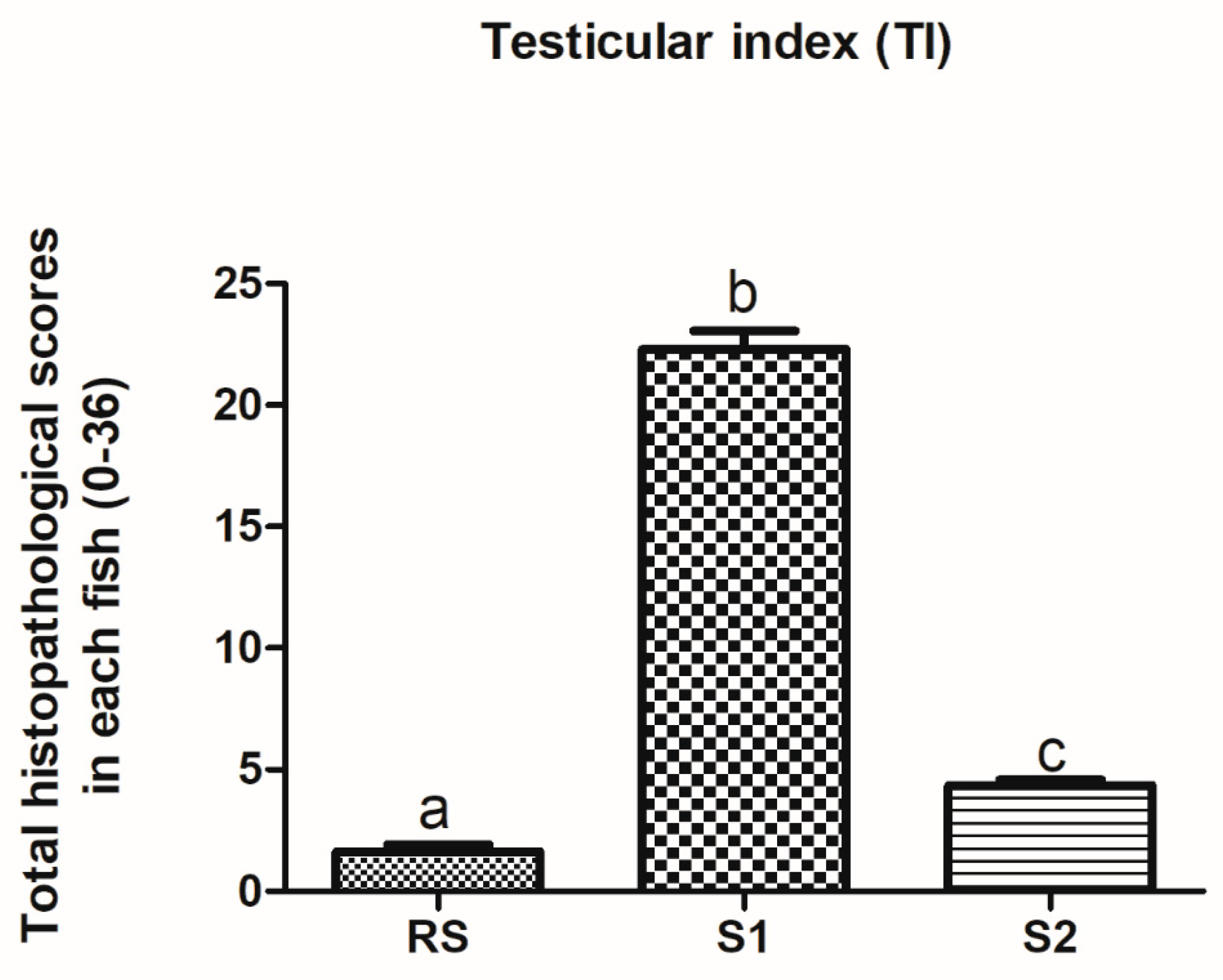
3.5. Testicular Histopathology
4. Discussion
4.1. Estrogenic Endocrine Disrupting Chemicals (E-EDCs) Analysis and Biometric Measurements
4.2. Biometric and Organosomatic Indices in Fish Exposed to Estrogenic Endocrine Disrupting Chemicals (E-EDCs)
4.3. Endocrine Gene Expression in Fish Exposed to Estrogenic Endocrine Disrupting Chemicals (E-EDCs)
4.4. Hormones Responses in Fish Exposed to Estrogenic Endocrine Disrupting Chemicals (E-EDCs)
4.5. Testicular Histopathology in Fish Exposed to Estrogenic Endocrine Disrupting Chemicals (E-EDCs)
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Gene Name | GenBank No. | Description | Annealing Temperature (°C) | Sequence (5′–3′) |
|---|---|---|---|---|
| er-type I | NM_001279770.1 | Forward Reverse | 55 | AGCGACAAATCAGTGCACCA ATTGCACGCTTCCTTCCATC |
| Er-type II | NM_001279774.1 | Forward Reverse | 55 | AGCATCCAAGGACACAACGA ATGCCCTTTGGTTCAGCTGT |
| cyp19a1 | NM_001279586.1 | Forward | 54 | TCAAACAAAACCCGCACGTG |
| Reverse | AAAACTCGGTGCGGTGCATT | |||
| sox9 | XM_003450119.4 | Forward Reverse | 56 | CGGGAGAGCATTCAGGTCAGTC CAGCTTTGCTGGAGGGAAGG |
| vtg | FJ709597 | Forward Reverse | 59 | AGACCCTCAGTTGCTGGAGT CGGTGTCGAGAGCTGAGTAG |
| β-actin | EU887951.1 | Forward Reverse | 60 | GTTGCCATCCAGGCTGTGCT TCTCGGCTGTGGTGGTGAAG |
References
- General Authority for Fish Resources Development GAFRD. Fish Statistics Year Book; Ministry of Agriculture and Land Reclamation: Cairo, Egypt, 2014.
- El-Sayed, A.-F.; Dickson, M.; El-Naggar, G.O. Value chain analysis of the aquaculture feed sector in Egypt. Aquaculture 2015, 437, 92–101. [Google Scholar] [CrossRef]
- El-Sayed, A.F.M. Regional Review on Status and Trends in Aquaculture Development in the Near East and North Africa-2015; FAO Fisheries and Aquaculture Circular; food and agriculture organization of the united nations (FAO): Rome, Italy, 2017; p. 43. [Google Scholar]
- Ojaveer, H.; Tomkiewicz, J.; Arula, T.; Klais, R. Female ovarian abnormalities and reproductive failure of autumn-spawning herring (Clupea harengus membras) in the Baltic Sea. ICES J. Mar. Sci. 2015, 72, 2332–2340. [Google Scholar] [CrossRef] [Green Version]
- Molnar, J.L.; Gamboa, R.L.; Revenga, C.; Spalding, M.D. Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ. 2008, 6, 485–492. [Google Scholar] [CrossRef]
- Peña-Mendoza, B.; Gómez-Márquez, J.L.; Salgado-Ugarte, I.H.; Ramirez-Noguera, D. Reproductive biology of Oreochromis niloticus (Perciformes: Cichlidae) at Emiliano Zapata dam, Morelos, Mexico. Rev. Biol. Trop. 2007, 53, 515–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swaminathan, M. Aquaculture and sustainable nutrition security in a warming planet. J. Farming Waters People 2012, 3, 19. [Google Scholar]
- Soliman, N.F. Aquaculture in Egypt under Changing Climate; Alexandria Research Center for Adaptation to Climate Change (ARCA): Alexandria, Egypt, 2017. [Google Scholar]
- Shaalan, M.; El-Mahdy, M.; Saleh, M.; El-Matbouli, M. Aquaculture in Egypt: Insights on the current trends and future perspectives for sustainable development. Rev. Fish. Sci. Aquac. 2017, 26, 99–110. [Google Scholar] [CrossRef]
- Muliari, M.; Akmal, Y.; Zulfahmi, I.; Karja, N.W.; Nisa, C.; Mahyana, M.; Humairani, R. Effect of exposure to palm oil mill effluent on reproductive impairment of male Nile Tilapia (Oreochromis niloticus, Linnaeus 1758). E3S Web Conf. 2020, 151, 01022. [Google Scholar] [CrossRef]
- Scholz, S.; Klüver, N. Effects of endocrine disrupters on sexual, gonadal development in fish. Sex. Dev. 2009, 3, 136–151. [Google Scholar] [CrossRef]
- Jeffries, K.M.; Jackson, L.J.; Ikonomou, M.G.; Habibi, H.R. Presence of natural and anthropogenic organic contaminants and potential fish health impacts along two river gradients in Alberta, Canada. Environ. Toxicol. Chem. 2010, 29, 2379–2387. [Google Scholar] [CrossRef]
- Peng, X.; Yu, Y.; Tang, C.; Tan, J.; Huang, Q.; Wang, Z. Occurrence of steroid estrogens, endocrine-disrupting phenols, and acid pharmaceutical residues in urban riverine water of the Pearl River Delta, South China. Sci. Total Environ. 2008, 397, 158–166. [Google Scholar] [CrossRef]
- Blazer, V.S.; Iwanowicz, L.R.; Henderson, H.; Mazik, P.M.; Jenkins, J.; Alvarez, D.A.; Young, J.A. Reproductive endocrine disruption in smallmouth bass (Micropterus dolomieu) in the Potomac River basin: Spatial and temporal comparisons of biological effects. Environ. Monit. Assess. 2011, 184, 4309–4334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.; Zhang, Y.; Zhou, R. Changes in extracellular osmolality initiate sperm motility in freshwater teleost rosy barb Puntius conchonius. Theriogenology 2009, 72, 704–710. [Google Scholar] [CrossRef] [PubMed]
- Desbrow, C.; Routledge, E.; Brighty, G.C.; Sumpter, J.P.; Waldock, M. Identification of estrogenic chemicals in STW effluent. 1. Chemical fractionation and In Vitro biological screening. Environ. Sci. Technol. 1998, 32, 1549–1558. [Google Scholar] [CrossRef]
- Wright-Walters, M.; Volz, C. Municipal wastewater concentrations of pharmaceutical and xeno-estrogens: Wildlife and human health implications. In Proceedings of the 2007 National Conference on Environmental Science and Technology, Greensboro, NC, USA, 12–14 September 2007; Springer: New York, NY, USA, 2009. [Google Scholar]
- Palanza, P.; Nagel, S.C.; Parmigiani, S.; vom Saal, F.S. Perinatal exposure to endocrine disruptors: Sex, timing and behavioral endpoints. Curr. Opin. Behav. Sci. 2016, 7, 69–75. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Xu, Y.-F.; Xu, S.; Li, J.; Tao, H.-C. Removal of estrogens in municipal wastewater treatment plants: A Chinese perspective. Environ. Pollut. 2012, 165, 215–224. [Google Scholar] [CrossRef]
- Behera, S.K.; Kim, H.W.; Oh, J.-E.; Park, H. Occurrence and removal of antibiotics, hormones and several other pharmaceuticals in wastewater treatment plants of the largest industrial city of Korea. Sci. Total Environ. 2011, 409, 4351–4360. [Google Scholar] [CrossRef]
- Quednow, K.; Püttmann, W. Endocrine disruptors in freshwater streams of Hesse, Germany: Changes in concentration levels in the time span from 2003 to 2005. Environ. Pollut. 2008, 152, 476–483. [Google Scholar] [CrossRef]
- Wee, S.Y.; Aris, A.Z. Endocrine disrupting compounds in drinking water supply system and human health risk implication. Environ. Int. 2017, 106, 207–233. [Google Scholar] [CrossRef]
- Zheng, W.; Li, X.; Yates, S.R.; Bradford, S.A. Anaerobic transformation kinetics and mechanism of steroid estrogenic hormones in dairy lagoon water. Environ. Sci. Technol. 2012, 46, 5471–5478. [Google Scholar] [CrossRef] [Green Version]
- Bergé, A.; Gasperi, J.; Rocher, V.; Gras, L.; Coursimault, A.; Moilleron, R. Phthalates and alkylphenols in industrial and domestic effluents: Case of Paris conurbation (France). Sci. Total Environ. 2014, 488, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Richardson, S.D.; Ternes, T.A. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2014, 86, 2813–2848. [Google Scholar] [CrossRef] [PubMed]
- Oehlmann, J.; Schulte-Oehlmann, U. Molluscs as bioindicators. In Trace Metals and Other Contaminants in the Environment; Breure, A.M., Markert, B.A., Zechmeister, H.G., Eds.; Elsevier: New York, NY, USA, 2002; Chapter 17; pp. 577–635. [Google Scholar]
- Frye, C.; Bo, E.; Calamandrei, G.; Calza, L.; Dessì-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocr. 2011, 24, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Yu, J.; Hu, X.; Yin, D. Characteristics of the alkylphenol and bisphenol A distributions in marine organisms and implications for human health: A case study of the East China Sea. Sci. Total Environ. 2016, 539, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-C.; Jiang, L.-Y.; Kuo, Y.-L.; Hsieh, C.-Y.; Chen, C.S.; Tien, C.-J. The potential role of water quality parameters on occurrence of nonylphenol and bisphenol A and identification of their discharge sources in the river ecosystems. Chemosphere 2013, 91, 904–911. [Google Scholar] [CrossRef]
- Shao, B.; Han, H.; Hu, J.; Zhao, J.; Wu, G.; Xue, Y.; Ma, Y.; Zhang, S. Determination of alkylphenol and bisphenol A in beverages using liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Chim. Acta 2005, 530, 245–252. [Google Scholar] [CrossRef]
- Wei, X.; Huang, Y.; Wong, M.H.; Giesy, J.P.; Wong, C.K.C. Assessment of risk to humans of bisphenol A in marine and freshwater fish from Pearl River Delta, China. Chemosphere 2011, 85, 122–128. [Google Scholar] [CrossRef]
- Villeneuve, D.L.; Garcia-Reyero, N.; Escalon, B.L.; Jensen, K.M.; Cavallin, J.E.; Makynen, E.A.; Durhan, E.J.; Kahl, M.D.; Thomas, L.M.; Perkins, E.J.; et al. Ecotoxicogenomics to support ecological risk assessment: A case study with bisphenol A in fish. Environ. Sci. Technol. 2011, 46, 51–59. [Google Scholar] [CrossRef]
- Diamanti-Kandarakis, E.; Bourguignon, J.-P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An Endocrine Society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, J.; Gu, L.-P.; Huang, B.; Pan, X. Biological effects of xenoestrogens and the functional mechanisms via genomic and nongenomic pathways. Environ. Rev. 2017, 25, 306–322. [Google Scholar] [CrossRef]
- Meng, S.; Qiu, L.; Hu, G.; Fan, L.; Song, C.; Zheng, Y.; Wu, W.; Qu, J.; Li, D.; Chen, J.; et al. Effects of methomyl on steroidogenic gene transcription of the hypothalamic-pituitary-gonad-liver axis in male tilapia. Chemosphere 2016, 165, 152–162. [Google Scholar] [CrossRef]
- Filby, A.L.; Thorpe, K.L.; Maack, G.; Tyler, C. gene expression profiles revealing the mechanisms of anti-androgen and estrogen-induced feminization in fish. Aquat. Toxicol. 2007, 81, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Chen, H.; Xu, X.-R.; Liu, S.-S.; Sun, K.-F.; Zhao, J.-L.; Ying, G.-G. Steroids in marine aquaculture farms surrounding Hailing Island, South China: Occurrence, bioconcentration, and human dietary exposure. Sci. Total Environ. 2015, 502, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, E.K.; Petersen, O.S.; Thompson, J.R.; Flower, R.J.; Ahmed, M.H. Hydrodynamic-ecological model analyses of the water quality of Lake Manzala (Nile Delta, Northern Egypt). Hydrobiologia 2009, 622, 195–220. [Google Scholar] [CrossRef]
- Ghanem, A.; Haggag, M. Assessment of the feasibility of using filter made of rice straw for treating aquaculture effluents in Egypt. Resour. Environ. 2015, 5, 135–145. [Google Scholar]
- Saad, A. Environmental Hydrogeologic Impacts of Ground Water Withdrawal in the Eastern Nile Delta Region with Emphasis on Ground-Water Pollution Potential. Ph.D. Thesis, Ain Shams Univeristy, Institute of Environmental Studies, Cairo, Egypt, 1997. [Google Scholar]
- Monem, A.M.A.; Gharieb, M.M.; Hussian, A.-E.M.; Flefil, N.S. Sensitivity of phytolankton to the wastewater quality discharging at lake Manzala, Egypt. J. Int. J. Ocean. 2017, 11, 231–247. [Google Scholar]
- Taha, A.; El-Mahmoudi, A.; El-Haddad, I. Pollution sources and related environmental impacts in the new communities Southeast Nile Delta, Egypt. J. Emir. J. Eng. Res. 2004, 9, 35–49. [Google Scholar]
- Elbana, T.A.; Bakr, N.; Karajeh, F.; El Quosy, F. Treated Wastewater Utilization for Agricultural Irrigation in Egypt. In The National Conference on Water Quality: Challenges and Solutions; National Research Centre: Cairo, Egypt, 2014; pp. 35–46. [Google Scholar]
- Zavala, M.A.L.; Arriaga, B.N.F.; Funamizu, N. Simultaneous determination of four estrogens in compost based on ultrasonic solvent extraction, solid-phase extraction clean-up and analysis by UHPLC-MS/MS. Am. J. Anal. Chem. 2016, 7, 434–445. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.; Li, Y.; Ouyang, H.; Xu, P.; Wu, D. High-performance liquid chromatographic analysis of bisphenol A and 4-nonylphenol in serum, liver and testis tissues after oral administration to rats and its application to toxicokinetic study. J. Chromatogr. B 2006, 830, 322–329. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative Pcr and the 2−ΔΔCT method. J. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Ijiri, S.; Kaneko, H.; Kobayashi, T.; Wang, D.; Sakai, F.; Paul-Prasanth, B.; Nakamura, M.; Nagahama, Y. Sexual dimorphic expression of genes in gonads during early differentiation of a teleost fish, the Nile Tilapia Oreochromis niloticus. Boil. Reprod. 2008, 78, 333–341. [Google Scholar] [CrossRef] [Green Version]
- Abo-Al-Ela, H.G.; El-Nahas, A.; Mahmoud, S.; Ibrahim, E.M. The extent to which immunity, apoptosis and detoxification gene expression interact with 17 alpha-methyltestosterone. Fish Shellfish. Immunol. 2017, 60, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Kevenaar, M.E.; Meerasahib, M.F.; Kramer, P.; Van De Lang-Born, B.M.N.; De Jong, F.H.; Groome, N.P.; Themmen, A.P.N.; Visser, J.A. Serum anti-müllerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 2006, 147, 3228–3234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques; Churchill Livingstone: London, UK, 2008. [Google Scholar]
- Zimmerli, S.; Bernet, D.; Burkhardt-Holm, P.; Schmidt-Posthaus, H.; Vonlanthen, P.; Wahli, T.; Segner, H. Assessment of fish health status in four Swiss rivers showing a decline of brown trout catches. Aquat. Sci. 2007, 69, 11–25. [Google Scholar] [CrossRef] [Green Version]
- Kolok, A.S.; Snow, D.D.; Kohno, S.; Sellin, M.K.; Guillette, L.J. Occurrence and biological effect of exogenous steroids in the Elkhorn River, Nebraska, USA. Sci. Total Environ. 2007, 388, 104–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowman, J.C.; Readman, J.W.; Zhou, J.L. Sorption of the natural endocrine disruptors, oestrone and 17β-oestradiol in the aquatic environment. Environ. Geochem. Health 2003, 25, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Kocaman, E.; Ozhan, K. Degradation of bisphenol A in natural and artificial marine and freshwaters in Turkey. Bull. Environ. Contam. Toxicol. 2019, 103, 496–500. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, R.; Huang, B.; Lin, C.; Zhou, J.; Pan, X. Biological effects and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in high-back crucian carp exposed to wastewater treatment plant effluents. Environ. Pollut. 2012, 162, 325–331. [Google Scholar] [CrossRef]
- Phumyu, N.; Boonanuntanasarn, S.; Jangprai, A.; Yoshizaki, G.; Na-Nakorn, U. Pubertal effects of 17α-methyltestosterone on GH–IGF-related genes of the hypothalamic–pituitary–liver–gonadal axis and other biological parameters in male, female and sex-reversed Nile tilapia. Gen. Comp. Endocrinol. 2012, 177, 278–292. [Google Scholar] [CrossRef]
- Fernandino, J.I.; Hattori, R.S.; Kishii, A.; Strüssmann, C.A.; Somoza, G.M. The cortisol and androgen pathways cross talk in high temperature-induced masculinization: The 11β-hydroxysteroid dehydrogenase as a key enzyme. Endocrinology 2012, 153, 6003–6011. [Google Scholar] [CrossRef] [Green Version]
- De Waal, P.P.; Wang, D.S.; Nijenhuis, W.A.; Schulz, R.; Bogerd, J. Functional characterization and expression analysis of the androgen receptor in zebrafish (Danio rerio) testis. Reproduction 2008, 136, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Leavy, M.; Trottmann, M.; Liedl, B.; Reese, S.; Stief, C.; Freitag, B.; Baugh, J.; Spagnoli, G.; Kölle, S. Effects of elevated β-estradiol levels on the functional morphology of the testis—New insights. Sci. Rep. 2017, 7, 39931. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, R.; Huang, B.; Lin, C.; Wang, Y.; Pan, X. Distribution and bioaccumulation of steroidal and phenolic endocrine disrupting chemicals in wild fish species from Dianchi Lake, China. Environ. Pollut. 2011, 159, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, S.; Bakhtiari, A.R.; Esmaili-Sari, A.; Bahramifar, N.; Rahbarizadeh, F. Occurrence of endocrine disruption chemicals (bisphenol A, 4-nonylphenol, and octylphenol) in muscle and liver of Cyprinus Carpino common from Anzali Wetland, Iran. Bull. Environ. Contam. Toxicol. 2013, 90, 578–584. [Google Scholar] [CrossRef] [PubMed]
- Hassanin, A.; Kuwahara, S.; Nurhidayat; Tsukamoto, Y.; Ogawa, K.; Hiramatsu, K.; Sasaki, F. Gonadosomatic index and testis morphology of common carp (Cyprinus carpio) in rivers contaminated with estrogenic chemicals. J. Vet. Med. Sci. 2002, 64, 921–926. [Google Scholar] [CrossRef] [Green Version]
- Al-Sakran, A.A.M.; Virk, P.; Elobeid, M.; Hamed, S.S.; Siddiqui, M.I.; Omer, S.; Mirghani, N.M. Histopathological effects on testis of adult male carp, Cyprinus carpio carpio, following exposure to graded concentrations of water-borne bisphenol A. Trop. J. Pharm. Res. 2016, 15, 73. [Google Scholar] [CrossRef] [Green Version]
- Rodgers-Gray, T.P.; Jobling, S.; Morris, S.; Kelly, C.; Kirby, S.; Janbakhsh, A.; Harries, J.E.; Waldock, M.J.; Sumpter, J.P.; Tyler, C.R. Long-term temporal changes in the estrogenic composition of treated sewage effluent and its biological effects on fish. Environ. Sci. Technol. 2000, 34, 1521–1528. [Google Scholar] [CrossRef]
- Hemming, J.M.; Allen, H.; Thuesen, K.A.; Turner, P.K.; Waller, W.T.; Lazorchak, J.M.; Lattier, D.; Chow, M.; Denslow, N.; Venables, B. Temporal and spatial variability in the estrogenicity of a municipal wastewater effluent. Ecotoxicol. Environ. Saf. 2004, 57, 303–310. [Google Scholar] [CrossRef]
- Bakhoum, S.; Faltas, S. The influence of water pollution upon the growth performance of Oreqchromis Aureus (Steinlj.) in lake Mariut, Egypt. Bull. Nat. Inst. Oceanogr. Fish. 1994, 20, 27–83. [Google Scholar]
- Khallaf, E.A.; Galal, M.; Authman, M.M. The biology of Oreochromis niloticus in a polluted canal. Ecotoxicology 2003, 12, 405–416. [Google Scholar] [CrossRef]
- Sohoni, P.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gargas, M.; et al. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef]
- Meng, S.; Qiu, L.; Hu, G.; Fan, L.; Song, C.; Zheng, Y.; Wu, W.; Qu, J.; Li, D.; Chen, J.; et al. Effect of methomyl on sex steroid hormone and vitellogenin levels in serum of male tilapia (Oreochromis niloticus) and recovery pattern. Environ. Toxicol. 2017, 32, 1869–1877. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.O.; Camp, J.M.; Maldonado, T.A.; Woodling, J.D. Some aspects of hepatic function in feral brown trout, Salmo trutta, living in metal contaminated water. Comp. Biochem. Physiol. Part C Pharmacol. Toxicol. Endocrinol. 2000, 127, 71–78. [Google Scholar] [CrossRef]
- Höger, B.; Taylor, S.; Hitzfeld, B.; Dietrich, D.; van den Heuvel, M.P. Stimulation of reproductive growth in rainbow trout (Oncorhynchus mykiss) following exposure to treated sewage effluent. Environ. Toxicol. Chem. 2006, 25, 2753–2759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemming, J.M.; Waller, W.T.; Chow, M.C.; Denslow, N.D.; Venables, B. Assessment of the estrogenicity and toxicity of a domestic wastewater effluent flowing through a constructed wetland system using biomarkers in male fathead minnows (pimephales promelas Rafinesque). Environ. Toxicol. Chem. 2001, 20, 2268. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, J.; Möller, G.; De Angelis, M.H.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [Green Version]
- Yadetie, F.; Arukwe, A.; Goksøyr, A.; Male, R. Induction of hepatic estrogen receptor in juvenile Atlantic salmon In Vivo by the environmental estrogen, 4-nonylphenol. Sci. Total Environ. 1999, 233, 201–210. [Google Scholar] [CrossRef]
- Arukwe, A. Differential biomarker gene and protein expressions in nonylphenol and estradiol-17β treated juvenile rainbow trout (Oncorhynchus mykiss). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 129, 1–10. [Google Scholar] [CrossRef]
- Soverchia, L.; Ruggeri, B.; Palermo, F.A.; Mosconi, G.; Cardinaletti, G.; Scortichini, G.; Gatti, G.; Polzonettimagni, A. Modulation of vitellogenin synthesis through estrogen receptor beta-1 in goldfish (Carassius Auratus) juveniles exposed to 17-β estradiol and nonylphenol. Toxicol. Appl. Pharmacol. 2005, 209, 236–243. [Google Scholar] [CrossRef]
- Bronson, M.W.; Hillenmeyer, S.; Park, R.W.; Brodsky, A.S. Estrogen coordinates translation and transcription, revealing a role for NRSF in human breast cancer cells. Mol. Endocrinol. 2010, 24, 1120–1135. [Google Scholar] [CrossRef] [Green Version]
- Gibert, Y.; Sassi-Messai, S.; Fini, J.-B.; Bernard, L.; Zalko, D.; Cravedi, J.-P.; Balaguer, P.; Andersson-Lendahl, M.; Demeneix, B.A.; Laudet, V. Bisphenol A induces otolith malformations during vertebrate embryogenesis. BMC Dev. Boil. 2011, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.-M.; Seo, J.S.; Kim, I.-C.; Yoon, Y.-D.; Lee, J.-S. Endocrine disrupting chemicals (bisphenol A, 4-nonylphenol, 4-tert-octylphenol) modulate expression of two distinct cytochrome P450 aromatase genes differently in gender types of the hermaphroditic fish Rivulus marmoratus. Biochem. Biophys. Res. Commun. 2006, 345, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Lyssimachou, A.; Jenssen, B.M.; Arukwe, A. Brain cytochrome P450 Aromatase gene isoforms and activity levels in Atlantic salmon after waterborne exposure to nominal environmental concentrations of the pharmaceutical ethynylestradiol and antifoulant tributyltin. Toxicol. Sci. 2006, 91, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.-H.; Aasi, D.; Katayama, Y. Bisphenol A in the aquatic environment and its endocrine-disruptive effects on aquatic organisms. Crit. Rev. Toxicol. 2007, 37, 607–625. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Shen, X.; Zhao, H.; Nagahama, Y. Genome-wide analysis of sox genes in Medaka (Oryzias latipes) and their expression pattern in embryonic development. Cytogenet. Genome Res. 2011, 134, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Freeman, J.L. Toxicogenomics to evaluate endocrine disrupting effects of environmental chemicals using the zebrafish model. Curr. Genom. 2016, 17, 515–527. [Google Scholar] [CrossRef]
- Shimasaki, Y.; Kitano, T.; Oshima, Y.; Inoue, S.; Imada, N.; Honjo, T. Tributyltin causes masculinization in fish. Environ. Toxicol. Chem. 2003, 22, 141–144. [Google Scholar] [CrossRef]
- Zhang, J.; Zuo, Z.; Chen, R.; Chen, Y.; Wang, C. Tributyltin exposure causes brain damage in Sebastiscus marmoratus. Chemosphere 2008, 73, 337–343. [Google Scholar] [CrossRef]
- Vandenberg, L.N.; Maffini, M.V.; Sonnenschein, C.; Rubin, B.S.; Soto, A.M. Bisphenol-A and the great divide: A review of controversies in the field of endocrine disruption. Endocr. Rev. 2008, 30, 75–95. [Google Scholar] [CrossRef]
- Wang, H.; Wang, J.; Wu, T.; Qin, F.; Hu, X.; Wang, L.; Wang, Z. Molecular characterization of estrogen receptor genes in Gobiocypris rarus and their expression upon endocrine disrupting chemicals exposure in juveniles. Aquat. Toxicol. 2011, 101, 276–287. [Google Scholar] [CrossRef]
- Yang, M.; Qiu, W.; Chen, B.; Chen, J.; Liu, S.; Wu, M.; Wang, K.-J. The In Vitro immune modulatory effect of bisphenol A on fish macrophages via estrogen receptor A and nuclear factor-Κb signaling. J. Environ. Sci. Technol. 2015, 49, 1888–1895. [Google Scholar] [CrossRef]
- Qin, F.; Wang, L.; Wang, X.; Liu, S.; Xu, P.; Wang, H.; Wu, T.; Zhang, Y.; Zheng, Y.; Li, M.; et al. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2013, 157, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Vosges, M.; Le Page, Y.; Chung, B.-C.; Combarnous, Y.; Porcher, J.; Kah, O.; Brion, F. 17α-Ethinylestradiol disrupts the ontogeny of the forebrain GnRH system and the expression of brain aromatase during early development of zebrafish. Aquat. Toxicol. 2010, 99, 479–491. [Google Scholar] [CrossRef] [PubMed]
- Harding, L.B.; Schultz, I.R.; Goetz, G.W.; Luckenbach, J.A.; Young, G.; Goetz, F.W.; Swanson, P. High-throughput sequencing and pathway analysis reveal alteration of the pituitary transcriptome by 17α-ethynylestradiol (EE2) in female coho salmon, Oncorhynchus kisutch. Aquat. Toxicol. 2013, 142, 146–163. [Google Scholar] [CrossRef] [PubMed]
- Pasmanik, M.; Schlinger, B.A.; Callard, G.V. In Vivo steroid regulation of aromatase and 5α-reductase in goldfish brain and pituitary. Gen. Comp. Endocrinol. 1988, 71, 175–182. [Google Scholar] [CrossRef]
- Behringer, R.R. 5 the In Vivo Roles of müllerian-inhibiting substance. In Current Topics in Developmental Biology; Elsevier Academic Press: San Diego, CA, USA, 1994; pp. 171–187. [Google Scholar]
- Yokota, H.; Tsuruda, Y.; Maeda, M.; Oshima, Y.; Tadokoro, H.; Nakazono, A.; Honjo, T.; Kobayashi, K. Effect of bisphenol A on the early life stage in Japanese Medaka (Oryzias Latipes). Environ. Toxicol. Chem. 2000, 19, 1925. [Google Scholar] [CrossRef]
- Metcalfe, D.C.; Metcalfe, T.L.; Kiparissis, Y.; Koenig, B.G.; Khan, C.; Hughes, R.J.; Croley, T.R.; March, E.R.; Potter, T. Estrogenic potency of chemicals detected in sewage treatment plant effluents as determined by In Vivo assays with Japanese Medaka (Oryzias Latipes). J. Environ. Toxicol. Chem. Biodivers. 2001, 20, 297–308. [Google Scholar] [CrossRef]
- Ali, T.; Abdel-Aziz, S.; El-Sayed, A.-F.; Zeid, S. Effects of nonylphenol on plasma steroids, vitellogenin synthesis and sex reversal in Nile Tilapia (Oreochromis Niloticus) fingerlings. Indian J. Geo Mar. Sci. 2017, 46, 521–528. [Google Scholar]
- Mandich, A.; Bottero, S.; Benfenati, E.; Cevasco, A.; Erratico, C.; Maggioni, S.; Massari, A.; Pedemonte, F.; Vigano, L. In Vivo exposure of carp to graded concentrations of bisphenol A. Gen. Comp. Endocrinol. 2007, 153, 15–24. [Google Scholar] [CrossRef]
- Lora, A.; Molina, A.; Bellido, C.; Blanco, A.; Monterde, J. Moyano Adverse effects of bisphenol A on the testicular parenchyma of zebrafish revealed using histomorphological methods. J. Veterinární Med. 2016, 61, 577–589. [Google Scholar] [CrossRef] [Green Version]


| Water Samples | Tissue Samples | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Target Chemical | RS | S1 | S2 | Testes | Muscle | Liver | ||||||
| RS | S1 | S2 | RS | S1 | S2 | RS | S1 | S2 | ||||
| Natural Hormones (µg/L) | Natural Hormones (µg/kg) | |||||||||||
| Estrone (E1) | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| 17β–Estradiol (E2) | <LOQ | <LOQ | <LOQ | 1.91 | <LOQ | 5.01 | <LOQ | 1.05 | <LOQ | <LOQ | <LOQ | <LOQ |
| Hexsterol | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Diethyl stilbesterol | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Dienesterol | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Testosterone | 0.12 | 0.2 | <LOQ | 0.16 | 6 | 1.1 | 0.19 | 0.23 | 0.57 | 0.68 | 0.41 | 3.8 |
| Progesterone | 0.21 | 0.13 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Veterinary Pharmaceuticals (µg L−1) | Veterinary Pharmaceuticals (µg kg−1) | |||||||||||
| Zeranol | 0.51 | <LOQ | 3.44 | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ | <LOQ |
| Industrial/Domestic Compound (µg/mL) | Industrial/Domestic Compound (µg/kg) | |||||||||||
| Bisphenol A | ND | 6.5 | ND | ND | 25.9 | ND | ND | 48.07 | ND | |||
| Site of Sample Collection | Reaction Pattern | Histopathological Examination | Frequency (n = 60) | % |
|---|---|---|---|---|
| RS | RC | vacuolated seminiferous epithelium | 10 | 16.6 |
| CD | congestion | 12 | 20 | |
| RC | Melano-macrophage centers | 10 | 16.6 | |
| S1 | RC | Vacuolization of the seminiferous epithelium | 35 | 58.3 |
| RC | No spermatozoa in the lumen of the testicular lobule | 18 | 30 | |
| RC | Melano-macrophage centers | 25 | 41.6 | |
| PC | Wall proliferation of lobular cysts | 25 | 41.6 | |
| I | Mononuclear leukocytes | 33 | 55 | |
| CD | congestion | 42 | 70 | |
| IS | Testicular oocytes | 8 | 13.3 | |
| S2 | RC | Vacuolization of the seminiferous epithelium | 15 | 25 |
| CD | congestion | 10 | 16.6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahran, E.; Elmetwally, M.; Awadin, W.; El-Matbouli, M. Multiple Xenosteroid Pollutants Biomarker Changes in Cultured Nile Tilapia Using Wastewater Effluents as Their Primary Water Source. Animals 2020, 10, 1475. https://doi.org/10.3390/ani10091475
Zahran E, Elmetwally M, Awadin W, El-Matbouli M. Multiple Xenosteroid Pollutants Biomarker Changes in Cultured Nile Tilapia Using Wastewater Effluents as Their Primary Water Source. Animals. 2020; 10(9):1475. https://doi.org/10.3390/ani10091475
Chicago/Turabian StyleZahran, Eman, Mohammed Elmetwally, Walaa Awadin, and Mansour El-Matbouli. 2020. "Multiple Xenosteroid Pollutants Biomarker Changes in Cultured Nile Tilapia Using Wastewater Effluents as Their Primary Water Source" Animals 10, no. 9: 1475. https://doi.org/10.3390/ani10091475






