Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Bone Biology of Broilers and Layers
2.1. Long Bone Growth
2.2. Bone Remodeling
3. Host-Immune Response in Coccidiosis and Necrotic Enteritis
4. Shift in Gut Microbiome and Immune Response
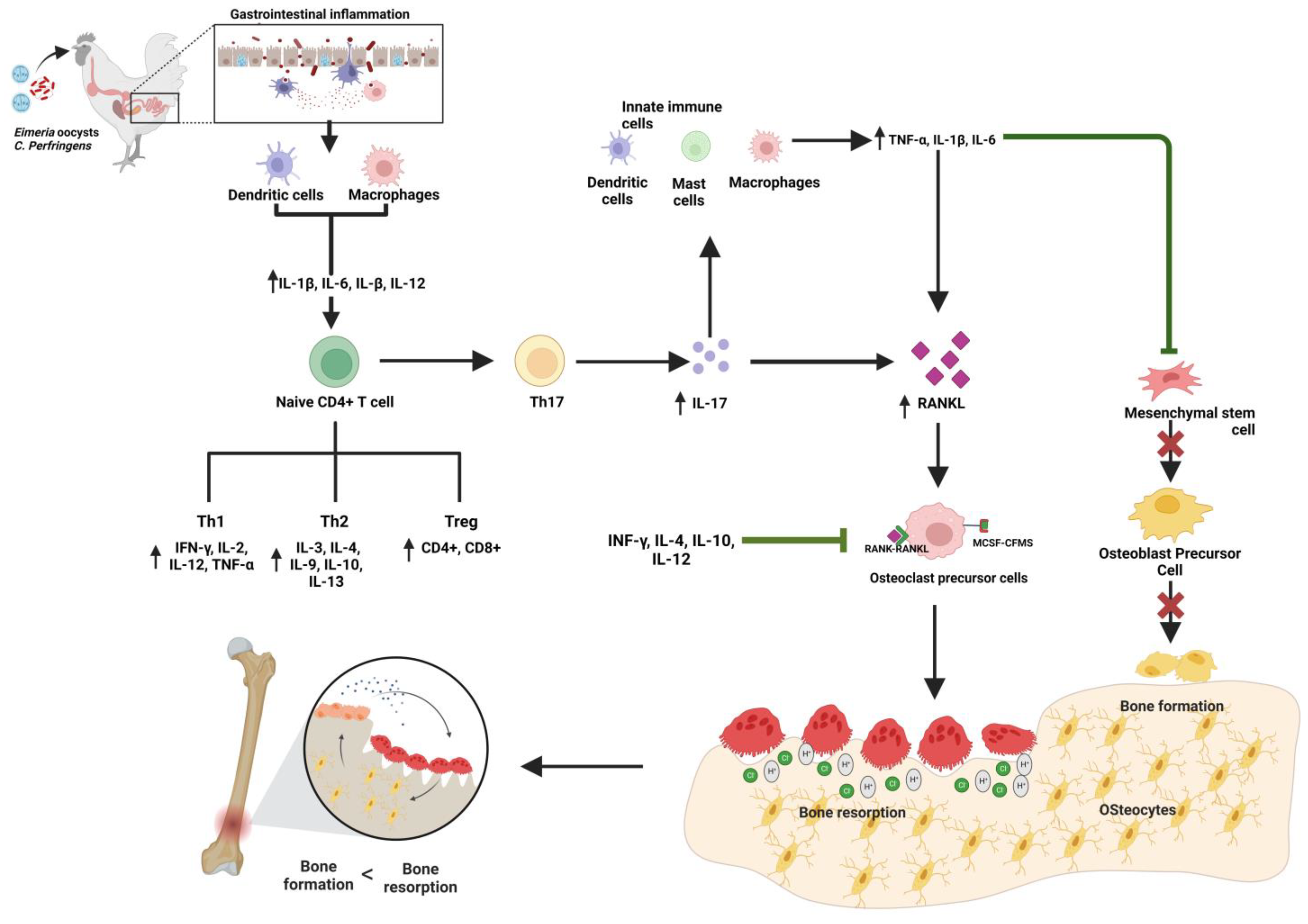
5. Interaction between Immune Response against Gastrointestinal Disorders and Bone Biology
6. Gut Microbiome and Bone Homeostasis
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitehead, C.C. Overview of Bone Biology in the Egg-Laying Hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Bloomfield, S.A.; Sugiyama, T.; Ricke, S.C. Concepts and Methods for Understanding Bone Metabolism in Laying Hens. Worlds Poult. Sci. J. 2012, 68, 71–82. [Google Scholar] [CrossRef]
- Regmi, P. Influence of Housing Systems on Bone Properties of Laying Hens; Michigan State University: Ann Arbor, MI, USA, 2015. [Google Scholar]
- Tsukasaki, M.; Takayanagi, H. Osteoimmunology: Evolving Concepts in Bone-Immune Interactions in Health and Disease. Nat. Rev. Immunol. 2019, 19, 626–642. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, J.; Horowitz, M.; Choi, Y. Osteoimmunology: Interactions of the Bone and Immune System. Endocr. Rev. 2008, 29, 403–440. [Google Scholar] [CrossRef]
- Dar, H.Y.; Azam, Z.; Anupam, R.; Mondal, R.K.; Srivastava, R.K. Osteoimmunology: The Nexus between Bone and Immune System. Front. Biosci. Landmark 2018, 23, 464–492. [Google Scholar]
- Okamoto, K.; Nakashima, T.; Shinohara, M.; Negishi-Koga, T.; Komatsu, N.; Terashima, A.; Sawa, S.; Nitta, T.; Takayanagi, H. Osteoimmunology: The Conceptual Framework Unifying the Immune and Skeletal Systems. Physiol. Rev. 2017, 97, 1295–1349. [Google Scholar] [CrossRef]
- Tompkins, Y.H.; Choi, J.; Teng, P.Y.; Yamada, M.; Sugiyama, T.; Kim, W.K. Reduced Bone Formation and Increased Bone Resorption Drive Bone Loss in Eimeria Infected Broilers. Sci. Rep. 2023, 13, 616. [Google Scholar] [CrossRef]
- Bradshaw, R.H.; Kirkden, R.D.; Broom, D.M. A Review of the Aetiology and Pathology of Leg Weakness in Broilers in Relation to Welfare. Avian Poult. Biol. Rev. 2002, 13, 45–104. [Google Scholar] [CrossRef]
- Kierończyk, B.; Rawski, M.; Józefiak, D.; Światkiewicz, S. Infectious and Non-Infectious Factors Associated with Leg Disorders in Poultry—A Review. Ann. Anim. Sci. 2017, 17, 645–669. [Google Scholar] [CrossRef]
- Wilson, S.; Duff, S.R.I.; Whitehead, C.C. Effects of Age, Sex and Housing on the Trabecular Bone of Laying Strain Domestic Fowl. Res. Vet. Sci. 1992, 53, 52–58. [Google Scholar] [CrossRef]
- Bishop, S.C.; Fleming, R.H.; Mccormagk, H.A.; Flock, D.K.; Whitehead, C.C. Inheritance of Bone Characteristics Affecting Osteoporosis in Laying Hens. Br. Poult. Sci. 2000, 41, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; White, D.; Chen, C.; Kim, W.K.; Adhikari, P. Effects of the Housing Environment and Laying Hen Strain on Tibia and Femur Bone Properties of Different Laying Phases of Hy-Line Hens. Poult. Sci. 2021, 100, 100933. [Google Scholar] [CrossRef] [PubMed]
- Chapman, H.D. Milestones in Avian Coccidiosis Research: A Review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [CrossRef]
- Chapman, H.D. Coccidiosis in Egg Laying Poultry; Elsevier Inc.: Amsterdam, The Netherlands, 2017; ISBN 9780128011515. [Google Scholar]
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Teng, P.Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of Increasing Challenge with Eimeria Maxima on the Growth Performance and Gene Expression of Biomarkers Associated with Intestinal Integrity and Nutrient Transporters. Vet. Res. 2021, 52, 81. [Google Scholar] [CrossRef]
- Teng, P.Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria Challenge Linearly Regulated Growth Performance, Dynamic Change of Gastrointestinal Permeability, Apparent Ileal Digestibility, Intestinal Morphology, and Tight Junctions of Broiler Chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Sharma, M.K.; Liu, G.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Effects of Mixed Eimeria Challenge on Performance, Body Composition, Intestinal Health, and Expression of Nutrient Transporter Genes of Hy-Line W-36 Pullets (0–6 Wks of Age). Poult. Sci. 2022, 101, 102083. [Google Scholar] [CrossRef]
- Poudel, S.; Tabler, G.T.; Lin, J.; Zhai, W.; Zhang, L. Riboflavin and Bacillus Subtilis Effects on Growth and Woody-Breast of Ross 708 Broilers with or without Eimeria Spp. Challenge. J. Anim. Sci. Technol. 2022, 64, 443. [Google Scholar] [CrossRef]
- Wade, B.; Keyburn, A.L.; Haring, V.; Ford, M.; Rood, J.I.; Moore, R.J. The Adherent Abilities of Clostridium Perfringens Strains Are Critical for the Pathogenesis of Avian Necrotic Enteritis. Vet. Microbiol. 2016, 197, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, Y.H.; Teng, P.; Pazdro, R.; Kim, W.K. Long Bone Mineral Loss, Bone Microstructural Changes and Oxidative Stress After Eimeria Challenge in Broilers. Front. Physiol. 2022, 13, 1344. [Google Scholar] [CrossRef]
- Zanu, H.K.; Kheravii, S.K.; Morgan, N.K.; Bedford, M.R.; Swick, R.A. Interactive Effect of Dietary Calcium and Phytase on Broilers Challenged with Subclinical Necrotic Enteritis: 3. Serum Calcium and Phosphorus, and Bone Mineralization. Poult. Sci. 2020, 99, 3617–3627. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.H.; Zhang, N.; Han, J.C.; Chang, C.W.; Hsiao, F.S.H.; Yu, Y.H. Optimization of Surfactin Production from Bacillus Subtilis in Fermentation and Its Effects on Clostridium Perfringens-Induced Necrotic Enteritis and Growth Performance in Broilers. J. Anim. Physiol. Anim. Nutr. 2018, 102, 1232–1244. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Wang, J.; Teng, P.Y.; Tompkins, Y.H.; Jordan, B.; Kim, W.K. Effects of Phytase and Coccidial Vaccine on Growth Performance, Nutrient Digestibility, Bone Mineralization, and Intestinal Gene Expression of Broilers. Poult. Sci. 2022, 101, 102124. [Google Scholar] [CrossRef] [PubMed]
- Oikeh, I.; Development, R. Coccidiosis in Modern Broiler Chickens: Targeted Nutritional Modulations for Consequences on Bone Quality; Newcastle University: Newcastle, UK, 2019. [Google Scholar]
- Oikeh, I.; Sakkas, P.; Blake, D.P.; Kyriazakis, I. Interactions between Dietary Calcium and Phosphorus Level, and Vitamin D Source on Bone Mineralization, Performance, and Intestinal Morphology of Coccidia-Infected Broilers. Poult. Sci. 2019, 98, 5679–5690. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, P.; Oikeh, I.; Blake, D.P.; Smith, S.; Kyriazakis, I. Dietary Vitamin D Improves Performance and Bone Mineralisation, but Increases Parasite Replication and Compromises Gut Health in Eimeria-Infected Broilers. Br. J. Nutr. 2019, 122, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Applegate, T.J.; Lilburn, M.S. Growth of the Femur and Tibia of a Commercial Broiler Line. Poult. Sci. 2002, 81, 1289–1294. [Google Scholar] [CrossRef]
- Gilbert, S.F. Early Vertebrate Development: Mesoderm and Endoderm. Developmental Biology, 5th ed.; Sinauer Assoc. Inc.: Sunderland, MA, USA, 1997; pp. 341–357. [Google Scholar]
- Regmi, P.; Deland, T.S.; Steibel, J.P.; Robison, C.I.; Haut, R.C.; Orth, M.W.; Karcher, D.M. Effect of Rearing Environment on Bone Growth of Pullets. Poult. Sci. 2015, 94, 502–511. [Google Scholar] [CrossRef]
- Beck, M.M.; Hansen, K.K. Role of Estrogen in Avian Osteoporosis. Poult. Sci. 2004, 83, 200–206. [Google Scholar] [CrossRef]
- Webster, A.B. Welfare Implications of Avian Osteoporosis. Poult. Sci. 2004, 83, 184–192. [Google Scholar] [CrossRef]
- Huang, S.; Kong, A.; Cao, Q.; Tong, Z.; Wang, X. The Role of Blood Vessels in Broiler Chickens with Tibial Dyschondroplasia. Poult. Sci. 2019, 98, 6527–6532. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Z.; Jin, D.; Cai, C.; Jia, C.; Liu, W.; Wang, T.; Li, S.; Zhang, H.; Huang, B.; et al. MTORC1 Regulates PTHrP to Coordinate Chondrocyte Growth, Proliferation and Differentiation. Nat. Commun. 2016, 7, 11151. [Google Scholar] [CrossRef] [PubMed]
- Kacena, M.A.; Nelson, T.; Clough, M.E.; Lee, S.K.; Lorenzo, J.A.; Gundberg, C.M.; Horowitz, M.C. Megakaryocyte-Mediated Inhibition of Osteoclast Development. Bone 2006, 39, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Aarden, E.M.; Nijweide, P.J.; Burger, E.H. Function of Osteocytes in Bone. J. Cell Biochem. 1994, 55, 287–299. [Google Scholar] [CrossRef]
- Sims, N.A.; Gooi, J.H. Bone Remodeling: Multiple Cellular Interactions Required for Coupling of Bone Formation and Resorption. Semin Cell Dev. Biol. 2008, 19, 444–451. [Google Scholar] [CrossRef]
- Takahashi, N.; Akatsu, T.; Udagawa, N.; Sasaki, T.; Yamaguchi, A.; Moseley, J.M.; Martin, T.J.; Suda, T. Osteoblastic Cells are Involved in Osteoclast Formation. Endocrinology 1988, 123, 2600–2602. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.H.; Van der Meer, J.W.M.; Nijweide, P.J. Osteoclast Formation from Mononuclear Phagocytes: Role of Bone-Forming Cells. J. Cell Biol. 1984, 99, 1901–1906. [Google Scholar] [CrossRef]
- Nakashima, T.; Hayashi, M.; Fukunaga, T.; Kurata, K.; Oh-Hora, M.; Feng, J.Q.; Bonewald, L.F.; Kodama, T.; Wutz, A.; Wagner, E.F.; et al. Evidence for Osteocyte Regulation of Bone Homeostasis through RANKL Expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef]
- Danks, L.; Komatsu, N.; Guerrini, M.M.; Sawa, S.; Armaka, M.; Kollias, G.; Nakashima, T.; Takayanagi, H. RANKL Expressed on Synovial Fibroblasts Is Primarily Responsible for Bone Erosions during Joint Inflammation. Ann. Rheum. Dis. 2016, 75, 1187–1195. [Google Scholar] [CrossRef]
- Knothe Tate, M.L.; Adamson, J.R.; Tami, A.E.; Bauer, T.W. The Osteocyte. Int. J. Biochem. Cell Biol. 2004, 36, 1–8. [Google Scholar] [CrossRef]
- Teitelbaum, S.L. Bone Resorption by Osteoclasts. Science 2000, 289, 1504–1508. [Google Scholar] [CrossRef]
- Newby, A.C. Metalloproteinase Expression in Monocytes and Macrophages and Its Relationship to Atherosclerotic Plaque Instability. Arter. Thromb. Vasc. Biol. 2008, 28, 2108–2114. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, X.; Lei, W.; Pang, L.; Wan, C.; Shi, Z.; Zhao, L.; Nagy, T.R.; Peng, X.; Hu, J.; et al. TGF-Β1–Induced Migration of Bone Mesenchymal Stem Cells Couples Bone Resorption with Formation. Nat. Med. 2009, 15, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Klein-Nulend, J.; Nijweide, P.J.; Burger, E.H. Osteocyte and Bone Structure. Curr. Osteoporos. Rep. 2003, 1, 5–10. [Google Scholar] [CrossRef]
- Conway, D.P.; Mckenzie, M.E.; Conway, D.P.; Mckenzie, M.E. Poultry Coccidiosis; John Wiley & Sons: Hoboken, NJ, USA, 2007; ISBN 9780813822020. [Google Scholar]
- Abdisa, T.; Hasen, R.; Tagesu, T.; Regea, G.; Tadese, G. Poultry Coccidiosis and Its Prevention, Control. J. Vet. Anim. Res. NF 2019, 2, 1–6. [Google Scholar]
- Fathima, S.; Al Hakeem, W.G.; Shanmugasundaram, R.; Selvaraj, R.K. Necrotic Enteritis in Broiler Chickens: A Review on the Pathogen, Pathogenesis, and Prevention. Microorganisms 2022, 10, 1958. [Google Scholar] [CrossRef]
- Daneshmand, A.; Kermanshahi, H.; Mohammed, J.; Sekhavati, M.H.; Javadmanesh, A.; Ahmadian, M.; Alizadeh, M.; Razmyar, J.; Kulkarni, R.R. Intestinal Changes and Immune Responses during Clostridium Perfringens-Induced Necrotic Enteritis in Broiler Chickens. Poult. Sci. 2022, 101, 101652. [Google Scholar] [CrossRef]
- Alizadeh, M.; Shojadoost, B.; Boodhoo, N.; Astill, J.; Taha-Abdelaziz, K.; Hodgins, D.C.; Kulkarni, R.R.; Sharif, S. Necrotic Enteritis in Chickens: A Review of Pathogenesis, Immune Responses and Prevention, Focusing on Probiotics and Vaccination. Anim. Health Res. Rev. 2021, 22, 147–162. [Google Scholar] [CrossRef]
- Moore, R.J. Necrotic Enteritis Predisposing Factors in Broiler Chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Kogut, M.H.; Klasing, K. An Immunologist’s Perspective on Nutrition, Immunity, and Infectious Diseases: Introduction and Overview. J. Appl. Poult. Res. 2009, 18, 103–110. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Poudel, S.; Zhang, L.; Tabler, G.T.; Lin, J.; Zhai, W. Effects of Riboflavin and Bacillus Subtilis on Internal Organ Development and Intestinal Health of Ross 708 Male Broilers with or without Coccidial Challenge. Poult. Sci. 2021, 100, 100973. [Google Scholar] [CrossRef] [PubMed]
- Yun, C.H.; Lillehoj, H.S.; Lillehoj, E.P. Intestinal Immune Responses to Coccidiosis. Immunology 2000, 24, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Lu, M.; Lillehoj, H.S. Coccidiosis: Recent Progress in Host Immunity and Alternatives to Antibiotic Strategies. Vaccines 2022, 10, 215. [Google Scholar]
- Shivaramaiah, C.; Barta, J.R.; Hernandez-Velasco, X.; Téllez, G.; Hargis, B.M. Coccidiosis: Recent Advancements in the Immunobiology of Eimeria Species, Preventive Measures, and the Importance of Vaccination as a Control Tool against These Apicomplexan Parasites. Vet. Med. Res. Rep. 2014, 5, 23–24. [Google Scholar]
- Lee, Y.; Lee, S.-H.; Deepthi Gadde, U.; Oh, S.-T.; Lee, S.-J.; Lillehoj, H.S. Dietary Allium Hookeri Reduces Inflammatory Response and Increases Expression of Intestinal Tight Junction Proteins in LPS-Induced Young Broiler Chicken. Res. Vet. Sci. 2017, 112, 149–155. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Lillehoj, E.P. Avian Coccidiosis. A Review of Acquired Intestinal Immunity and Vaccination Strategies: American Association of Avian Pathologists Stable. Avian Dis. 2016, 44, 408–425. [Google Scholar]
- Hong, Y.H.; Lillehoj, H.S.; Lee, S.H.; Dalloul, R.A.; Lillehoj, E.P. Analysis of Chicken Cytokine and Chemokine Gene Expression Following Eimeria Acervulina and Eimeria Tenella Infections. Vet. Immunol. Immunopathol. 2006, 114, 209–223. [Google Scholar] [CrossRef]
- Lillehoj, H.S.; Trout, J.M. Avian Gut-Associated Lymphoid Tissues and Intestinal Immune Responses to Eimeria Parasites. Clin. Microbiol. Rev. 1996, 9, 349–360. [Google Scholar] [CrossRef]
- Kim, W.H.; Chaudhari, A.A.; Lillehoj, H.S. Involvement of T Cell Immunity in Avian Coccidiosis. Front. Immunol. 2019, 10, 2732. [Google Scholar]
- Hong, Y.H.; Lillehoj, H.S.; Lillehoj, E.P.; Lee, S.H. Changes in Immune-Related Gene Expression and Intestinal Lymphocyte Subpopulations Following Eimeria Maxima Infection of Chickens. Vet. Immunol. Immunopathol. 2006, 114, 259–272. [Google Scholar] [CrossRef]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 Family of Heterodimeric Cytokines: New Players in the Regulation of T Cell Responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef]
- Scott, P. IL-12: Initiation Cytokine for Cell-Mediated Immunity. Science 1993, 260, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-Induced Mucogenesis Promotes the Onset of Necrotic Enteritis by Supporting Clostridium Perfringens Growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian Necrotic Enteritis: Experimental Models, Host Immunity, Pathogenesis, Risk Factors, and Vaccine Development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef]
- Ruhnke, I.; Andronicos, N.M.; Swick, R.A.; Hine, B.; Sharma, N.; Kheravii, S.K.; Wu, S.B.; Hunt, P. Immune Responses Following Experimental Infection with Ascaridia Galli and Necrotic Enteritis in Broiler Chickens. Avian Pathol. 2017, 46, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Lillehoj, H.S. Characterization of Intestinal Immune Response to Clostridium Perfringens Infection in Broiler Chickens. Poult. Sci. 2019, 98, 188–198. [Google Scholar] [CrossRef]
- Kolls, J.K.; Khader, S.A. The Role of Th17 Cytokines in Primary Mucosal Immunity. Cytokine Growth Factor. Rev. 2010, 21, 443–448. [Google Scholar] [CrossRef]
- Walliser, I.; Göbel, T.W. Chicken IL-17A Is Expressed in Aβ and Γδ T Cell Subsets and Binds to a Receptor Present on Macrophages, and T Cells. Dev. Comp. Immunol. 2018, 81, 44–53. [Google Scholar] [CrossRef]
- Kogut, M.H. The Gut Microbiota and Host Innate Immunity: Regulators of Host Metabolism and Metabolic Diseases in Poultry? J. Appl. Poult. Res. 2013, 22, 637–646. [Google Scholar] [CrossRef]
- Broom, L.J.; Kogut, M.H. The Role of the Gut Microbiome in Shaping the Immune System of Chickens. Vet. Immunol. Immunopathol. 2018, 204, 44–51. [Google Scholar] [CrossRef]
- Kogut, M.H.; Lee, A.; Santin, E. Microbiome and Pathogen Interaction with the Immune System. Poult. Sci. 2020, 99, 1906–1913. [Google Scholar] [CrossRef] [PubMed]
- Broom, L.J. Host–Microbe Interactions and Gut Health in Poultry—Focus on Innate Responses. Microorganisms 2019, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Crowley, S.M.; Law, H.T.; Chang, S.Y.; Ko, H.J.; Vallance, B.A. The Intestinal Epithelium: Central Coordinator of Mucosal Immunity. Trends Immunol. 2018, 39, 677–696. [Google Scholar] [CrossRef]
- Cheled-Shoval, S.L.; Gamage, N.S.W.; Amit-Romach, E.; Forder, R.; Marshal, J.; Van Kessel, A.; Uni, Z. Differences in Intestinal Mucin Dynamics between Germ-Free and Conventionally Reared Chickens after Mannan-Oligosaccharide Supplementation. Poult. Sci. 2014, 93, 636–644. [Google Scholar] [CrossRef]
- Shira, E.B.; Sklan, D.; Friedman, A. Impaired Immune Responses in Broiler Hatchling Hindgut Following Delayed Access to Feed. Vet. Immunol. Immunopathol. 2005, 105, 33–45. [Google Scholar] [CrossRef]
- Kaspers, B.; Lettmann, S.; Roell, S. Development of the Gut Associated Immune System. In Proceedings of the European Symposium on Poultry Nutrition, Prague, Czech Republic, 24–27 August 2015; Volume 20. [Google Scholar]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, Metabolites and Host Immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef]
- Wu, S.B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two Necrotic Enteritis Predisposing Factors, Dietary Fishmeal and Eimeria Infection, Induce Large Changes in the Caecal Microbiota of Broiler Chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Caballero, S.; Pamer, E.G. Microbiota-Mediated Inflammation and Antimicrobial Defense in the Intestine. Ann. Rev. Immunol. 2015, 33, 227–256. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Wang, X.; Robinson, K.; Whitmore, M.A.; Stewart, S.N.; Zhao, J.; Zhang, G. Identification of an Intestinal Microbiota Signature Associated with the Severity of Necrotic Enteritis. Front. Microbiol. 2021, 12, 2377. [Google Scholar] [CrossRef]
- Antonissen, G.; Eeckhaut, V.; Van Driessche, K.; Onrust, L.; Haesebrouck, F.; Ducatelle, R.; Moore, R.J.; Van Immerseel, F. Microbial Shifts Associated with Necrotic Enteritis. Avian Pathol. 2016, 45, 308–312. [Google Scholar] [CrossRef]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus Licheniformis Normalize the Ileum Microbiota of Chickens Infected with Necrotic Enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Wang, W.; Gao, X.; Zheng, S.; Lancuo, Z.; Li, Y.; Zhu, L.; Hou, J.; Hai, J.; Long, X.; Chen, H.; et al. The Gut Microbiome and Metabolome of Himalayan Griffons (Gyps Himalayensis): Insights into the Adaptation to Carrion-Feeding Habits in Avian Scavengers. Avian. Res. 2021, 12, 52. [Google Scholar] [CrossRef]
- Stanley, D.; Wu, S.-B.; Rodgers, N.; Swick, R.A.; Moore, R.J. Differential Responses of Cecal Microbiota to Fishmeal, Eimeria and Clostridium Perfringens in a Necrotic Enteritis Challenge Model in Chickens. PLoS ONE 2014, 9, e104739. [Google Scholar] [CrossRef]
- Pietruska, A.; Bortoluzzi, C.; Hauck, R. A Meta-Analysis of the Effect of Eimeria Spp. and/or Clostridium Perfringens Infection on the Microbiota of Broiler Chickens. Poult. Sci. 2023, 102, 102652. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The Immunomodulatory Effects of Lactic Acid Bacteria for Improving Immune Functions and Benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 Functions as an Osteoclastogenic Helper T Cell Subset That Links T Cell Activation and Bone Destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, H.; Ogasawara, K.; Hida, S.; Chiba, T.; Murata, S.; Sato, K.; Takaoka, A.; Yokochi, T.; Oda, H.; Tanaka, K.; et al. T-Cell-Mediated Regulation of Osteoclastogenesis by Signaling Crosstalk between RANKL and IFN-γ. Nature 2000, 408, 600–605. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Nakashima, T.; Takayanagi, H. Osteocyte Control of Osteoclastogenesis. Bone 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Sharma, M.K.; White, D.L.; Tompkins, Y.H.; Kim, W.K. Effect of mixed Eimeria challenge on skeletal health of Hy-Line W-36 pullets. In Proceedings of the International Poultry Scientific Forum, Atlanta, GA, USA, 25–27 January 2022; p. 70. [Google Scholar]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional Specialization of Interleukin-17 Family Members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor Necrosis Factor α Stimulates Osteoclast Differentiation by a Mechanism Independent of the Odf/Rankl–Rank Interaction. J. Exp. Med. 2000, 191, 275–286. [Google Scholar] [CrossRef]
- Gilbert, L.; He, X.; Farmer, P.; Rubin, J.; Drissi, H.; Van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Nanes, M.S. Expression of the Osteoblast Differentiation Factor RUNX2 (Cbfa1/AML3/Pebp2αA) Is Inhibited by Tumor Necrosis Factor-α. J. Biol. Chem. 2002, 277, 2695–2701. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Udagawa, N.; Takahashi, N.; Matsuzaki, K.; Itoh, K.; Ishiyama, S.; Saito, S.; Inoue, K.; Kamatani, N.; Gillespie, M.T.; et al. IL-17 in Synovial Fluids from Patients with Rheumatoid Arthritis Is a Potent Stimulator of Osteoclastogenesis. J. Clin. Investig. 1999, 103, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Kotake, S.; Nanke, Y. Effect of TNFα on Osteoblastogenesis from Mesenchymal Stem Cells. Biochim. Biophys. Acta BBA Gen. Subj. 2014, 1840, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Dhodapkar, K.M.; Barbuto, S.; Matthews, P.; Kukreja, A.; Mazumder, A.; Vesole, D.; Jagannath, S.; Dhodapkar, M.V. Dendritic Cells Mediate the Induction of Polyfunctional Human IL17-Producing Cells (Th17-1 Cells) Enriched in the Bone Marrow of Patients with Myeloma. Blood 2008, 112, 2878–2885. [Google Scholar] [CrossRef]
- Uluçkan, Ö.; Jimenez, M.; Karbach, S.; Jeschke, A.; Graña, O.; Keller, J.; Busse, B.; Croxford, A.L.; Finzel, S.; Koenders, M.; et al. Chronic Skin Inflammation Leads to Bone Loss by IL-17-Mediated Inhibition of Wnt Signaling in Osteoblasts. Sci. Transl. Med. 2016, 8, 330ra37. [Google Scholar] [CrossRef] [PubMed]
- Bendre, M.S.; Montague, D.C.; Peery, T.; Akel, N.S.; Gaddy, D.; Suva, L.J. Interleukin-8 Stimulation of Osteoclastogenesis and Bone Resorption Is a Mechanism for the Increased Osteolysis of Metastatic Bone Disease. Bone 2003, 33, 28–37. [Google Scholar] [CrossRef]
- Sun, H.; Zhao, Y.; Wang, K.; Zhu, L.; Dong, J.; Zhao, J.; Wang, Y.; Li, H.; Sun, X.; Lu, Y. Low Dose IL-2 Suppress Osteoclastogenesis in Collagen-Induced Arthritis via JNK Dependent Pathway. Immun. Inflamm. Dis 2020, 8, 727–735. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Tomar, G.B.; Barhanpurkar, A.P.; Gupta, N.; Pote, S.T.; Mishra, G.C.; Wani, M.R. IL-3 Attenuates Collagen-Induced Arthritis by Modulating the Development of Foxp3+ Regulatory T Cells. J. Immunol. 2011, 186, 2262–2272. [Google Scholar] [CrossRef]
- Stein, N.C.; Kreutzmann, C.; Zimmermann, S.P.; Niebergall, U.; Hellmeyer, L.; Goettsch, C.; Schoppet, M.; Hofbauer, L.C. Interleukin-4 and Interleukin-13 Stimulate the Osteoclast Inhibitor Osteoprotegerin by Human Endothelial Cells through the STAT6 Pathway. J. Bone Miner. Res. 2008, 23, 750–758. [Google Scholar] [CrossRef]
- Kelchtermans, H.; Geboes, L.; Mitera, T.; Huskens, D.; Leclercq, G.; Matthys, P. Activated CD4+CD25+ Regulatory T Cells Inhibit Osteoclastogenesis and Collagen-Induced Arthritis. Ann. Rheum. Dis. 2009, 68, 744–750. [Google Scholar] [CrossRef]
- Hernandez, C.J.; Guss, J.D.; Luna, M.; Goldring, S.R. Links Between the Microbiome and Bone. J. Bone Miner. Res. 2016, 31, 1638–1646. [Google Scholar] [CrossRef] [PubMed]
- Ibáñez, L.; Rouleau, M.; Wakkach, A.; Blin-Wakkach, C. Gut Microbiome and Bone. Jt. Bone Spine 2019, 86, 43–47. [Google Scholar] [CrossRef]
- Chen, P.; Xu, T.; Zhang, C.; Tong, X.; Shaukat, A.; He, Y.; Liu, K.; Huang, S. Effects of Probiotics and Gut Microbiota on Bone Metabolism in Chickens: A Review. Metabolites 2022, 12, 1000. [Google Scholar] [CrossRef]
- Wideman, R.F. Bacterial Chondronecrosis with Osteomyelitis and Lameness in Broilers: A Review. Poult. Sci. 2016, 95, 325–344. [Google Scholar] [CrossRef]
- Wideman, R.F.; Prisby, R.D. Bone Circulatory Disturbances in the Development of Spontaneous Bacterial Chondronecrosis with Osteomyelitis: A Translational Model for the Pathogenesis of Femoral Head Necrosis. Front. Endocrinol. 2013, 3, 183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lu, Y.; Wang, Y.; Ren, X.; Han, J. The impact of the intestinal microbiome on bone health. Intractable Rare Dis. Res. 2018, 7, 148–155. [Google Scholar] [CrossRef] [PubMed]

| Disease | Immune Responses | Cytokines | Effect on Immunity | Bone Resorption | Bone Formation | Possible Mechanism |
|---|---|---|---|---|---|---|
| Coccidiosis Necrotic enteritis | Innate Immunity | Natural killer cells | Antigen recognition and phagocytosis | Osteoclast differentiation, maturation, and activation | ||
| Macrophages Dendritic cells | IL-1β | Proinflammation | Direct activates the RANK signaling to promote osteoclastogenesis [92,93] | |||
| IL-6 | Th17 induction | Activation of osteoclastogenesis [99,101,102] | ||||
| IL-8 | Proinflammation | Osteoclastic activation through RANKL [103] | ||||
| IL-12 | Proinflammation | Inhibition of RANKL-mediated osteoclast formation [92] | ||||
| TNF-α | Proinflammation | Indirect osteoclastic activation through RANKL [97,98,100] | ||||
| Coccidiosis Necrotic enteritis | Adaptive Immune Response | |||||
| Th1 | IFN-γ | Cellular immunity | Inhibit osteoclastogenesis [92] | |||
| IL-2 | Proinflammation | Inhibition of RANKL [104] | ||||
| IL-12 | Proinflammation | Inhibition of RANKL-initiated osteoclastogenesis [92] | ||||
| TNF-α | Proinflammation | Indirect osteoclastic activation through RANKL [97,98,100] | ||||
| Th2 | IL-3 | Proinflammation | Blocks RANKL-induced osteoclastogenesis [105] | |||
| IL-4 | Humoral immunity | Inhibit osteoclastogenesis, Osteoprotegerin [106] | ||||
| IL-9 | Antiinflammation | Unknown | ||||
| IL-10 | Antiinflammation | Suppress bone resorption [92] | ||||
| IL-13 | Antiinflammation | Inhibit osteoclastogenesis, Osteoprotegerin [106] | ||||
| Th17 | IL-17 | Proinflammation | Induction of RANK and RANKL expression [99,101,102] | |||
| IL-22 | Proinflammation | Unknown [99,101,102] | ||||
| Tregs | CD4+ | Helper T cells | TGF, IL-4, and IL10 [107] | |||
| CD8+ | Cytotoxic T cells | Production of OPG [107] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, M.K.; Regmi, P.; Applegate, T.; Chai, L.; Kim, W.K. Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens. Animals 2023, 13, 1816. https://doi.org/10.3390/ani13111816
Sharma MK, Regmi P, Applegate T, Chai L, Kim WK. Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens. Animals. 2023; 13(11):1816. https://doi.org/10.3390/ani13111816
Chicago/Turabian StyleSharma, Milan Kumar, Prafulla Regmi, Todd Applegate, Lilong Chai, and Woo Kyun Kim. 2023. "Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens" Animals 13, no. 11: 1816. https://doi.org/10.3390/ani13111816
APA StyleSharma, M. K., Regmi, P., Applegate, T., Chai, L., & Kim, W. K. (2023). Osteoimmunology: A Link between Gastrointestinal Diseases and Skeletal Health in Chickens. Animals, 13(11), 1816. https://doi.org/10.3390/ani13111816






