Use of GnRH Agonist in Dogs Affected with Leishmaniosis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Ethical Statement
2.3. Clinical Evaluation
2.4. Samples Collection
2.5. Laboratory Testing
2.6. Treatment Protocol
2.7. Statistical Analysis
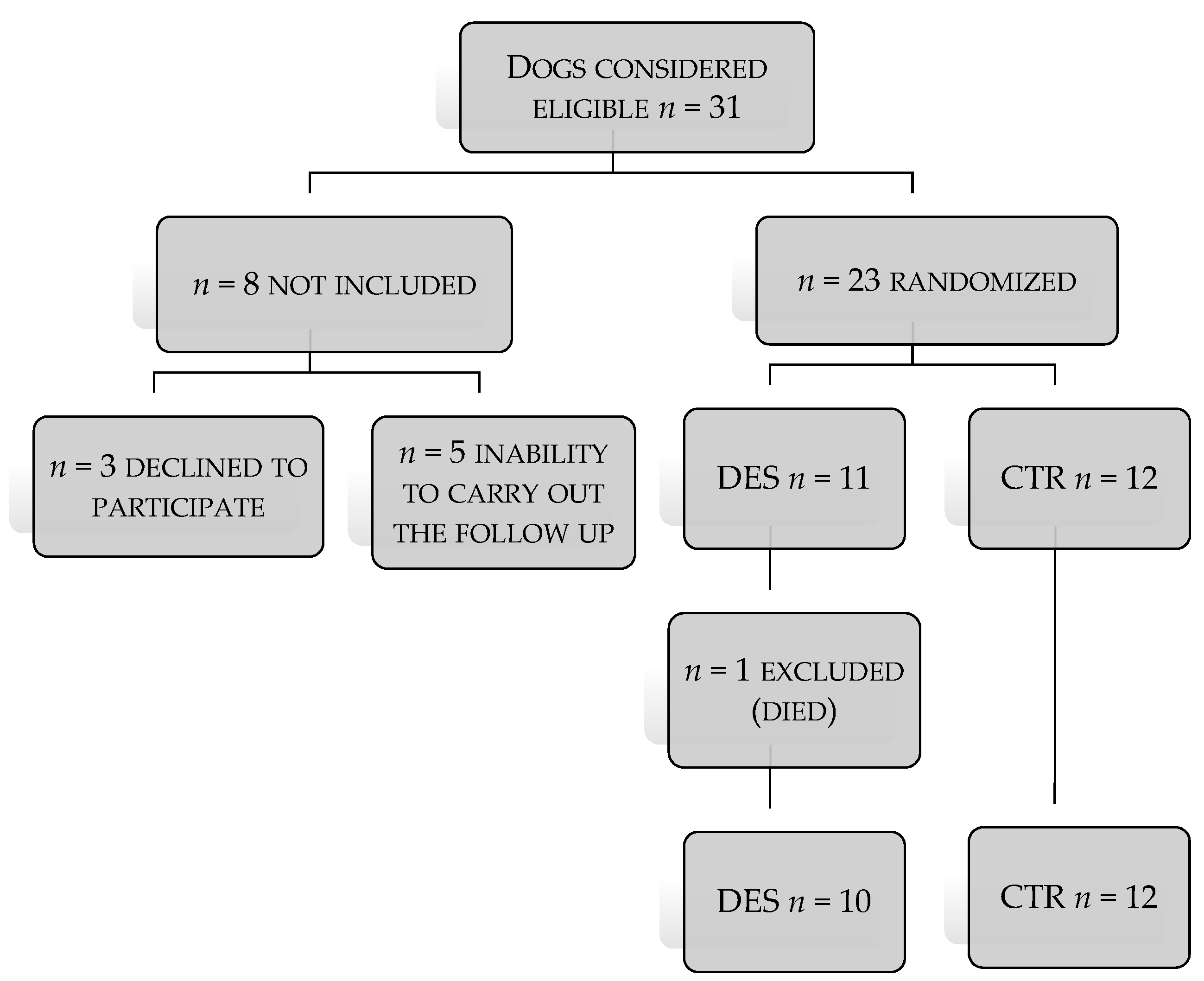
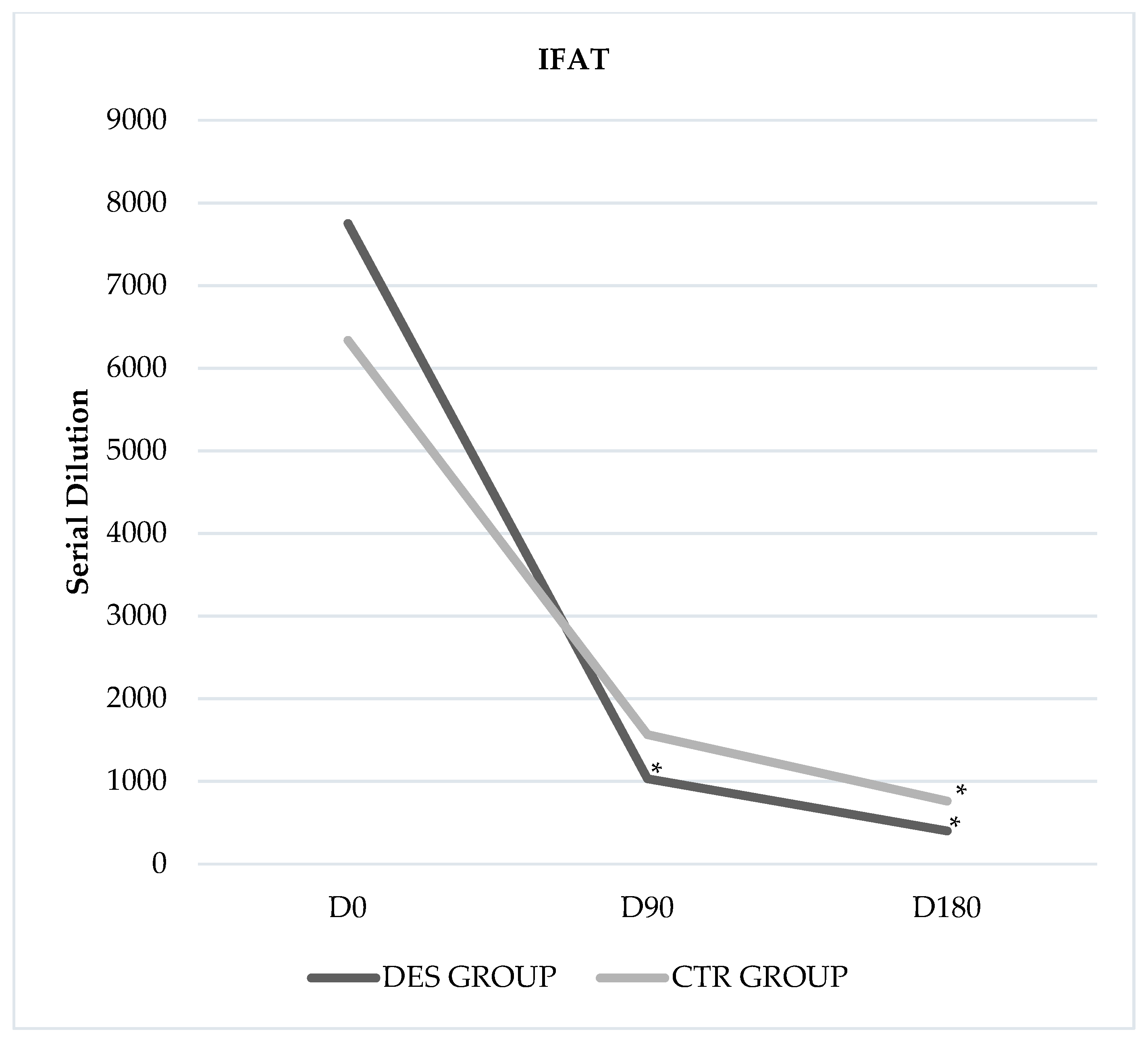
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gradoni, L. Epizootiology of Canine Leishmaniasis in Southern Europe. In Canine Leishmaniasis: An Update; Killick-Kendrick, R., Ed.; Hoechst Roussel Vet: Wiesbaden, Germany, 1999; pp. 32–39. [Google Scholar]
- Travi, B.L.; Ferro, C.; Cadena, H.; Montoya-lerma, J.; Adler, G.H. Canine visceral leishmaniasis: Dog infectivity to sand flies from non-endemic areas. Res. Vet. Sci. 2002, 72, 83–86. [Google Scholar] [CrossRef]
- De Lima, V.M.F.; Goncalves, M.E.; Ikeda, F.A.; Luvizotto, M.C.R.; Feitosa, M.M. Anti-Leishmania antibodies in cerebrospinal fluid from dogs with visceral leishmaniasis. Braz. J. Med. Biol. Res. 2003, 36, 485–489. [Google Scholar] [CrossRef]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, F.; Cano, J.J.; Jannin, J.; den Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Maroli, M.; Feliciangeli, M.D.; Bichaud, L.; Charrel, R.N.; Gradoni, L. Phlebotomine sandflies and the spreading of leishmaniasis and other diseases of public health concern. Med. Vet. Entomol. 2013, 27, 123–147. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Koutinas, A.; Mirò, G.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009, 165, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, E.; Ahmed, S.; Goldsmith-Pestana, K.; Nieto, J.; Osorio, Y.; Travi, B.; Moreno, J.; McMahon-Pratt, D. Immunogenicity of the P-8 amastigote antigen in the experimental model of canine visceral leishmaniasis. Vaccine 2007, 25, 1534–1543. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Barbiéri, C. Immunology of canine leishmaniasis. Parasite Immunol. 2006, 28, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Reis, A.; Giunchetti, R.; Carrillo, E.; Martins-Filho, O.; Moreno, J. Immunity to Leishmania and the rational search for vaccines against canine leishmaniasis. Trends Parasitol. 2010, 26, 341–349. [Google Scholar] [CrossRef]
- Foglia Manzillo, V.; Di Muccio, T.; Cappiello, S.; Scalone, A.; Paparcone, R.; Fiorentino, E.; Gizzarelli, M.; Gramiccia, M.; Gradoni, L.; Oliva, G. Prospective study on the incidence and progression of clinical signs in naïve dogs naturally infected by Leishmania infantum. PLoS Negl. Trop. Dis. 2013, 7, e2225. [Google Scholar] [CrossRef]
- Hosein, S.; Blake, D.P.; Solano-Gallego, L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitology 2017, 144, 95–115. [Google Scholar] [CrossRef]
- Oliva, G.; Roura, X.; Crotti, A.; Maroli, M.; Castagnaro, M.; Gradoni, L.; Lubas, G.; Paltrinieri, S.; Zatelli, A.; Zini, E. Guidelines for treatment of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1192–1198. [Google Scholar] [CrossRef]
- Koutinas, A.F.; Koutinas, C.K. Pathologic mechanisms underlying the clinical findings in canine Leishmaniosis due to Leishmania infantum/chagasi. Vet. Pathol. 2014, 51, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.D.T.; Alonso, D.P.; Vendrame, C.M.V.; Costa, D.L.; Costa, C.H.N.; Werneck, G.L.; Ribolla, P.E.M.; Goto, H. Lipoprotein lipase and PPAR alpha gene polymorphisms, increased very-low-density lipoprotein levels, and decreased high-density lipoprotein levels as risk markers for the development of visceral leishmaniasis by Leishmania infantum. Mediat. Inflamm. 2014, 2014, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Foo, Y.Z.; Nakagawa, S.; Rhodes, G.; Simmons, L.W. The effects of sex hormones on immune function: A meta-analysis. Biol. Rev. Camb. Philos. Soc. 2017, 92, 551–571. [Google Scholar] [CrossRef] [PubMed]
- Roden, A.C.; Moser, M.T.; Tri, S.D.; Mercader, M.; Kuntz, S.M.; Dong, H.; Hurwitz, A.A.; McKean, D.J.; Celis, E.; Leibovich, B.C.; et al. Augmentation of T cell levels and responses induced by androgen deprivation. J. Immunol. 2004, 173, 6098–6108. [Google Scholar] [CrossRef] [PubMed]
- Fontaine, E.; Fontbonne, A. Clinical use of GnRH agonists in canine and feline species. Reprod. Domest. Anim. 2011, 46, 344–353. [Google Scholar] [CrossRef] [PubMed]
- Trigg, T.E.; Doyle, A.G.; Walsh, J.D.; Swangchan-Uthai, T. A review of advances in the use of the GnRH agonist deslorelin in control of reproduction. Theriogenology 2006, 66, 1507–1512. [Google Scholar] [CrossRef]
- Junaidi, A.; Williamson, P.E.; Cummins, J.M.; Martin, G.B.; Blackberry, M.A.; Trigg, T.E. Use of a new drug delivery formulation of the gonadotrophin-releasing hormone analogue Deslorelin for reversible long-term contraception in male dogs. Reprod. Fertil. Dev. 2003, 15, 317–322. [Google Scholar] [CrossRef]
- Adel, A.; Berkvens, D.; Abatih, E.; Soukehal, A.; Bianchini, J.; Saegerman, C. Evaluation of Immunofluorescence Antibody Test Used for the Diagnosis of Canine Leishmaniasis in the Mediterranean Basin: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- World Organisation for Animal Health OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. Cap. 2.1.8, Par. B.2. 2008. Available online: https://www.oie.int/fileadmin/Home/eng/Health_standards/tahm/3.01.11_LEISHMANIOSIS.pdf (accessed on 25 October 2019).
- Reguera, R.M.; Morán, M.; Pérez-Pertejo, Y.; García-Estrada, C.; Balaña-Fouce, R. Current status on prevention and treatment of canine leishmaniasis. Vet. Parasitol. 2016, 227, 98–114. [Google Scholar] [CrossRef]
- Roberts, C.W.; Walker, W.; Alexander, J. Sex-associated hormones and immunity to protozoan parasites. Clin. Microbiol. Rev. 2001, 14, 476–488. [Google Scholar] [CrossRef]
- Cloots, K.; Burza, S.; Malaviya, P.; Hasker, E.; Kansal, S.; Mollett, G.; Chakravarty, J.; Roy, N.; Lal, B.K.; Rijal, S.; et al. Male predominance in reported Visceral Leishmaniasis cases: Nature or nurture? A comparison of population-based with health facility-reported data. PLoS Negl. Trop. Dis. 2020, 14, e0007995. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Suprelorin 5 June 2012. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/veterinary/medicines/000109/vet_med_000189.jsp&mid=WC0b01ac058001fa1c (accessed on 27 October 2019).
- Courtenay, O.; Quinnell, R.J.; Garcez, L.M.; Dye, C. Low infectiousness of a wildlife host of Leishmania infantum: The crab-eating fox is not important for transmission. Parasitology 2002, 125, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Borja, L.S.; De Souza, O.M.F.; Solcá, M.S.; Bastos, l.A.; Bordoni, M.; Magalhães, J.T.; Larangeira, D.F.; Barrouin-Melo, S.M.; Fraga, D.B.M.; Veras, P.S.T. Parasite load in the blood and skin of dogs naturally infected by Leishmania infantum is correlated with their capacity to infect sand fly vectors. Vet. Parasitol. 2016, 229, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Mock, B.A.; Nacy, C.A. Hormonal modulation of sex differences in resistance to Leishmania major systemic infections. Infect. Immun. 1988, 56, 3316–3319. [Google Scholar] [CrossRef]
- Alexander, J. Sex differences and cross-immunity in DBA/2 mice infected with L. mexicana and L. major. Parasitology 1988, 96, 297–302. [Google Scholar] [CrossRef]
- Satoskar, A.; Al-Quassi, H.H.; Alexander, J. Sex-determined resistance against Leishmania mexicana is associated with the preferential induction of a Th1-like response and IFN-gamma production by female but not male DBA/2 mice. Immun. Cell Biol. 1988, 76, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kobets, T.; Havelková, H.; Grekov, I.; Volkova, V.; Vojtíšková, J.; Slapničková, M.; Kurey, I.; Sohrabi, Y.; Svobodová, M.; Demant, P.; et al. Genetics of host response to Leishmania tropica in mice—Different control of skin pathology, chemokine reaction, and invasion into spleen and liver. PLoS Negl. Trop. Dis. 2012, 6, e1667. [Google Scholar] [CrossRef]
- Munoz, G.; Davies, C.R. Leishmania panamensis transmission in the domestic environment: The results of a prospective epidemiological survey in Santander, Columbia. Biomedica 2006, 26, 131–144. [Google Scholar] [CrossRef][Green Version]
- Turetz, M.L.; Machado, P.R.; Ko, A.I.; Alves, F.; Bittencourt, A.; Almeida, R.P.; Mobashery, N.; Johnson, W.D.; Carvalho, E.M. Disseminated leishmaniasis: A new and emerging form of leishmaniasis observed in northeastern Brazil. J. Infect. Dis. 2002, 186, 1829–1834. [Google Scholar] [CrossRef]
- Brilhante, A.F.; Melchior, L.A.K.; Nunes, V.L.B.; Cardoso, C.d.O.; Galati, E.A.B. Epidemiological aspects of American cutaneous leishmaniasis (ACL) in an endemic area of forest extractivist culture in western Brazilian Amazonia. Rev. Inst. Med. Trop. São Paulo 2017, 59. [Google Scholar] [CrossRef] [PubMed]
- Johannsen, T.H.; Main, K.M.; Ljubicic, M.L.; Jensen, T.K.; Andersen, H.R.; Andersen, M.S.; Petersen, J.H.; Andersson, A.-M.; Juul, A. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. J. Clin. Endocrinol. Metab. 2018, 103, 3028–3037. [Google Scholar] [CrossRef] [PubMed]
| Clinical Signs | Scores | |||
|---|---|---|---|---|
| 0 | 1 | 2 | 3 | |
| Appetite | Normal | Slight decrease | Moderate decrease | Anorexia |
| Mentation | Normal | Slight depression | Depression | Prostration |
| Exercise Intolerance | No | Slight | Moderate | Refusal to move |
| Weight Loss | No | Slight | Moderate | Severe |
| Polyuria | No | Slight | Moderate | Severe |
| Polydipsia | No | Slight | Moderate | Severe |
| Urine Protein/Urine Creatinine | No | <1 | >1 < 2 | >2 |
| Localized Muscle Atrophy (Temporal Muscles) | No | Slight | Moderate | Severe |
| Generalized Muscle Atrophy | No | Slight | Moderate | Severe |
| Lymph Adenomegaly | No | 1–2 nodes | >2 < 4 nodes | Generalized |
| Splenomegaly | No | - | Yes | - |
| Conjunctivitis and/or Blepharitis | No | Unilateral and slight | Bilateral or unilateral severe | Bilateral and severe |
| Uveitis and/or Keratitis | No | Unilateral and slight | Bilateral or unilateral severe | Bilateral and severe |
| Pale Mucous Membranes | No | Slight | Moderate | Severe |
| Epistaxis | Never presented | Sporadic | Frequent | Persistent |
| Mouth Ulcers or Nodules | No | 1 or 2 small ulcers or nodules | >2 small ulcers or nodules | >1/4 of oral cavity covered by ulcers or nodules |
| Vomiting | No | Sporadic | Frequent | Frequent with blood |
| Diarrhoea | No | Sporadic | Frequent | Persistent |
| Lameness | No | Sporadic | Frequent | Constant |
| Erythema | No | <10% body surface or slight generalized erythema | 10–25% body surface or moderate generalized erythema | >25% body surface |
| Dry Exfoliative Dermatitis | No | <10% body surface or slight generalized erythema | 10–25% body surface or moderate generalized erythema | >25% body surface |
| Ulcerative Dermatitis | No | 1–2 ulcers | 3–5 ulcers | >5 ulcers |
| Nodular Dermatitis | No | 1–2 nodules | 3–5 nodules | >5 nodules |
| Sterile Pustular Dermatitis | No | 1–2 pustules | 3–5 pustules | >5 pustules |
| Alopecia | No | <10% body surface | 10–25% body surface erythema | >25% body surface |
| Altered Pigmentation | No | Localized | Multifocal | Generalized |
| Hyperkeratosis Truffle and Pads | No | Slight | Moderate | Severe |
| Generalized Hyperkeratosis | No | Slight | Moderate | Severe |
| Variable | DES Group | CTR Group | p-Value |
|---|---|---|---|
| Age (Months) | 58.7 ± 29.3 | 62.6 ± 18.1 | 0.94 |
| Clinical Score (Points) | 14.8 ± 5.8 | 11.2 ± 5.2 | 0.82 |
| Body Temperature (°C) | 39.4 ± 0.8 | 39.6 ±−0.81 | 0.46 |
| Weight (kg) | 18.6 ± 10.4 | 21 ± 8.4 | 0.79 |
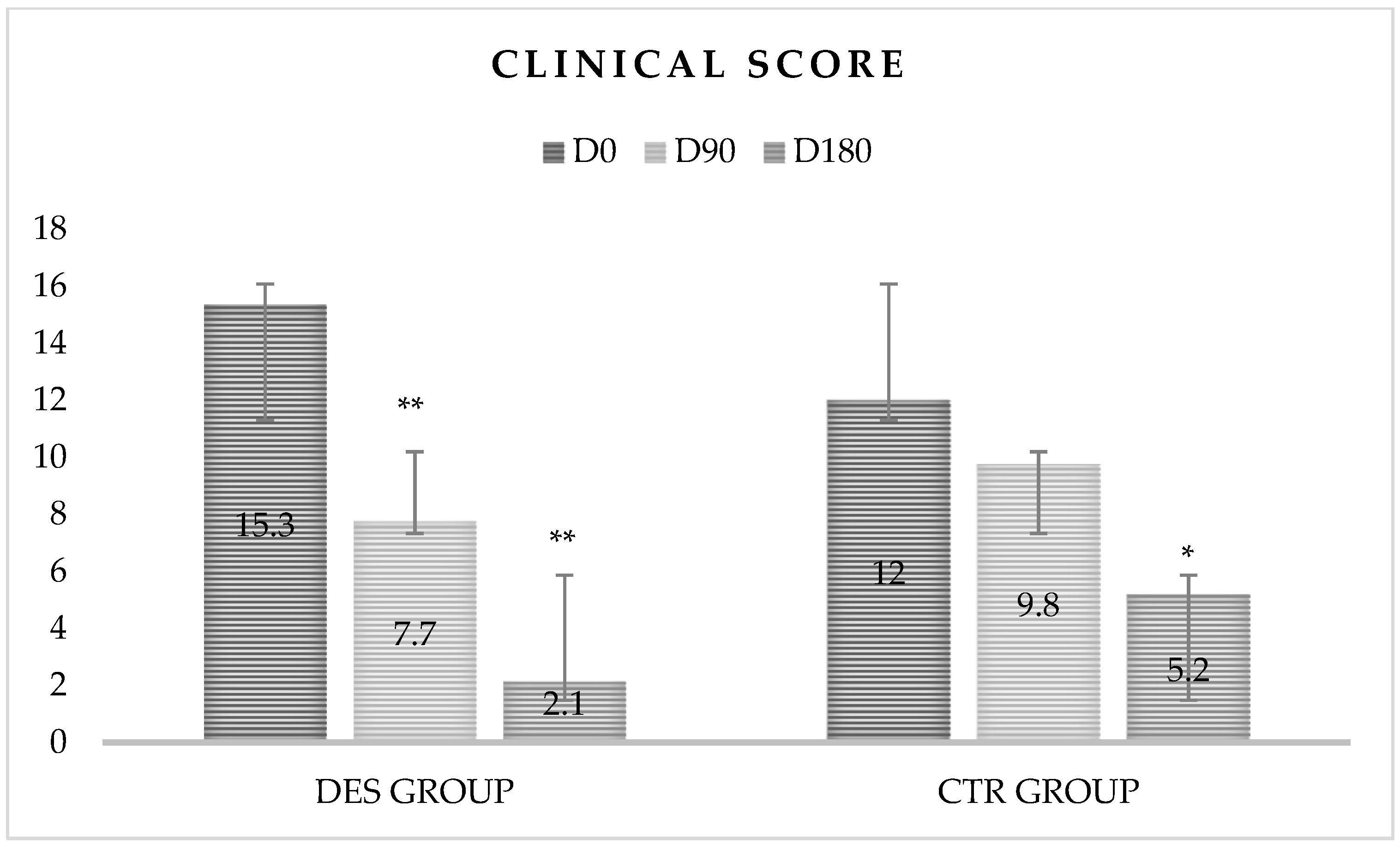
| Time | Clinical Score | |
|---|---|---|
| Day | DES Group | CTR Group |
| D0 | 15.3 ± 6.3 ABC | 12 ± 4.9 |
| D90 | 7.7 ± 5.9 ACa | 9.8 ± 4.3 Bb |
| D180 | 2.1 ± 4.3 ABa | 5.2 ± 4.1 Ab |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, M.; Biondi, V.; Quartuccio, M.; Cristarella, S.; Emmanuele, G.; Marino, G.; Liotta, L.; Passantino, A. Use of GnRH Agonist in Dogs Affected with Leishmaniosis. Animals 2021, 11, 432. https://doi.org/10.3390/ani11020432
Pugliese M, Biondi V, Quartuccio M, Cristarella S, Emmanuele G, Marino G, Liotta L, Passantino A. Use of GnRH Agonist in Dogs Affected with Leishmaniosis. Animals. 2021; 11(2):432. https://doi.org/10.3390/ani11020432
Chicago/Turabian StylePugliese, Michela, Vito Biondi, Marco Quartuccio, Santo Cristarella, Giovanni Emmanuele, Gabriele Marino, Luigi Liotta, and Annamaria Passantino. 2021. "Use of GnRH Agonist in Dogs Affected with Leishmaniosis" Animals 11, no. 2: 432. https://doi.org/10.3390/ani11020432
APA StylePugliese, M., Biondi, V., Quartuccio, M., Cristarella, S., Emmanuele, G., Marino, G., Liotta, L., & Passantino, A. (2021). Use of GnRH Agonist in Dogs Affected with Leishmaniosis. Animals, 11(2), 432. https://doi.org/10.3390/ani11020432










