Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Diet, and Experimental Design
2.2. Meat Quality Analysis
2.3. Biomarkers for Oxidative Stress and Tissue Damages
2.4. Serum and Liver Lipids
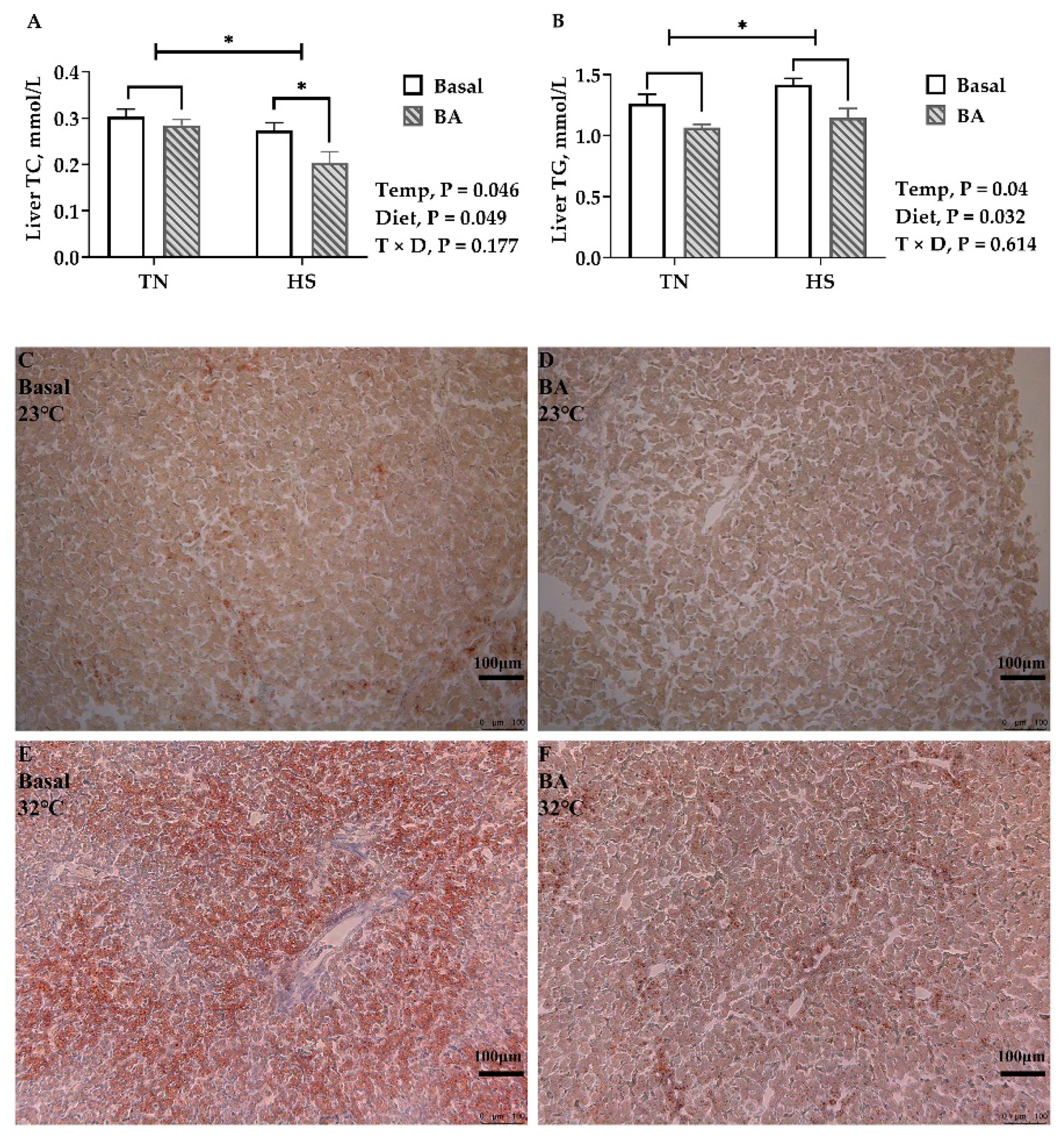
2.5. Oil Red O Staining of Liver Tissues
2.6. RNA Isolation and qPCR to Quantify Genes Related to Lipid Metabolism
2.7. Statistical Analysis
3. Results
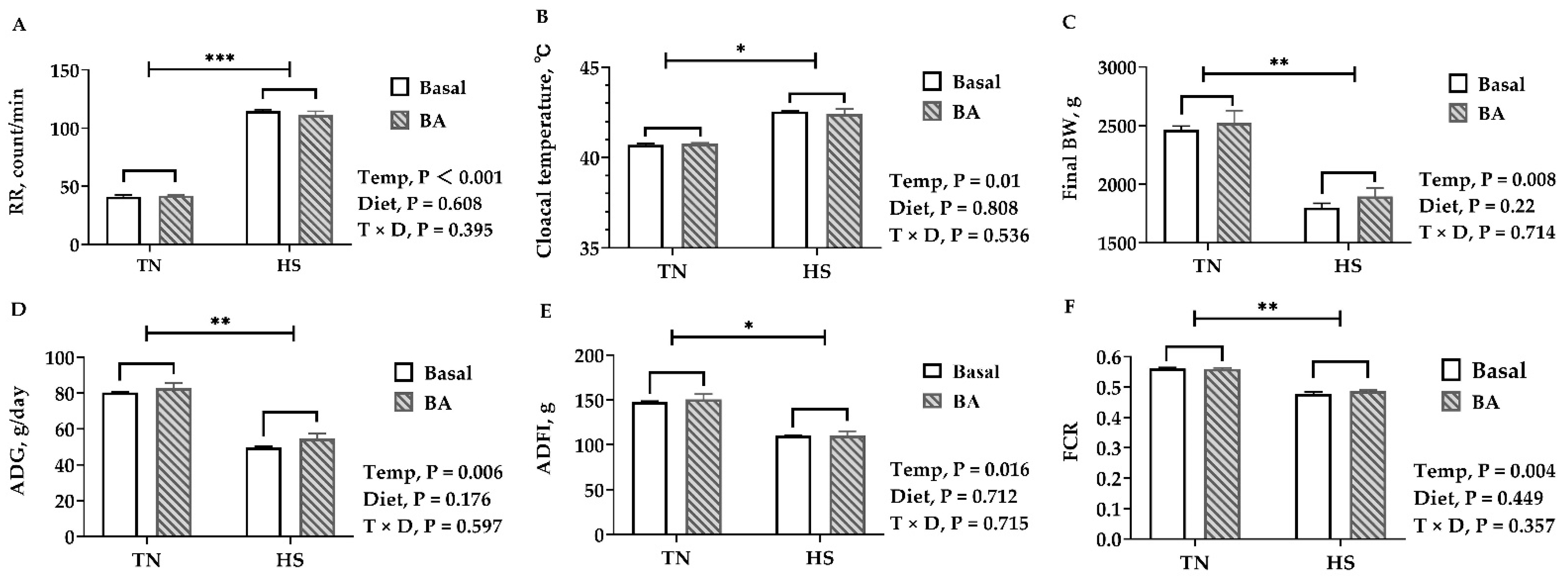
3.1. Growth and Behavioral Performance
3.2. Carcass and Meat Quality
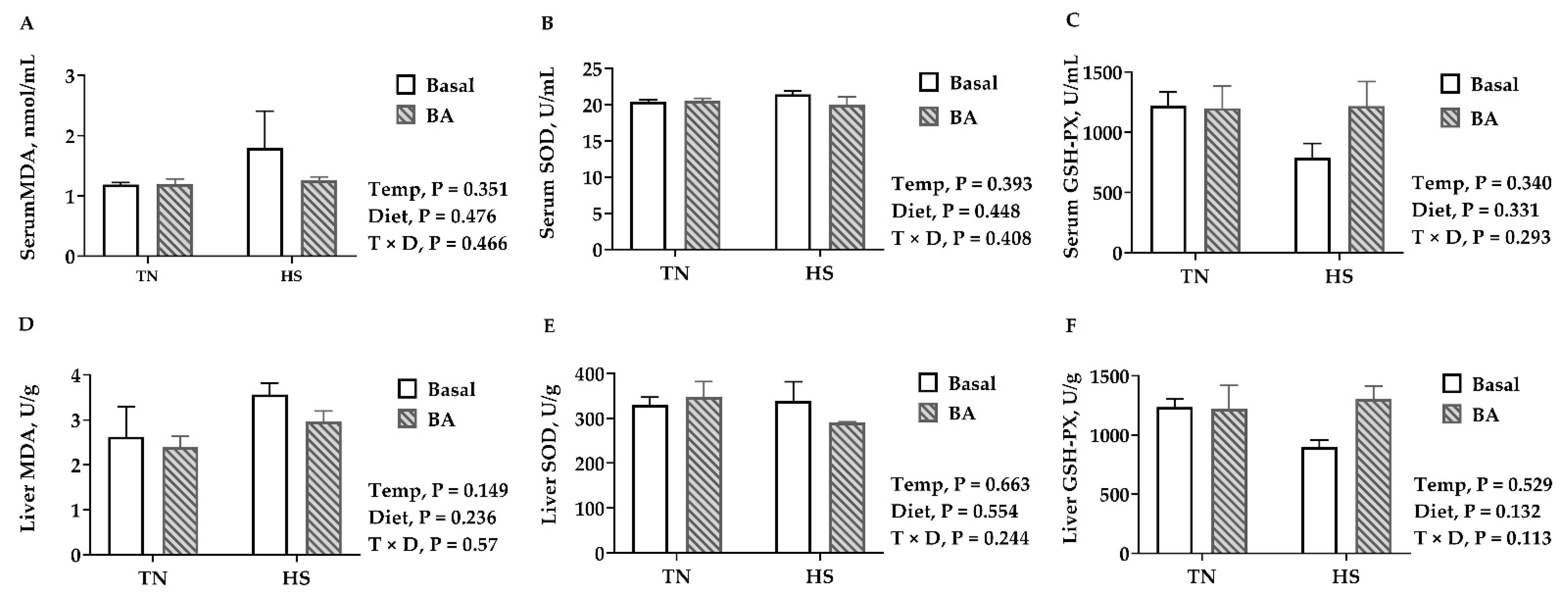
3.3. Serum and Liver Oxidative Stress and Damages
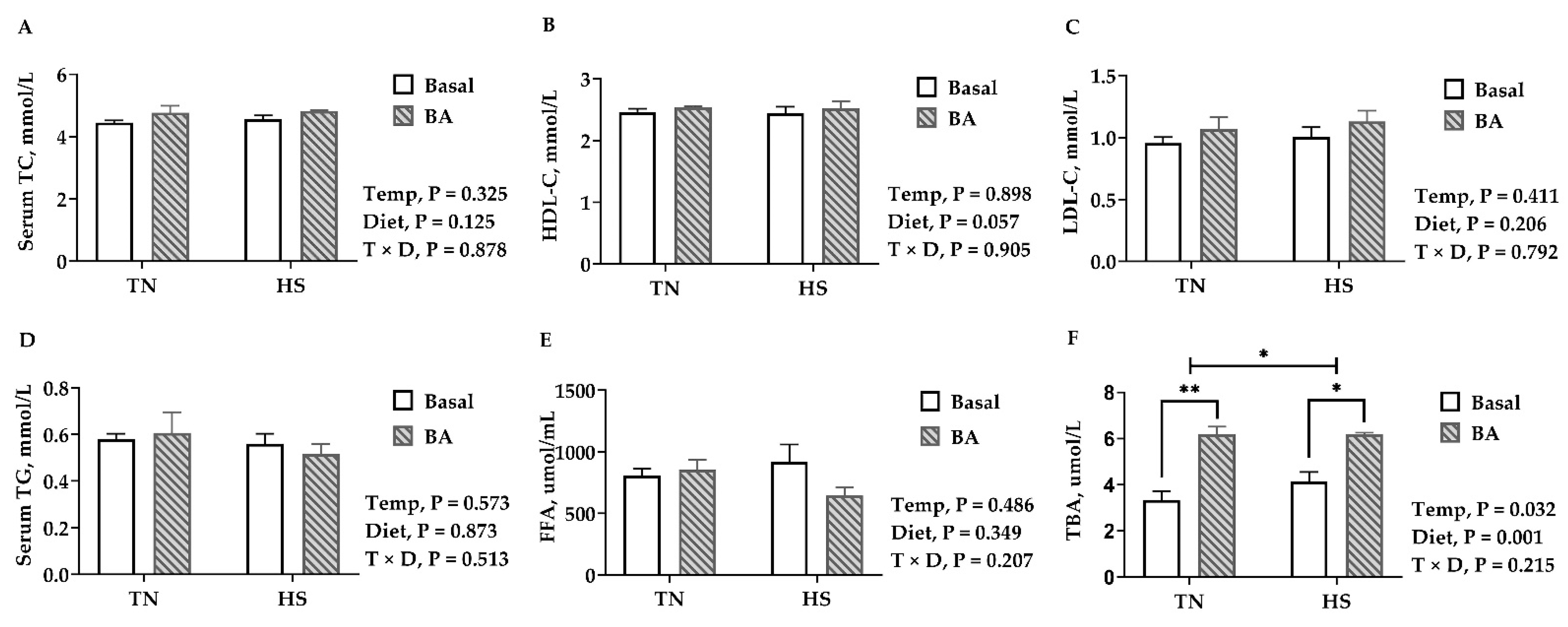
3.4. Serum, Liver Lipids, and Total Bile Acids
3.5. Expression of Gene Associated with Lipid and Bile Acid Synthesis in the Liver
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- St-Pierre, N.R.; Cobanov, B.; Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy. Sci. 2003, 86, E52–E77. [Google Scholar] [CrossRef] [Green Version]
- Halevy, O.; Geyra, A.; Barak, M.; Uni, Z.; Sklan, D. Early posthatch starvation decreases satellite cell proliferation and skeletal muscle growth in chicks. J. Nutr. 2000, 130, 858–864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gisolfi, C.V. Is the GI system built for exercise? News. Physiol. Sci. 2000, 15, 114–119. [Google Scholar] [CrossRef]
- Lin, H.; Decuypere, E.; Buyse, J. Acute heat stress induces oxidative stress in broiler chickens. Comp. Biochem. Phys. A 2006, 144, 11–17. [Google Scholar] [CrossRef]
- Lu, Z.; He, X.; Ma, B.; Zhang, L.; Li, J.; Jiang, Y.; Zhou, G.; Gao, F. Chronic heat stress impairs the quality of breast-muscle meat in broilers by affecting redox status and energy-substance metabolism. J. Agric. Food. Chem. 2017, 65, 11251–11258. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; He, X.F.; Ma, B.B.; Zhang, L.; Li, J.L.; Jiang, Y.; Zhou, G.H.; Gao, F. Increased fat synthesis and limited apolipoprotein B cause lipid accumulation in the liver of broiler chickens exposed to chronic heat stress. Poult. Sci. 2019, 98, 3695–3704. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S. Heat Stress Alters Animal Physiology and Post-Absorptive Metabolism During Pre- and Postnatal Development. Ph.D.Thesis, Iowa State University, Ames, IA, USA, 2014. [Google Scholar] [CrossRef]
- Zhang, M.; Li, C.C.; Li, F.; Li, H.; Liu, X.J.; Loor, J.J.; Kang, X.T.; Sun, G.R. Estrogen promotes hepatic synthesis of long-chain polyunsaturated fatty acids by regulating ELOVL5 at post-transcriptional level in laying hens. Int. J. Mol. Sci. 2017, 18, 1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashizawa, Y.; Kubota, M.; Kadowaki, M.; Fujimura, S. Effect of dietary vitamin E on broiler meat qualities, color, water-holding capacity and shear force value, under heat stress conditions. Anim. Sci. J. 2013, 84, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Windisch, W.; Schedle, K.; Plitzner, C. Use of phytogenic products as feed additives for swine and poultry. J. Anim. Sci. 2008, 86 (Suppl. 14), 140–148. [Google Scholar] [CrossRef] [PubMed]
- Attia, Y.A.; Hassan, S.S. Broiler tolerance to heat stress at various dietary protein/energy levels. Eur. Poult. Sci. 2017, 81, 171. [Google Scholar] [CrossRef]
- Molinaro, A.; Wahlstrom, A.; Marschall, H.U. Role of bile acids in metabolic control. Trends. Endocrin. Metab. 2018, 29, 31–41. [Google Scholar] [CrossRef]
- Lai, W.; Huang, W.; Dong, B.; Cao, A.; Zhang, W.; Li, J.; Wu, H.; Zhang, L. Effects of dietary supplemental bile acids on performance, carcass characteristics, serum lipid metabolites and intestinal enzyme activities of broiler chickens. Poult. Sci. 2018, 97, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Wen, X.; Meng, Q.; Wu, W.; Everaert, N.; Xie, J.; Zhang, H. Alteration in bile acids profile in Large White pigs during chronic heat exposure. J. Therm. Biol. 2019, 84, 375–383. [Google Scholar] [CrossRef]
- Borgstrom, B. Influence of bile salt, pH, and time on the action of pancreatic lipase; physiological implications. J. Lipid. Res. 1964, 5, 522–531. [Google Scholar] [CrossRef]
- Fiorucci, S.; Mencarelli, A.; Palladino, G.; Cipriani, S. Bile-acid-activated receptors: Targeting TGR5 and farnesoid-X-receptor in lipid and glucose disorders. Trends. Pharmacol. Sci. 2009, 30, 570–580. [Google Scholar] [CrossRef]
- Watanabe, M.; Houten, S.M.; Wang, L.; Moschetta, A.; Mangelsdorf, D.J.; Heyman, R.A.; Moore, D.D.; Auwerx, J. Bile acids lower triglyceride levels via a pathway involving FXR, SHP, and SREBP-1c. J. Clin. Investig. 2004, 113, 1408–1418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khadke, S.P.; Kuvalekar, A.A.; Harsulkar, A.M.; Mantri, N. High energy intake induced overexpression of transcription factors and its regulatory genes involved in acceleration of hepatic lipogenesis: A rat model for type 2 diabetes. Biomedicines 2019, 7, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaczuk, M. Tauroursodeoxycholate-bile acid with chaperoning activity: Molecular and cellular effects and therapeutic perspectives. Cells 2019, 8, 1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mueller, M.; Castro, R.E.; Thorell, A.; Marschall, H.U.; Auer, N.; Herac, M.; Rodrigues, C.M.P.; Trauner, M. Ursodeoxycholic acid: Effects on hepatic unfolded protein response, apoptosis and oxidative stress in morbidly obese patients. Liver Int. 2018, 38, 523–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, L. Study on the Effects and Molecular Mechanism of TUDCA on Anti-Heat Stress Ability in Yellow Feathe Broilers. Master’s Thesis, South China Agricultural University, Guangzhou, China, 2013. (In Chinese). [Google Scholar]
- Liu, Q.; Hu, Y.; Cao, Y.; Song, G.; Liu, Z.; Liu, X. Chicoric acid ameliorates lipopolysaccharide-induced oxidative stress via promoting the Keap1/Nrf2 transcriptional signaling pathway in BV-2 microglial cells and mouse brain. J. Agric. Food. Chem. 2017, 65, 338–347. [Google Scholar] [CrossRef]
- Zhen, L.; Zhang, S.; Shi, Y.; Feng, J.; Zhang, M. Water/Feed: A Thermal Comfort Evaluation Index for Broilers at Moderate Temperatures. Chin. J. Anim. Nutr. 2015, 27, 1750–1758. [Google Scholar] [CrossRef]
- Gao, T.; Li, J.; Zhang, L.; Jiang, Y.; Yin, M.; Liu, Y.; Gao, F.; Zhou, G. Effect of different tumbling marination methods and time on the water status and protein properties of prepared pork chops. Asian Australas J. Anim. Sci. 2015, 28, 1020–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Xie, J.; Wu, W.; Yi, B.; Liu, L.; Zhang, H. High ammonia exposure regulates lipid metabolism in the pig skeletal muscle via mTOR pathway. Sci. Total. Environ. 2020, 740, 139917. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, Y.; Cui, J. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J. Gastroenterol. 2013, 19, 9439–9446. [Google Scholar] [CrossRef] [PubMed]
- Qu, H.; Yan, H.; Lu, H.; Donkin, S.S.; Ajuwon, K.M. Heat stress in pigs is accompanied by adipose tissue-specific responses that favor increased triglyceride storage. J. Anim. Sci. 2016, 94, 1884–1896. [Google Scholar] [CrossRef] [PubMed]
- Aksit, M.; Yalcin, S.; Ozkan, S.; Metin, K.; Ozdemir, D. Effects of temperature during rearing and crating on stress parameters and meat quality of broilers. Poult. Sci. 2006, 85, 1867–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef]
- Song, D.J.; King, A.J. Effects of heat stress on broiler meat quality. World. Poultry. Sci. J. 2015, 71, 701–709. [Google Scholar] [CrossRef]
- Miao, Q.; Si, X.; Xie, Y.; Chen, L.; Liu, Z.; Liu, L.; Tang, X.; Zhang, H. Effects of acute heat stress at different ambient temperature on hepatic redox status in broilers. Poult. Sci. 2020, 99, 4113–4122. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.K.; Wang, A.A.; Ying, Z.X.; Zhang, L.G.; Su, W.P.; Cheng, K.; Feng, C.C.; Zhou, Y.M.; Zhang, L.L.; Wang, T. Effects of diets with different energy and bile acids levels on growth performance and lipid metabolism in broilers. Poult. Sci. 2019, 98, 887–895. [Google Scholar] [CrossRef]
- Okouchi, R.; Shuang, E.; Yamamoto, K.; Ota, T.; Seki, K.; Imai, M.; Ota, R.; Asayama, Y.; Nakashima, A.; Suzuki, K.; et al. Simultaneous intake of euglena gracilis and vegetables exerts synergistic anti-obesity and anti-inflammatory effects by modulating the gut microbiota in diet-induced obese mice. Nutrients 2019, 11, 204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piekarski, A.; Decuypere, E.; Buyse, J.; Dridi, S. Chenodeoxycholic acid reduces feed intake and modulates the expression of hypothalamic neuropeptides and hepatic lipogenic genes in broiler chickens. Gen. Comp. Endocrinol. 2016, 229, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Vlahcevic, Z.R.; Heuman, D.M.; Hylemon, P.B. Regulation of bile acid synthesis. Hepatology 1991, 13, 590–600. [Google Scholar] [CrossRef]
- Chiang, J.Y. Bile acid metabolism and signaling. Compr. Physiol. 2013, 3, 1191–1212. [Google Scholar] [CrossRef] [Green Version]
- Martinot, E.; Sedes, L.; Baptissart, M.; Lobaccaro, J.M.; Caira, F.; BeauDOIn, C.; Volle, D.H. Bile acids and their receptors. Mol. Aspects. Med. 2017, 56, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Song, K.H.; Ellis, E.; Strom, S.; Chiang, J.Y. Hepatocyte growth factor signaling pathway inhibits cholesterol 7alpha-hydroxylase and bile acid synthesis in human hepatocytes. Hepatology 2007, 46, 1993–2002. [Google Scholar] [CrossRef]
- Henkel, A.S.; Anderson, K.A.; Dewey, A.M.; Kavesh, M.H.; Green, R.M. A chronic high-cholesterol diet paradoxically suppresses hepatic CYP7A1 expression in FVB/NJ mice. J. Lipid Res. 2011, 52, 289–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Kong, X.; Owsley, E.; Ellis, E.; Strom, S.; Chiang, J.Y. Insulin regulation of cholesterol 7alpha-hydroxylase expression in human hepatocytes: Roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J. Biol. Chem. 2006, 281, 28745–28754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Rivas, P.A.; Chauhan, S.S.; Ha, M.; Fegan, N.; Dunshea, F.R.; Warner, R.D. Effects of heat stress on animal physiology, metabolism, and meat quality: A review. Meat. Sci. 2020, 162, 108025. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Khaoustov, V.I.; Chung, C.C.; Sohn, J.; Krishnan, B.; Lewis, D.E.; Yoffe, B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology 2002, 36, 592–601. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Briz, O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009, 15, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Pellicciari, R.; Pruzanski, M.; Auwerx, J.; Schoonjans, K. Targeting bile-acid signalling for metabolic diseases. Nat. Rev. Drug Discov. 2008, 7, 678–693. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, C.; Tang, S.; Liu, L.; Cao, A.; Xie, J.; Zhang, H. Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens. Animals 2021, 11, 630. https://doi.org/10.3390/ani11030630
Yin C, Tang S, Liu L, Cao A, Xie J, Zhang H. Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens. Animals. 2021; 11(3):630. https://doi.org/10.3390/ani11030630
Chicago/Turabian StyleYin, Chang, Shanlong Tang, Lei Liu, Aizhi Cao, Jingjing Xie, and Hongfu Zhang. 2021. "Effects of Bile Acids on Growth Performance and Lipid Metabolism during Chronic Heat Stress in Broiler Chickens" Animals 11, no. 3: 630. https://doi.org/10.3390/ani11030630





