Redox Status in Canine Leishmaniasis
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Measurement of Reactive Oxygen Species (ROS) and Reactive Nitrogen Species (RNS)
2.2.1. Assay for Superoxide Anion (O2−)
2.2.2. Assay for Nitric Oxide (NO)
2.2.3. Assay for Hydroperoxides (ROOH)
2.3. Scavenging Enzyme Activity
2.3.1. Assay for Superoxide Dismutase (SOD)
2.3.2. Scavenging Nonenzymatic Activity: Ferric Reducing-Antioxidant Power (FRAP)
2.4. Statistical Analysis
3. Results
3.1. Reactive Oxygen Species (ROS)
3.2. Scavenging Activity of ROS
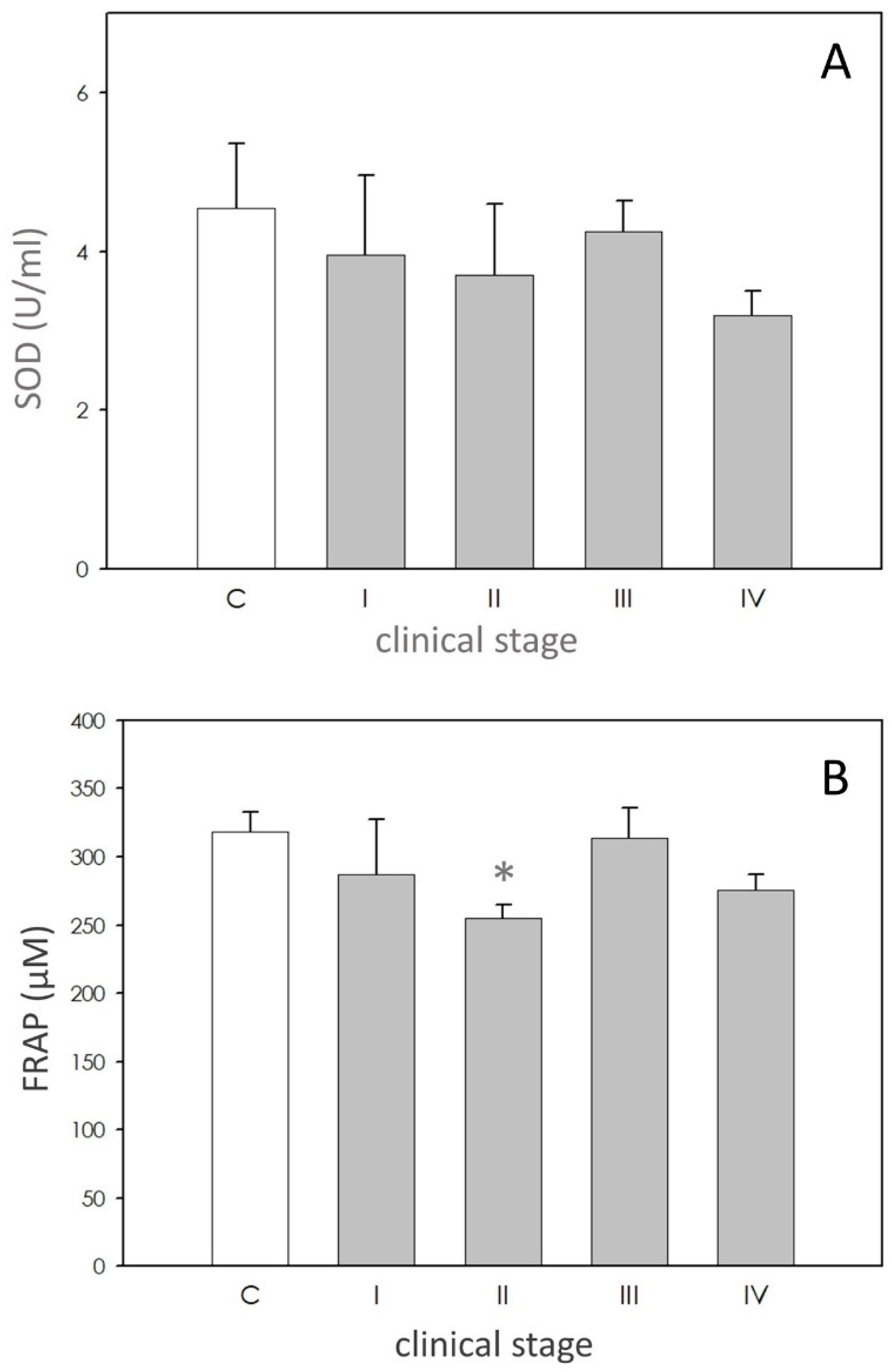
3.2.1. Superoxide Dismutase (SOD)
3.2.2. Scavenging Nonenzymatic activity: Ferric Reducing-Antioxidant Power (FRAP)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- van Griensven, J.; Diro, E. Visceral Leishmaniasis: Recent Advances in Diagnostics and Treatment Regimens. Infect Dis. Clin. N. Am. 2019, 33, 79–99. [Google Scholar] [CrossRef]
- Ruiz-Postigo, J.A.; Grouta, L.; Jaina, S. World Health Organization [WHO] Global Leishmaniasis Surveillance, 2017–2018, and First Report on 5 Additional Indicators. Available online: https://www.who.int/health-topics/leishmaniasis#tab=tab_1 (accessed on 18 September 2020).
- Giraud, E.; Martin, O.; Yakob, L.; Rogers, M. Quantifying Leishmania metacyclic promastigotes from individual sandfly bites reveals the efficiency of vector transmission. Commun. Biol. 2019, 2, 84. [Google Scholar] [CrossRef]
- Iatta, R.; Furlanello, T.; Colella, V.; Tarallo, V.D.; Latrofa, M.S.; Brianti, E.; Trerotoli, P.; Decaro, N.; Lorusso, E.; Schunack, B.; et al. A nationwide survey of Leishmania infantum infection in cats and associated risk factors in Italy. PLoS Negl. Trop. Dis. 2019, 13, e0007594. [Google Scholar] [CrossRef] [Green Version]
- Pearson, R.D.; Romito, R.; Symes, P.H.; Harcus, J.L. Interaction of Leishmania donovani promastigotes with human monocyte derived macrophages: Parasite entry, intracellular survival, and multiplication. Infect. Immun. 1981, 32, 1249–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodruff, A.W.; Topley, E.; Knight, R.; Campbell-Browine, G.B. The anemia of Kala-Azar. Br. J. Hematol. 1972, 22, 319–329. [Google Scholar] [CrossRef] [PubMed]
- Stocks, J.; Dormandy, T.L. The autooxidation of human red cell lipids induced by hydrogen peroxide. Br. J. Hematol. 1971, 20, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Vural, H.; Aksoy, N.; Ozbilge, H. Alterations of oxidative–antioxidative status in human cutaneous leishmaniasis. Cell Biochem. Funct. 2004, 22, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Keles, H.; Selek, S.; Guzel, S.; Celik, H.; Ozcan Erel, O. Increased DNA damage and oxidative stress in patients with cutaneous leishmaniasis. Mutat. Res. 2005, 585, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Serarslan, G.; Yılmaz, H.R.; Sogut, S. Serum antioxidant activities, malondialdehyde and nitric oxide levels in human cutaneous leishmaniasis. Clin. Exp. Dermatol. 2005, 30, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Van Assche, T.; Deschacht, M.; Inocêncio da Luz, R.A.; Maes, L.; Cos, P. Leishmania–macrophage interactions: Insights into the redox biology. Free Rad. Biol. Med. 2011, 51, 337–351. [Google Scholar] [CrossRef]
- Daneshvar, H.; Wyllie, S.; Phillips, S.; Hagan, P.; Burchmore, R. Comparative proteomics profiling of a gentamicin-attenuated Leishmania infantum cell line identifies key changes in parasite thiol-redox metabolism. J. Proteom. 2012, 75, 1463–1471. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Lisi, S.; Mitolo, V.; Acquafredda, A.; Fasanella, A.; Carelli, M.G.; Brandonisio, O. Evaluation of killing, superoxide anion and nitric oxide production by Leishmania infantum-infected dog monocytes. Cytobios 1998, 95, 151–160. [Google Scholar]
- Britti, D.; Sconza, S.; Morittu, V.M.; Santori, D.; Boari, A. Superoxide dismutase and Glutathione peroxidase in the blood of dogs with Leishmaniasis. Vet. Res. Commun. 2008, 32, S251–S254. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Ravicini, S.; Rossi, G.; Roura, X. Serum concentrations of the derivatives of reactive oxygen metabolites (d-ROMs) in dogs with leishmaniosis. Vet. J. 2010, 186, 393–395. [Google Scholar] [CrossRef] [PubMed]
- Heidarpour, M.; Soltani, S.; Mohri, M.; Khoshnegah, J. Canine visceral leishmaniasis: Relationships between oxidative stress, liver and kidney variables, trace elements, and clinical status. Parasitol. Res. 2012, 111, 1491–1496. [Google Scholar] [CrossRef]
- Almeida, B.F.M.; Narciso, L.G.; Melo, L.M.; Preve, P.P.; Bosco, A.M.; Lima, V.M.F.; Ciarlini, P.C. Leishmaniasis causes oxidative stress and alteration of oxidative metabolism and viability of neutrophils in dogs. Vet. J. 2013, 198, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Longoni, S.S.; Sanchez-Moreno, M.; Rivera Lopez, J.E.; Marin, C. Leishmania infantum secreted iron superoxide dismutase purification and its application to the diagnosis of canine Leishmaniasis. Comparative Immunology. Microbiol. Infect. Dis. 2013, 36, 499–506. [Google Scholar]
- Souza, C.C.; Barreto, T.D.O.; da Silva, S.M.; Pinto, A.W.J.; Figueiredo, M.M.; Ferreira Rocha, O.G.; Silvia, D.; Cangussu, S.D.; Tafuri, W.L. A potential link among antioxidant enzymes, histopathology and trace elements in canine visceral leishmaniasis. Int. J. Exp. Path 2014, 95, 260–270. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Sousa, K.C.M.; André, M.R.; Guarda, N.S.; Moresco, R.N.; Herrera, H.M.; Machado, R.Z.; Jaques, J.A.; Tinucci-Costa, M.; Da Silva, A.S. Nitric Oxide, Protein Oxidation and Total Antioxidant Levels in Serum of Dogs Naturally Infected by Ehrlichia canis, Leishmania infantum and Babesia vogeli. Acta Sci. Vet. 2015, 43, 1320. [Google Scholar]
- Laskay, T.; van Zandbergen, G.; Solbach, W. Neutrophil granulocytes—Trojan horses for Leishmania major and other intracellular microbes? Trends Microbiol. 2003, 1, 210–214. [Google Scholar] [CrossRef]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors 2011, 4, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berni, M.; Gigante, P.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Bisphenol S, a Bisphenol A alternative, impairs swine ovarian and adipose cell functions. Domest Anim. Endocrinol. 2019, 66, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Grolli, S.; Ramoni, R.; Conti, V.; Quintavalla, F.; Grasselli, F. Platelets are involved in in vitro swine granulosa cell luteinization and angiogenesis. Anim. Reprod. Sci. 2018, 188, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Bussolati, S.; Santini, S.E.; Grasselli, F. Reactive oxygen species and anti-oxidant defences in swine follicular fluids. Reprod. Fertil. Dev. 2008, 20, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Gigante, P.; Berni, M.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ramoni, R.; Basini, G. Glyphosate affects swine ovarian and adipose stromal cell functions. Anim. Reprod. Sci. 2018, 195, 185–196. [Google Scholar] [CrossRef]
- Ciccimarra, R.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ragionieri, L.; Ravanetti, F.; Botti, M.; Gazza, F.; Cacchioli, A.; Di Lecce, R.; et al. Orexin system in swine ovarian follicles. Domest Anim. Endocrinol. 2018, 62, 49–59. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasit Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [Green Version]
- Alvar, J.; Canavate, C.; Molina, R.; Moreno, J.; Nieto, J. Canine leishmaniasis. Adv. Parasitol. 2004, 57, 1–88. [Google Scholar]
- Pereira, M.A.; Santos, R.; Oliveira, R.; Costa, L.; Prata, A.; Goncalves, V.; Roquette, M.; Vala, H.; Santos-Gomes, G. Prognostic factors and life expectancy in canine leishmaniosis. Vet. Sci. 2020, 7, 128. [Google Scholar] [CrossRef]
- Castagnaro, M.; Crotti, A.; Fondati, A.; Gradoni, L.; Lubas, G.; Maroli, M.; Oliva, G.; Paltrinieri, S.; Solano-Gallego, L.; Roura, X.; et al. Leishmaniosi canina: Linee guida su diagnosi, stadiazione, terapia, monitoraggio e prevenzione. Veterinaria 2007, 21, 19–32. [Google Scholar]
- Gradoni, L.; Gramiccia, M. Leishmaniasis. In OIE Manual of Standards for Diagnostic Tests and Vaccine, 4th ed.; Office International des Epizooties: Paris, France, 2000; pp. 803–812. [Google Scholar]
- Lopes-Neto, B.E.; Santos, G.J.L.; Lima, A.L.; Barbosae, M.C. Catalase and glutathione peroxidase in dogs naturally infected by Leishmania infantum. Acta Sci. Vet. 2016, 44, 1360. [Google Scholar] [CrossRef] [Green Version]
- Deschacht, M.; Van Assche, T.; Hendrickx, S.; Bult, H.; Maes, L.; Cos, P. Role of oxidative stress and apoptosis in the cellular response of murine macrophages upon Leishmania infection. Parasitology 2012, 139, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Kocyigit, A.; Gur, S.; Erel, O.; Gurel, M.S. Associations among plasma selenium, zinc, copper, and iron concentrations and immunoregulatory cytokine levels in patients with cutaneous leishmaniasis. Biol. Trace Elem. Res. 2002, 90, 47–55. [Google Scholar] [CrossRef]
- Rubio, C.P.; Martinez-Subiela, S.; Hernández-Ruiz, J.; Tvarijonaviciute, A.; Ceron, J.J. Analytical validation of an automated assay for ferric-reducing ability of plasma in dog serum. J. Vet. Diagn Investig. 2017, 29, 574–578. [Google Scholar] [CrossRef] [Green Version]
- Erel, O.; Kocyigit, A.; Bulut, V.; Gurel, M.S. Reactive nitrogen and oxygen intermediates in patients with cutaneous leishmaniasis. Mem. Inst. Oswaldo Cruz 1999, 94, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Biswas, T.; Ghosh, D.K.; Mukherjee, N.; Ghosal, J. Lipid peroxidation of erythrocytes in visceral leishmaniasis. J. Parasitol. 1997, 83, 151–152. [Google Scholar] [CrossRef]
- Solcà, M.S.; Andrade, B.B.; Abbehusen, M.M.C.; Teixeira, C.R.; Khouri, R.; Valenzuela, J.C.; Kamhawi, S.; Bozza, P.T.; Bittencourt Mothé Fraga, D.; Matos Borges, V.; et al. Circulating Biomarkers of Immune Activation, Oxidative Stress and Inflammation Characterize Severe Canine Visceral Leishmaniasis. Sci. Rep. 2016, 6, 32619. [Google Scholar] [CrossRef]
- Holzmuller, P.; Bras-Goncalves, R.; Lemesre, J.L. Phenotypical characteristics, biochemical pathways, molecular targets and putative role of nitric oxide-mediated programmed cell death in Leishmania. Parasitology 2006, 132, S19–S32. [Google Scholar] [CrossRef]
- Rubio, C.P.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Hernández-Ruiz, J.; Pardo-Marin, L.; Segarra, S.; Ceron, J.J. Changes in serum biomarkers of oxidative stress after treatment for canine leishmaniosis in sick dogs. Comp. Immunol. Microbiol. Infect. Dis. 2016, 49, 51–57. [Google Scholar] [CrossRef]

| Dog | Breed | Age (Years) | Sex |
|---|---|---|---|
| 1 | Mongrel | 4 | F |
| 2 | Mongrel | 10 | F |
| 3 | Beagle | 7 | F |
| 4 | Mongrel | 5 | F |
| 5 | Beagle | 4 | M |
| 6 | Beagle | 9 | M |
| 7 | English setter | 3 | M |
| 8 | Mongrel | 4 | M |
| 9 | Mongrel | 3.5 | M |
| 10 | Mongrel | 6 | M |
| 11 | Mongrel | 5 | M |
| 12 | Mongrel | 4 | M |
| 13 | Poddle, Miniature | 9 | M |
| 14 | Mongrel | 4 | M |
| 15 | Mongrel | 3 | F |
| 16 | Mongrel | 7.5 | F |
| 17 | Mongrel | 9 | F |
| 18 | Mongrel | 4 | F |
| 19 | Riesenschnauzer | 5 | F |
| 20 | Mongrel | 6 | M |
| 21 | Mongrel | 3 | M |
| 22 | Mongrel | 4 | M |
| 23 | Dachshund | 10.5 | M |
| 24 | Labrador retriever | 8 | M |
| Breed | Age (Years) | Sex | BW (kg) | |
|---|---|---|---|---|
| Stage I mild disease with negative to low positive antibody levels | Epagneul Breton | 5 | F | 14 |
| German Shepherd dog | 3.5 | M | 46 | |
| Mongrel | 6 | Fs | 12 | |
| Mongrel | 5 | M | 12 | |
| Mongrel | 5 | M | 23 | |
| Mongrel | 6 | F | 26 | |
| Dachshund | 10 | M | 12 | |
| Pug | 2 | F | 10 | |
| Stage II moderate disease with low to high positive antibody levels | Pug | 3 | F | 9 |
| German Shepherd dog | 4 | F | 40 | |
| Mongrel | 3.5 | F | 8 | |
| English Pointer | 3 | F | 18 | |
| English Cocker Spaniel | 6.5 | M | 14 | |
| Mongrel | 1.3 | F | 9 | |
| Bernese mountain dog | 7 | M | 37 | |
| Epagneul Breton | 4.6 | F | 17 | |
| French Bulldog | 4.8 | M | 13 | |
| Stage III severe disease with medium to high positive antibody levels | Dogo Argentino | 2 | M | 30 |
| German Shepherd dog | 4 | M | 27 | |
| Boxer | 3 | F | 27 | |
| Pinscher | 6 | M | 3.2 | |
| Mongrel | 3 | M | 17 | |
| Epagneul Breton | 2.5 | M | 13 | |
| Mongrel | 4 | F | 12 | |
| Epagneul Breton | 3 | Fs | 32 | |
| Mongrel | 8.5 | M | 26 | |
| Bracco italiano | 1.5 | M | 26 | |
| Mongrel | 5.5 | F | 32 | |
| Stage IV very severe disease with medium to high positive antibody levels | Labrador retriever | 3 | M | 38 |
| American Staffordshire terrier | 1 | M | 23 | |
| Pinscher | 3 | M | 6 | |
| Pinscher | 5 | M | 3.5 | |
| German Shepherd dog | 5 | M | 36 | |
| Epagneul Breton | 2.5 | F | 11 | |
| Irish Setter | 4.5 | M | 29 | |
| St Bernard dog | 3 | F | 67 | |
| Mongrel | 5 | M | 22 | |
| Cane da Pastore maremmano abruzzese | 4 | Fs | 44 | |
| German Shepherd dog | 1.5 | M | 36 | |
| Mongrel | 7 | Fs | 25 | |
| German Shepherd dog | 2 | M | 39 | |
| German Shepherd dog | 3.5 | M | 31 | |
| Shar Pei | 3 | M | 12 | |
| Jack Russell terrier | 3 | F | 8.5 | |
| Mongrel | 5 | M | 30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintavalla, F.; Basini, G.; Bussolati, S.; Carrozzo, G.G.; Inglese, A.; Ramoni, R. Redox Status in Canine Leishmaniasis. Animals 2021, 11, 119. https://doi.org/10.3390/ani11010119
Quintavalla F, Basini G, Bussolati S, Carrozzo GG, Inglese A, Ramoni R. Redox Status in Canine Leishmaniasis. Animals. 2021; 11(1):119. https://doi.org/10.3390/ani11010119
Chicago/Turabian StyleQuintavalla, Fausto, Giuseppina Basini, Simona Bussolati, Gennaro Giuseppe Carrozzo, Antonio Inglese, and Roberto Ramoni. 2021. "Redox Status in Canine Leishmaniasis" Animals 11, no. 1: 119. https://doi.org/10.3390/ani11010119





