1. Introduction
Health status and wellness of animals in vivo has been related to the meat quality of final products [
1,
2]. Consequently, many dietary interventions have been investigated recently in order to ensure the increasing demand of high nutritional value and high-quality meat [
3].
Oleuropein is one of the most abundant polyphenol compounds present in the olive leaves (
Olea europaea) but it has also isolated from other plant species and fruits [
4]. Recent studies show the positive effects of olive by-products, not only thanks to their antioxidant properties [
4], but also due to their capacity to modify nutrient metabolism, with changes in glucose uptake, lipid, and amino acid profiles [
5] that may affect meat quality [
6,
7]. Thus, oleuropein has shown capacity to catch free radicals and metal ions and it has been related with an increase in the glutathione route, cystine and monounsaturated blood levels, as well as reduced cortisol under stressful conditions [
5] and other interesting pharmacological and anti-inflammatory effects [
4]. In addition, the combination of olive extracts such as oleuropein has been reported to have synergistic effects in vivo with other antioxidants such as vitamin E [
5,
6]. Vitamin E is one of the most studied compounds in the literature because of its effects in vivo [
5,
8] and post-mortem [
9], explained by its incorporation into biological membranes and consequent accumulation in different tissues. Vitamin E has been shown to be effective in improving colour and lipid stability, muscle proteolysis [
10] or in reducing drip loss. However, their presence or function can be modified by the interaction with other compounds [
9,
11,
12].
Betaine is the most effective methyl group donor [
13] involved in energy and protein metabolism [
14]. It is commonly added to diets because its supplementation increases energy value [
13] and performance [
15]. Dietary betaine supplementation has also been reported to modify some meat quality characteristics [
16] and could have interesting effects in terms of controlling stress [
14]. Recently, it has been found that the regulating fatty acid metabolism effect of betaine is associated with the up-regulation of genes involved in fatty-acid transportation and fatty-acid oxidation [
17], which may modify the fatty acid profile. Moreover, the combination of dietary betaine and vitamin E has been reported to reduce meat TBARS and improve the oxidative status to a greater extent than betaine alone [
18].
Another additive that participates in fat and glucose metabolism is magnesium. In this way, magnesium is involved in many enzymatic reactions in the organism and is essential for oxidation-reduction reactions and phosphorylation processes in charge of high-energy compounds [
19]. Levels of 0.05–0.06% are recommended to fulfil requirements [
20]. Magnesium has also been reported to improve meat quality, mainly by reducing drip loss [
3], and it modifies the fatty acid profile in combination with selenium [
21]. Hence, its in vivo effects on dyslipidaemia are explained by lipoprotein lipase and desaturase stimulation [
22].
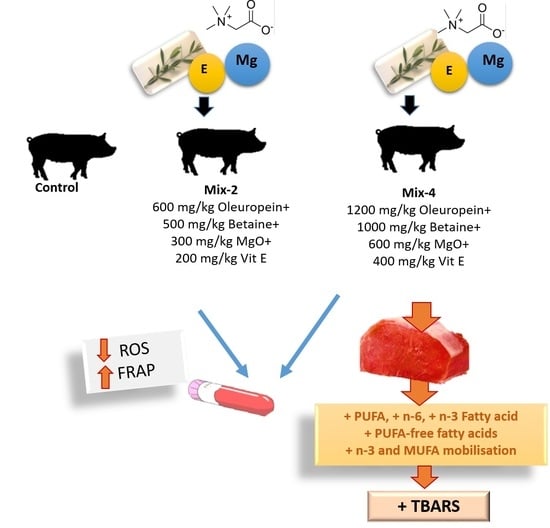
These compounds show antioxidant and/or metabolic effects on the organism that are reflected, in many cases, in meat quality. However, to our knowledge, the combination of these nutrients have not been evaluated together. Consequently, the aim of the present investigation was to study the effect of the dietary mixture at a higher dose of oleuropein extract (1200 mg/kg) with betaine (1000 mg/kg), magnesium oxide (600 mg/kg) and α–tocopheryl acetate (400 mg/kg), or a half-dose of these compounds (600 mg of oleuropein extract/kg; with 500 mg betaine/kg, 300 mg magnesium oxide/kg and 200 mg α–tocopheryl acetate/kg), on pigs’ performance and meat quality characteristics (drip loss, TBARS, colour changes and fatty acid profile of intramuscular fat).
3. Results
The supplementation of two treatments in fattening pigs diet did not modify (
p > 0.05) the pigs’ performance (body weight: BW or average daily gain: ADWG, average daily intake: ADFI and feed conversion ratio: FCR) (
Table 2). Carcass yield (carcass lean %, lean % of ham and loin, carcass fat thickness, and weight or percentage of ham, shoulder, loin, or belly) was changed by neither dietary supplementation of the two additive-mixture (
p > 0.05).
Glucose was reduced with the high-dose mixture (
p = 0.03) (
Table 3), whereas the use of the lower doses did not affect (
p > 0.05) glucose levels when compared to the control group. However, the use of the half-dose mixture increased the concentration of α-tocopherol in serum (
p = 0.0001) when compared with the control group, but no differences were observed with the high-dose mixture. Moreover, supplemented groups resulted in higher serum FRAP (
p = 0.0026) and a tendency to have lower serum TBARS (
p = 0.07) when compared to the control.
The muscle composition of
longissimus lumborum is presented in
Table 3. Percentage of drip loss, moisture content and intramuscular fat were not modified by treatments (
p > 0.05). On the contrary, α-tocopherol in the muscle increased with the supplementation dose (
p = 0.0011), whereas TBARS production increased in the group supplemented with the high-dose mixture at day 7 of refrigerated storage when compared to the control (
p = 0.033).
In terms of the texture parameters of muscle (
Table 3), no changes were found with dietary supplementation in muscles’ hardness, adhesiveness, springiness, cohesiveness, gumminess and chewiness when compared to the control group (
p > 0.05).
The intramuscular fatty acid profiles of neutral, free and polar fatty acids are presented in
Table 4,
Table 5 and
Table 6, respectively. The high-dose mixture resulted in higher C18:2n-6 (
p = 0.0001), C20:3n-6 (
p = 0.0006), C20:4n-6 (
p = 0.0001), C22:4n-6 (
p = 0.0001), and C22:6n-3 (
p = 0.0001) in the neutral lipid fraction, whereas C18:1n-9 (
p = 0.0092) and C22:5n-3 (
p = 0.0001) were lower in neutral lipids when compared with the other groups. The half-dose mixture did not differ in most of the fatty acids of the neutral lipid fraction compared with the control group, except for C20:5n-3 and C22:5n-3 that had a lower proportion compared with the control (
p < 0.0001). These effects resulted in a higher proportion of PUFA (
p = 0.0001), n-6 (
p = 0.0001), n-3 (
p = 0.0095), and n6/n3 (
p = 0.0001) in the neutral fraction of the high-dose mixture supplemented pigs, whereas the proportion of total MUFA was lower (
p = 0.0064). No changes between the control and half-dose mixture were detected in the main groups of fatty acids.
Changes were also observed in the free fatty acid fraction (
Table 5). Pigs supplemented with the high-dose mixture had a higher proportion of C20:0 (
p = 0.0001), C20:3n-6 (
p = 0.0001), C22:4n-6 (
p = 0.0034), C22:5n-3 (
p = 0.0003), and C22:6n-3 (
p = 0.0003) in the free fatty acid fraction compared with the other groups. Consequently, pigs that received the high-dose mixture had a higher proportion of n-6 (
p = 0.033) and n-3 (
p = 0.0009) free fatty acids when compared to the other groups, and tended to have higher free PUFA (
p = 0.06) when compared to the control. Pigs given the half-dose mixture had a similar free fatty acid proportion to the control (
p > 0.05).
The fatty acid composition of the polar lipid fraction is presented in
Table 6. Pigs supplemented with the high-dose mixture had lower C16:1n-9 (
p = 0.0001), C16:1n-7 (
p = 0.0002), C17:0 (
p = 0.0001), C17:1 (
p = 0.0001), C18:2n-6 (
p = 0.0021), C18:3n-3 (
p = 0.043) and PUFA (
p = 0.0074) and tended to have higher MUFA (
p = 0.056) in the polar lipid fraction when compared to the control group. Pigs fed the half-dose mixture did not differ in SAT or MUFA polar lipid fractions with the aforementioned fatty acids, except for C18:2 n-6 (
p = 0.0021) and PUFA proportions (
p = 0.0074), which were lower compared with the control group and similar to those reported for the higher-dose mixture.
The ratio free-fatty acids/neutral lipids as indicator of relative mobilization/hydrolysis is presented in
Figure 1. Pigs supplemented with the high-dose mixture had higher n-3 and MUFA values (
p < 0.05) and lower SAT and PUFA ratios. Pigs fed the half-dose mixture had intermediate values of n-3 and MUFA and did not differ in PUFA ratio when compared with the control group.
4. Discussion
Most studies have evaluated the effects of betaine, oleuropein, vitamin E, or magnesium on meat quality separately. This is the first study evaluating the combined effects of these nutrients. These compounds were chosen due to their positive effects as antioxidant agents, so they would be expected to have additive effects on pig’s meat quality. Two different combinations of doses (double and half dose) were therefore investigated in order to give the most recommended mixture’s dose.
In the present study, no differences in ADWG, ADFI, or FCR were observed in pigs supplemented with the experimental diets and a lack of effects on lean or fat thickness of carcass yield was observed. Similar results have been reported through the single administration of vitamin E and/or oleuropein extract [
6,
30]. Other feed additives, such as magnesium, have been reported to improve the performance at 0.3% (3000 mg/kg) of MgO on the diets of swine over 7 days [
31]. Moreover, these authors also found decreased backfat thickness and improved carcass yield depending on the magnesium supplementation dose. However, the doses used in the present study were lower (300 and 600 mg/kg) than those used by Tarsitano et al. [
31]. In terms of betaine supplementation, there are contradictory results; Fernandez-Figares et al. [
32] observed no effects on ADWG and FCR in pigs (from 36 to 64 kg) using betaine at 0.12, 0.25, or 0.5% (1200, 2500, or 5000 mg/kg), but found fat concentration to be reduced by treatments. However, Siljander-Rasi et al. [
33] found that the use of betaine at 250, 500, and 1000 mg/kg resulted in a linearly improvement of the ADWG and F/G ratio. In a meta-analysis carried out by Sales [
34], betaine supplementation (from 1000 to 5000 mg/kg) in growing-finishing pigs decreased carcass fat, whereas no significant effect was found on ADWG. In the present study, the combination of these four compounds did not show additive effects on these parameters.
Concerning the effect of the half-dose or high-dose mixture on blood parameters as indicators of the glycemic muscle state that could modify meat characteristics, a decrease of serum glucose was observed with the high-dose mixture supplementation, which might be explained by the described hypoglycemic effect of oleuropein extract [
35] or its combination with other compounds such as magnesium or betaine. The lack of effects of the half-dose mixture on diminishing glucose levels was also observed in other works when lower doses of oleuropein (192 mg/kg) were combined with vitamin E (100 mg/kg) and selenium (0.26 mg/kg) [
5], and this was attributed to the possible contrary effects of these compounds on glucose concentrations. A lower accumulation of vitamin E has therefore been associated with lower blood glucose [
36]. Meanwhile, it has been reported that lower serum levels of magnesium are related to increased glycaemia in patients with diabetes [
37,
38]. However, betaine supplementation has shown effects on decreasing glucose at lower doses (15 g/d vs. 30 g/d) [
39] with possible effects depending on the physiological state [
40]. The half-dose mixture of oleuropein, vitamin E, magnesium, and betaine did not, therefore, modify glucose when compared to the control and would need a high-dose mixture to reduce this blood parameter.
The accumulation of α-tocopherol in serum and muscle was related with the supplementation dose and higher numbers were detected in those pigs that received more of this vitamin in feed. These differences in serum α-tocopherol could result in a favourable oxidative status in mixture-supplemented animals, however, a dose effect was not observed and half-dose and high-dose supplemented pigs had similar serum α-tocopherol and total antioxidant power. Other studies have shown the relationship between increased serum α-tocopherol concentration and antioxidant power [
8,
27], which is explained by the potent antioxidant effect of this vitamin [
8]. Moreover, lower doses of oleuropein extract (192 mg/kg) and a similar supplementation time than those used in the present study have shown antioxidant effects in vivo, explained by their contribution of the glutathione route [
5] as one of the most interesting antioxidant enzyme complexes in the organism. Other nutrients such as magnesium have been negatively associated with oxidative stress and increased TBARS production [
41], and it has been suggested that, in humans, magnesium may modulate antioxidant defenses in the organism [
42]. However, betaine supplementation has shown certain prooxidant effects [
43], whereas other studies show antioxidant [
44] or prooxidant effects depending on the dose administered [
39]. Finally, the combination of all these dietary nutrients together in the present research resulted in a favourable blood oxidative status of supplemented animals that was surprisingly not reflected in the oxidative status of meat, since the high-dose mixture supplemented pigs had a higher content of TBARS production at day 7 of refrigerated storage when compared to the control. This result was not expected taking into consideration that vitamin E concentration was higher in those pigs supplemented with the high-dose mixture, as this group also had the highest concentration of serum α-tocopherol and the relationship found between serum α-tocopherol and their accumulation in tissues [
8,
27]. Vitamin E is one of the most potent antioxidants in the organism that can be accumulated in muscle membranes, so a higher presence of this vitamin has been associated with lower TBARS production and higher lipid stability [
9]. In addition, the use of oleuropein extract has been reported to delay lipid oxidation in meat from birds [
30] and pork [
6], and similar results have been found in pork through the use of olive leaves that may contain not only oleuropein but also other antioxidant components [
45]. Lower TBARS values have also been observed in meat from pigs given a short-term feeding of magnesium supplements [
46], although supplementation doses were much higher (1500 mg/kg) than those used in the present research. In addition, the use of betaine (1000 mg/kg) alone [
47] or in combination with 200 IU/kg of vitamin E improved the TBARS production of meat [
18]. It has been reported that betaine participates in the methionine cycle through the remethylation of homocysteine to methionine, which is a precursor of cysteine, taurine, and glutathione, which have been reported to have antioxidant effects [
5,
18]. Despite the antioxidant effects of the different nutrients used in the present study, results indicate an imbalance in oxidative status between antioxidant–prooxidant components in the muscle that might be explained by differences in muscle composition not only depending on vitamin E accumulation as reported later. Moreover, the accumulation of these antioxidants in meat could be affected by the supplementation dose; however, in the present study their concentrations were not quantified in muscle with the exception of vitamin E. Hence, previous studies reported that bioavailability and tissue utilization of lipophilic antioxidants maybe affected by other antioxidant compounds of hydrophilic characteristics [
48,
49]. Since there is not previous studies in which these four compounds were combined it is difficult to assess the single supplement responsible of the specific effect or the interaction with other constituents. So further investigations would be needed to dilucidate this fact.
Concerning other meat quality characteristics, no differences were observed in the present study on the combination of different nutrients in drip loss or texture parameters. Pigs supplemented with the high-dose or low-dose mixture only showed a tendency to have higher water-holding capacity but no significant changes were found. The use of olive by-products at 5 or 10% [
45], or their combination with vitamin E [
6], has been reported to have positive effects on drip loss. Dietary supplementation with MgO at doses of 0.3% (3000 mg/kg) [
31] or higher (3.6 g/pig/day) [
50] has also been effective in reducing the drip loss of pork, however, other authors using doses of 5 g/pig/day in the form of magnesium oxide did not find clear effects [
46]. On the other hand, betaine has also shown positive effects on the water retention of meat [
47,
51]. Doses of magnesium and betaine in the present study were lower than those in which positive effects were found. However, vitamin E and oleuropein were higher than those in which positive effects on these parameters were reported (100 mg/kg of vitamin E and 192 mg/kg of oleuropein). Since water retention is related with proteolysis and texture of muscle [
10], these measurements were also quantified. As with drip loss, no significant changes were found by treatments. Other authors found a lack of effect in meat texture through the single dietary administration of magnesium oxide [
46] or betaine [
47,
51], even though it affected drip loss. There is no previous information on the combined effect of oleuropein extract, vitamin E, MgO, and betaine, but in terms of the present results, the combination of these nutrients did not show additive effects on water retention or texture parameters.
The fatty acid profile of meat was one of the characteristics most affected by the combination of the different nutrients at different doses. It has been reported that it is mainly oleuropein, betaine and magnesium that have effects on lipid metabolism through different mechanisms that result in faster lipid utilization. In the present research, pigs supplemented with the high-dose mixture had higher PUFA, n-6, n-3 and lower MUFA in the neutral lipid fraction of intramuscular fat. Free PUFA were also higher in the meat from high-dose mixture supplemented animals when compared to the others. In a previous study, doses of oleuropein around 200 mg/kg decreased total PUFA proportion and increased relative mobilization index (free-fatty acids/neutral lipids) in blood [
5], whereas the proportion of PUFA free fatty acid of muscle decreased [
6]. This was attributed to a faster glucose uptake and lipolysis initiation. However, according to the results of the present study, the combination of oleuropein at 1200 or 2400 mg/kg with other three compounds would result in a different fatty acid profile. The fatty acid profile could also be affected by betaine supplementation, although probably to a lesser extent due to the supplementation dose. According to Xu et al. [
52], the efficiency of betaine supplementation on decreasing unsaturated fatty acids is reduced at levels rising above 0.08% (800 mg/kg), which is lower than the high-dose supplementation of the present study 1000 mg/kg. In other studies, it has been reported that betaine at doses of 0.2% (2000 mg/kg) [
16] or higher decreased unsaturated fatty acids and increased saturated fatty acids in pork. Similar results were observed by Yang et al. [
53], who reported lower PUFA in the loin of pigs supplemented with 2000 mg/kg of betaine, but did not find changes in the PUFA proportion of meat from pigs fed 4000 or 6000 mg/kg. Li et al. [
17] found that betaine at 1250 or 2500 mg/kg (0.1–0.2%) increased muscle free fatty acids (similarly to what was observed in the present study for the high-dose mixture). This was attributed to differences in the balance of fatty acid uptake and oxidation, since betaine promotes fatty acid uptake, increasing the expression of transporters and enhancing fatty acid oxidation through AMPK activation. Moreover, betaine indirectly stimulates the synthesis of carnitine necessary for the transport of long-chain fatty acids to mitochondria, where they are oxidized [
54]. So according to the effects reported for oleuropein or betaine supplementation, PUFA proportions should have been depleted in pigs supplemented with the mixture. A higher uptake of n-6 and n-3 PUFA in the triglycerides fraction of intramuscular fat was observed, however, a higher hydrolysis of n-3 fatty acids was only observed in the muscle from pigs fed a high-dose mixture. It is possible that their effects could be counteracted by magnesium supplementation. Therefore, the higher proportion of PUFA in meat from the high-dose mixture might be attributed in part to the effects of this mineral on the fatty acid profile, as reported previously [
21]. The authors using 300 mg/kg of magnesium oxide reported higher proportions of PUFA and n-6 and n-3 fatty acids in pig muscle that was attributed to their effects on some desaturase enzymes. Hence, magnesium has been reported to be a cofactor for Δ5 and Δ6-desaturases [
55]. However, because in the present study four compounds were used in the dietary mixtures it is not possible to quantify the specific effect of each component on fatty acid profile.
A higher mobilization was also observed in MUFA in accordance with previous findings on oleuropein supplementation [
5], but this group of fatty acids was also found in the present study at higher proportions in polar lipids of muscle membranes of pigs fed the high-dose mixture, which would indicate that its utilization for energy supply was not as important as other unsaturated fatty acids [
56]. In addition, antioxidants have been reported to protect Δ9 desaturase enzymes [
9,
57] mainly in the lipid fraction [
9].
Despite the high potential of lipid mobilization of oleuropein and betaine, muscle from pigs supplemented with the high-dose mixture had higher PUFA in the triglyceride and free fatty acid fractions. This specific fatty acid composition of meat from pigs supplemented with the high-dose mixture could be responsible for the highest lipid oxidation observed in this group when compared to the control. Phospholipid fraction has been considered to be the main agent responsible for lipid oxidation initiation [
58], since PUFA are mainly located in lipid membranes. However, other authors have found a direct relationship between muscle free fatty acids and TBARS production [
59] and an inverse or direct correlation between serum free n-3 and free MUFA fatty acids and muscle lipid stability [
6].