Curcumin Supplementation Improves Growth Performance and Anticoccidial Index by Improving the Antioxidant Capacity, Inhibiting Inflammatory Responses, and Maintaining Intestinal Barrier Function in Eimeria tenella-Infected Broilers
Abstract
:Simple Summary
Abstract
1. Introducti0on
2. Materials and Methods
2.1. Oocyst Procurement
2.2. Birds and Their Management
2.3. Sample Collection
2.4. Estimation of the Anticoccidial Index
2.5. Determination of Antioxidant Indicators
2.6. Gene Expression Analyses
2.7. Pyrosequencing of 16S rDNA Amplicon
2.8. Statistical Analysis
3. Results
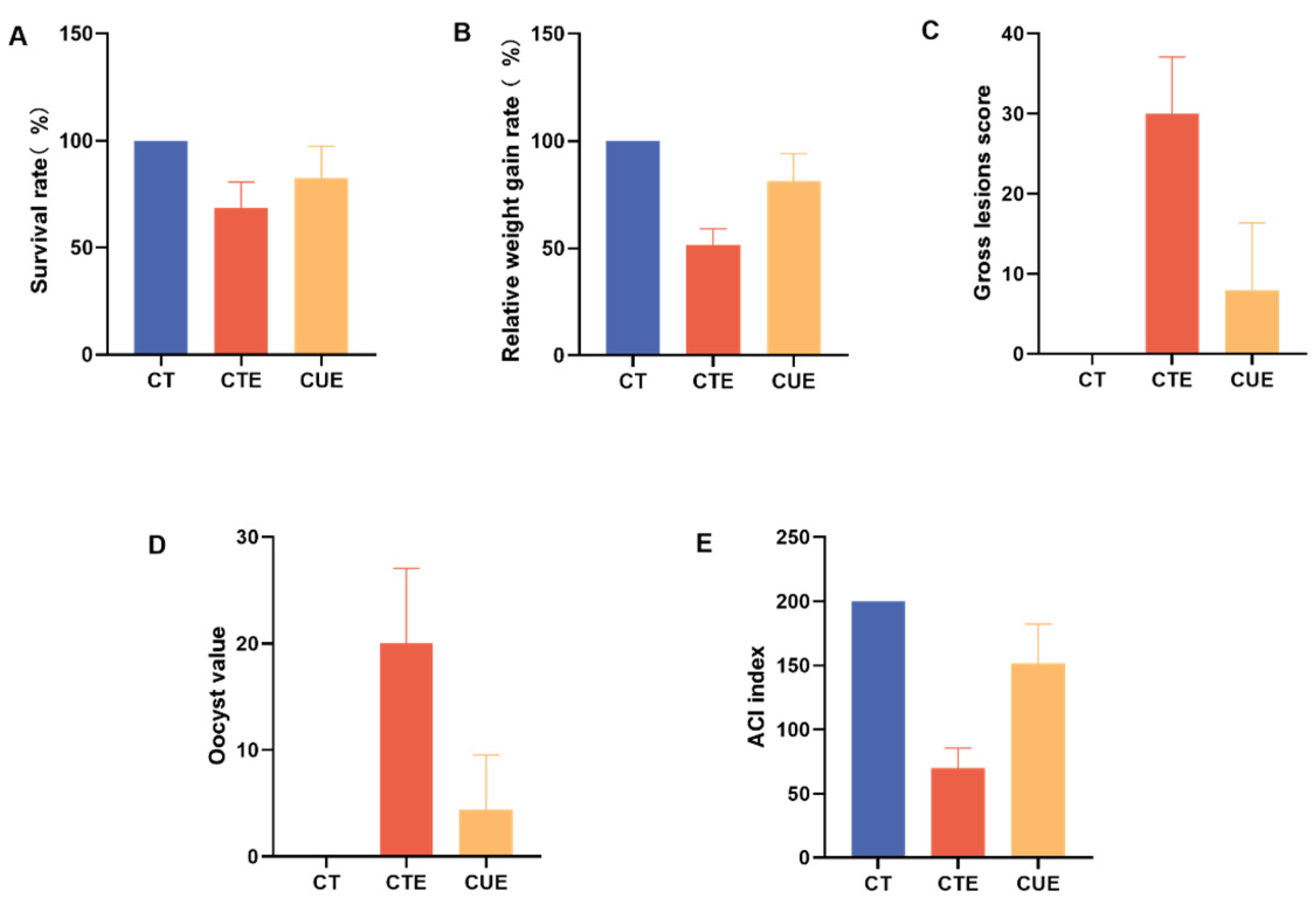
3.1. Curcumin Supplement Improved Growth Performance in Coccidia-Infected Broilers
3.2. Curcumin Supplementation Can Enhance the Anticoccidial Index
3.3. Curcumin Supplement Can Improve Antioxidant Capacity
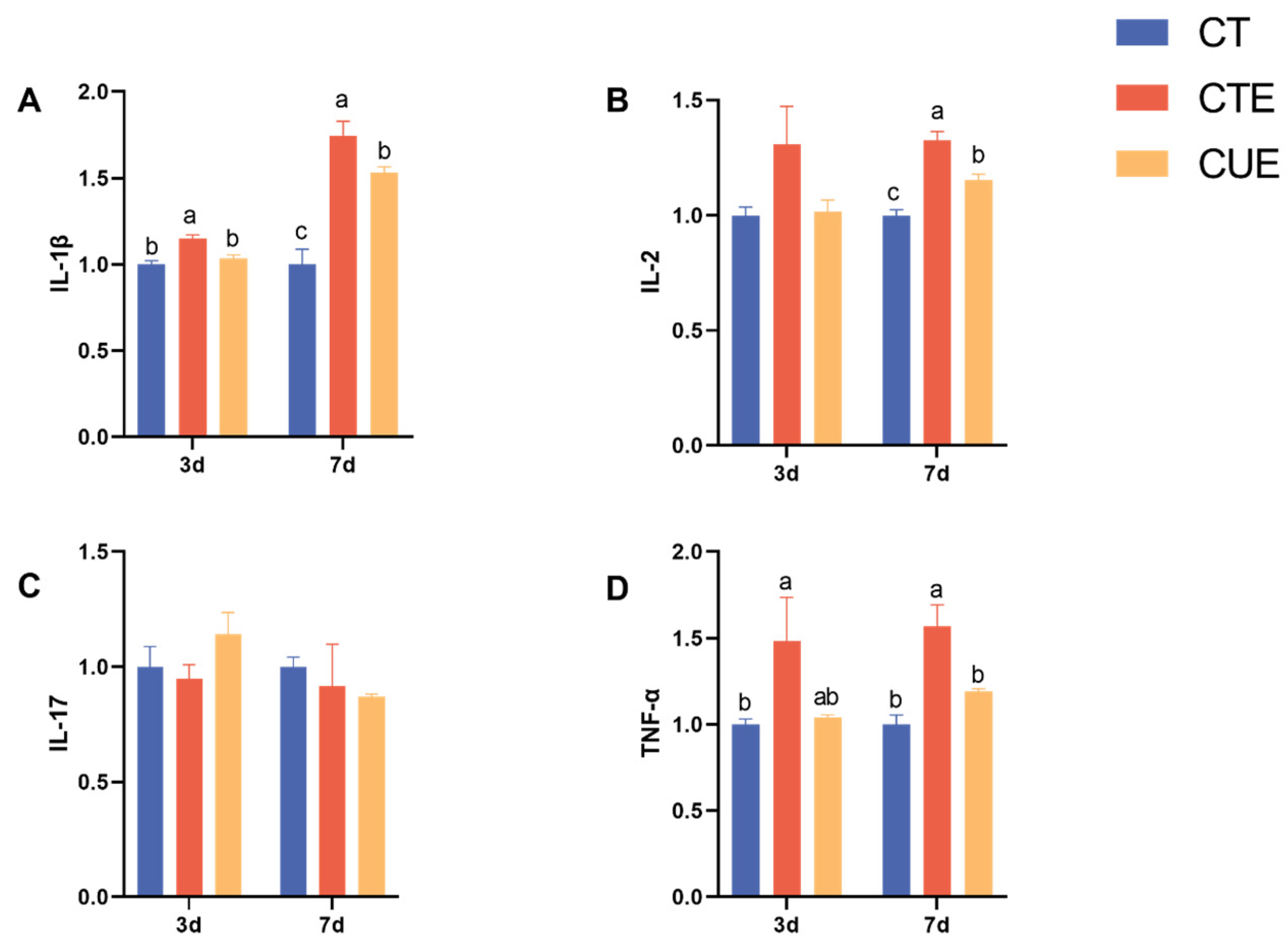
3.4. Effect of Curcumin on the Gene Expression of Cecal Mucosal Cytokines
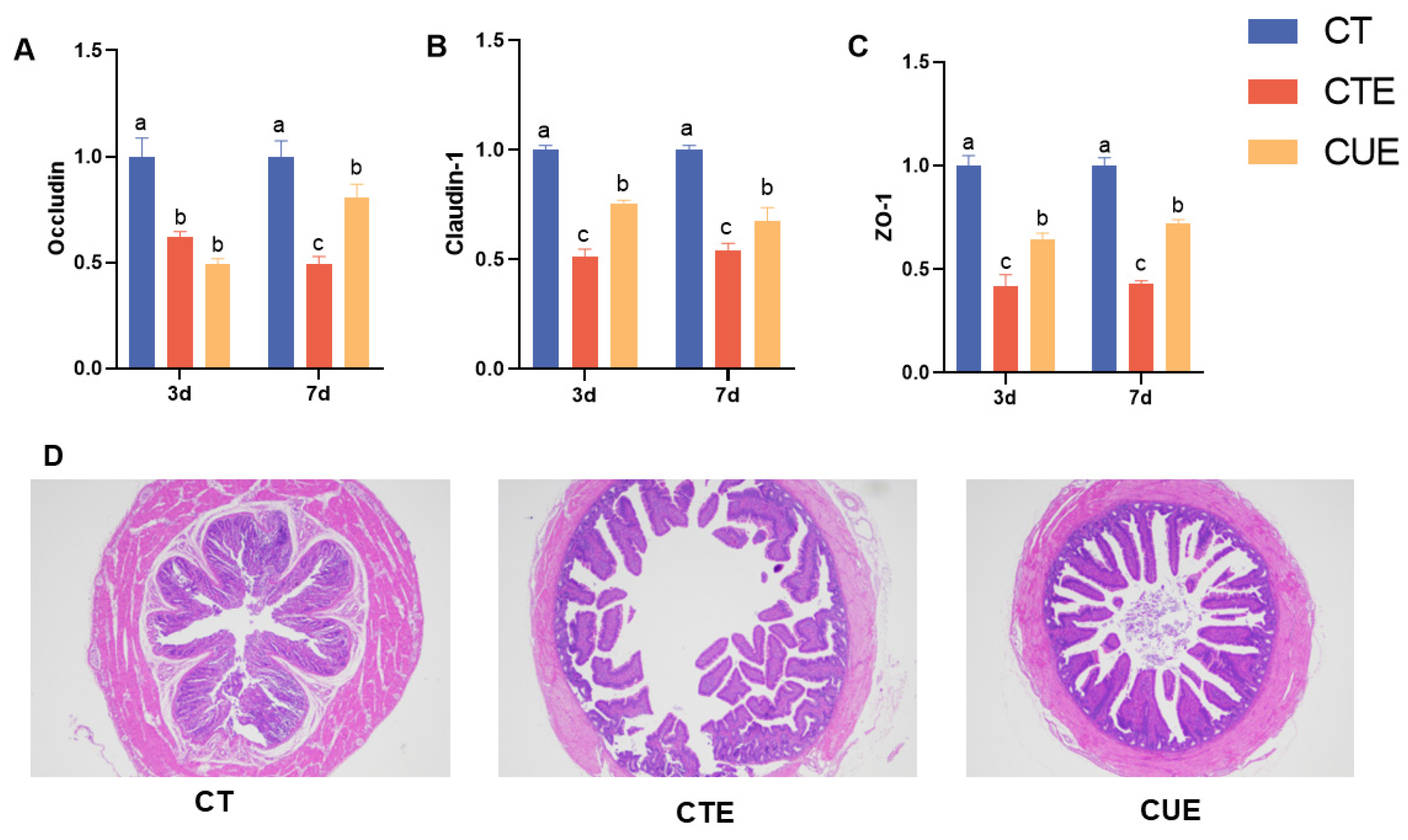
3.5. Curcumin Supplementation Maintained the Integrity of the Intestinal Epithelial Barrier
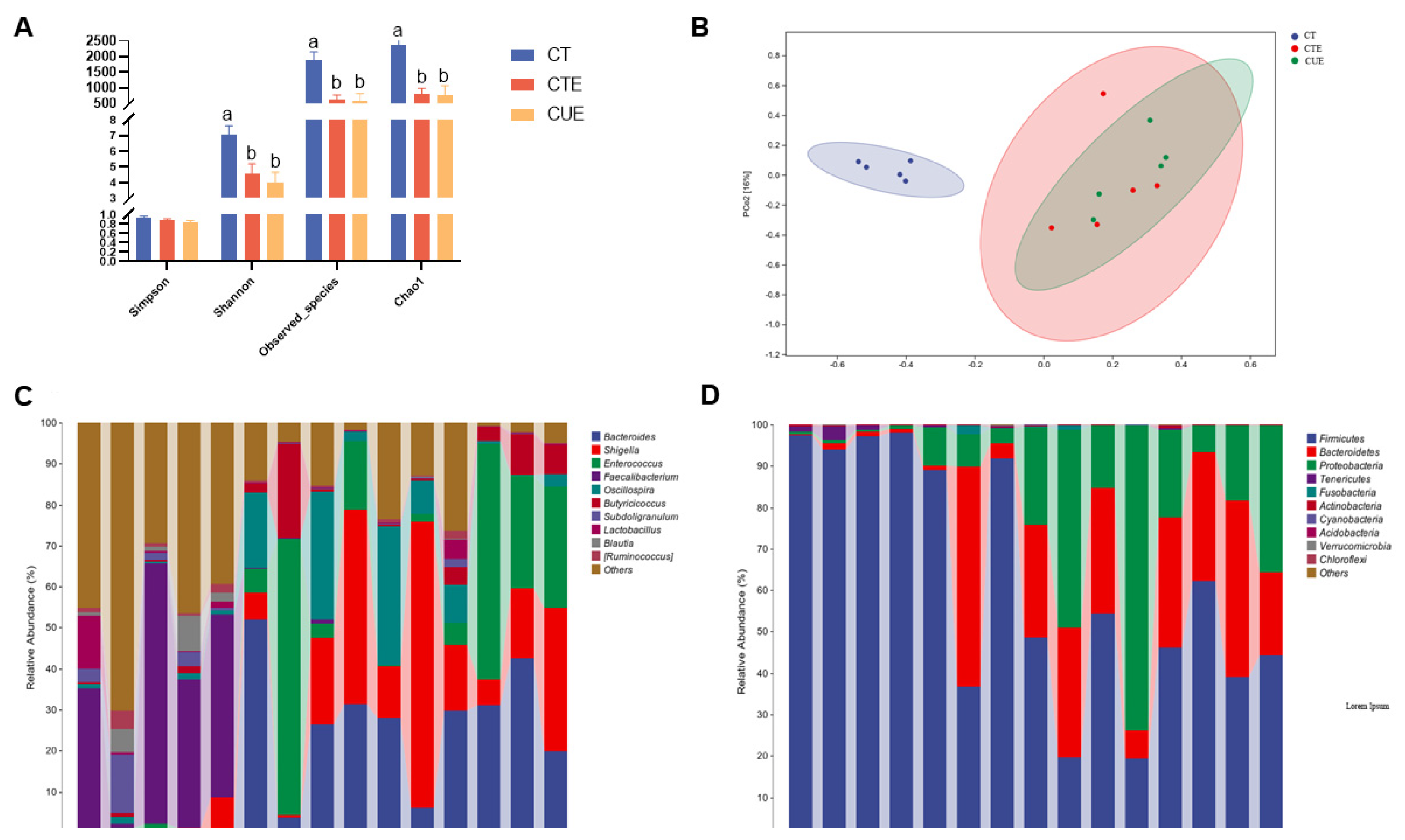
3.6. Effect of Curcumin on Cecal Microbiota Structure
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mesa, C.; Gomez-Osorio, L.M.; Lopez-Osorio, S.; Williams, S.M.; Chaparro-Gutiérrez, J.J. Survey of coccidia on commercial broiler farms in Colombia: Frequency of Eimeria species, anticoccidial sensitivity, and histopathology. Poult. Sci. 2021, 100, 101239. [Google Scholar] [PubMed]
- Chapman, H.D. Milestones in avian coccidiosis research: A review. Poult. Sci. 2014, 93, 501–511. [Google Scholar] [PubMed]
- Schaeffer, M.; Schroeder, J.; Heckeroth, A.R. Identification of lead compounds targeting the cathepsin B-like enzyme of Eimeria tenella. Antimicrob. Agents Chemother. 2012, 56, 1190–1201. [Google Scholar] [PubMed]
- Kaingu, F.; Liu, D.; Wang, L.; Tao, J. Anticoccidial effects of Aloe secundiflora leaf extract against Eimeria tenella in broiler chicken. Trop. Anim. Health Prod. 2017, 49, 823–828. [Google Scholar] [PubMed]
- Wu, S.B.; Stanley, D.; Rodgers, N.; Swick, R.A.; Moore, R.J. Two necrotic enteritis predisposing factors, dietary fishmeal and Eimeria infection, induce large changes in the caecal microbiota of broiler chickens. Vet. Microbiol. 2014, 169, 188–197. [Google Scholar] [PubMed]
- Huang, G.; Tang, X.; Bi, F. Eimeria tenella infection perturbs the chicken gut microbiota from the onset of oocyst shedding. Vet. Parasitol. 2018, 258, 30–37. [Google Scholar] [PubMed]
- Zhang, Y.; Zuo, R.; Song, X.; Gong, J.; Wang, J.; Lin, M.; Yang, F. Optimization of Maduramicin Ammonium-Loaded Nanostructured Lipid Carriers Using Box-Behnken Design for Enhanced Anticoccidial Effect against Eimeria tenella in Broiler Chickens. Pharmaceutics 2022, 14, 1330. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Huang, B.; Dong, H. Molecular Characterization and Immune Protection of a New Conserved Hypothetical Protein of Eimeria tenella. PLoS ONE 2016, 11, e157678. [Google Scholar]
- Huyghebaert, G.; Ducatelle, R.; Van Immerseel, F. An update on alternatives to antimicrobial growth promoters for broilers. Vet. J. 2011, 187, 182–188. [Google Scholar]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar]
- Habibi, H.; Firouzi, S.; Nili, H. Anticoccidial effects of herbal extracts on Eimeria tenella infection in broiler chickens: In vitro and in vivo study. J. Parasit. Dis. 2016, 40, 401–407. [Google Scholar] [PubMed]
- Li, R.; Yao, Y.; Gao, P.; Bu, S. The Therapeutic Efficacy of Curcumin vs. Metformin in Modulating the Gut Microbiota in NAFLD Rats: A Comparative Study. Front. Microbiol. 2021, 11, 555293. [Google Scholar]
- Tanaudommongkon, I.; Tanaudommongkon, A.; Prathipati, P. Curcumin Nanoparticles and Their Cytotoxicity in Docetaxel-Resistant Castration-Resistant Prostate Cancer Cells. Biomedicines 2020, 8, 253. [Google Scholar] [CrossRef]
- Zhang, J.F.; Bai, K.W.; Su, W.P.; Wang, A.; Zhang, L. Curcumin attenuates heat-stress-induced oxidant damage by simultaneous activation of GSH-related antioxidant enzymes and Nrf2-mediated phase II detoxifying enzyme systems in broiler chickens. Poult. Sci. 2018, 97, 1209–1219. [Google Scholar] [PubMed]
- Alizadeh, M.; Kheirouri, S. Curcumin reduces malondialdehyde and improves antioxidants in humans with diseased conditions: A comprehensive meta-analysis of randomized controlled trials. Biomedicine 2019, 9, 23. [Google Scholar]
- Li, S.; You, J.; Wang, Z. Curcumin alleviates high-fat diet-induced hepatic steatosis and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2021, 143, 110270. [Google Scholar]
- Khalafalla, R.E.; Muller, U.; Shahiduzzaman, M.; Dyachenko, V.; Desouky, A.Y.; Alber, G.; Daugschies, A. Effects of curcumin (diferuloylmethane) on Eimeria tenella sporozoites in vitro. Parasitol. Res. 2011, 108, 879–886. [Google Scholar] [PubMed]
- Partoazar, A.; Kianvash, N.; Darvishi, M.H.; Nasoohi, S.; Rezayat, S.M.; Bahador, A. Ethosomal Curcumin Promoted Wound Healing and Reduced Bacterial Flora in Second Degree Burn in Rat. Drug Res. 2016, 66, 660–665. [Google Scholar]
- María Eugenia, C. Influence of Curcumin (Curcuma longa) as a Natural Anticoccidial Alternative in Adult Rabbits: First Results. Ital. J. Anim. Sci. 2015, 14, 3–7. [Google Scholar]
- Cervantes-Valencia, M.E.; Alcalá-Canto, Y.; Sumano-Lopez, H. Effects of Curcuma longa dietary inclusion against Eimeria spp. in naturally-infected lambs. Small Rumin. Res. 2016, 136, 27–35. [Google Scholar]
- Yadav, S.; Teng, P.Y.; Souza, D.S.T.; Gould, R.L.; Craig, S.W.; Lorraine Fuller, A. The effects of different doses of curcumin compound on growth performance, antioxidant status, and gut health of broiler chickens challenged with Eimeria species. Poult. Sci. 2020, 99, 5936–5945. [Google Scholar]
- Song, X.; Li, Y.; Chen, S.; Jia, R.; Huang, Y.; Zou, Y. Anticoccidial Effect of Herbal Powder “Shi Ying Zi” in Chickens Infected with Eimeria tenella. Animals 2020, 10, 1484. [Google Scholar] [CrossRef] [PubMed]
- Jin, H. Coccidiosis: Oocyst counting technique for coccidiostat evaluation. Exp. Parasitol. 1970, 28, 99–102. [Google Scholar]
- Song, J.; Duan, C.; Sang, Y. Effects of Graphene on Bacterial Community Diversity and Soil Environments of Haplic Cambisols in Northeast China. Forests 2018, 9, 677. [Google Scholar] [CrossRef]
- Kim, D.K.; Lillehoj, H.S.; Lee, S.H.; Jang, S.I.; Lillehoj, E.P.; Bravo, D. Dietary Curcuma longa enhances resistance against Eimeria maxima and Eimeria tenella infections in chickens. Poult. Sci. 2013, 92, 2635–2643. [Google Scholar]
- Rochell, S.J.; Helmbrecht, A.; Parsons, C.M.; Dilger, R.N. Interactive effects of dietary arginine and Eimeria acervulina infection on broiler growth performance and metabolism. Poult. Sci. 2017, 96, 659–666. [Google Scholar] [PubMed]
- Teng, P.Y.; Fuller, A.L.; Kim, W.K. Evaluation of nitro compounds as feed additives in diets of Eimeria-challenged broilers in vitro and in vivo. Poult. Sci. 2020, 99, 1320–1325. [Google Scholar] [PubMed]
- Rajput, N.; Muhammad, N.; Yan, R.; Pang, Q.; Shan, A.; Feng, X. Effect of Dietary Supplementation of Curcumin on Growth Performance, Intestinal Morphology and Nutrients Utilization of Broiler Chicks. J. Poult. Sci. 2013, 50, 44–52. [Google Scholar]
- Xun, W.; Shi, L.; Zhou, H.; Hou, G.; Cao, T.; Zhao, C. Effects of curcumin on growth performance, jejunal mucosal membrane integrity, morphology and immune status in weaned piglets challenged with enterotoxigenic Escherichia coli. Int. Immunopharmacol. 2015, 27, 46–52. [Google Scholar]
- Platel, K.; Srinivasan, K. Influence of dietary spices and their active principles on pancreatic digestive enzymes in albino rats. Food/Nahrung 2000, 44, 42–46. [Google Scholar]
- Georgieva, N.V.; Gabrashanska, M.; Koinarski, V. Zinc Supplementation against Eimeria acervulina-Induced Oxidative Damage in Broiler Chickens. Vet. Med. Int. 2011, 2011, 647124. [Google Scholar] [PubMed]
- Xiang, L.; Zhang, Q.; Chi, C. Curcumin analog A13 alleviates oxidative stress by activating Nrf2/ARE pathway and ameliorates fibrosis in the myocardium of high-fat-diet and streptozotocin-induced diabetic rats. Diabetol. Metab. Syndr. 2020, 12, 1. [Google Scholar] [PubMed]
- Carpentier, R.; Lipka, E.; Howsam, M.; Rodolphe, C. Evolution of availability of curcumin inside poly-lactic-co-glycolic acid nanoparticles: Impact on antioxidant and antinitrosant properties. Int. J. Nanomed. 2015, 10, 5355. [Google Scholar]
- Medina-Pizano, M.Y.; Medina-Rosales, M.N.; Martinez-Hernandez, S.L. Protective Effect of Curcumin against Doxazosin- and Carvedilol-Induced Oxidative Stress in HepG2 Cells. Oxid. Med. Cell. Longev. 2022, 2022, 6085515. [Google Scholar] [PubMed]
- Jin, S.; Yang, H.; Jiao, Y. Dietary Curcumin Alleviated Acute Ileum Damage of Ducks (Anas platyrhynchos) Induced by AFB1 through Regulating Nrf2-ARE and NF-kappaB Signaling Pathways. Foods 2021, 10, 1370. [Google Scholar] [PubMed]
- Fang, H.; Liu, A.; Chen, X. The severity of LPS induced inflammatory injury is negatively associated with the functional liver mass after LPS injection in rat model. J. Inflamm. 2018, 15, 21. [Google Scholar]
- Yazdanabadi, F.I.; Moghaddam, G.H.; Nematollahi, A. Effect of arginine supplementation on growth performance, lipid profile, and inflammatory responses of broiler chicks challenged with coccidiosis. Prev. Vet. Med. 2020, 180, 105031. [Google Scholar]
- Moraes, P.O.; Andretta, I.; Cardinal, K.M. Effect of functional oils on the immune response of broilers challenged with Eimeria spp. Animal 2019, 13, 2190–2198. [Google Scholar]
- Laurent, F.; Mancassola, R.; Lacroix, S.; Menezes, R.; Naciri, M. Analysis of chicken mucosal immune response to Eimeria tenella and Eimeria maxima infection by quantitative reverse transcription-PCR. Infect. Immun. 2001, 69, 2527–2534. [Google Scholar]
- Wu, N.; Wang, J.J. Curcumin attenuates liver warm ischemia and reperfusion-induced combined restrictive and obstructive lung disease by reducing matrix metalloprotease 9 activity. Transplant. Proc. 2014, 46, 1135–1138. [Google Scholar]
- Soliman, M.M.; Abdo, N.M.; Ismail, T.A. Immunohistochemical and molecular study on the protective effect of curcumin against hepatic toxicity induced by paracetamol in Wistar rats. BMC Complement. Altern. Med. 2014, 14, 457. [Google Scholar]
- Shehzad, A.; Rehman, G.; Lee, Y.S. Curcumin in inflammatory diseases. BioFactors 2013, 39, 69–77. [Google Scholar] [PubMed]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Seco Calvo, J.; Córdova Martínez, A.; Caballero García, A.; Fernandez-Lazaro, C.I. Modulation of Exercise-Induced Muscle Damage, Inflammation, and Oxidative Markers by Curcumin Supplementation in a Physically Active Population: A Systematic Review. Nutrients 2020, 12, 501. [Google Scholar] [CrossRef] [PubMed]
- Klawitter, M.; Quero, L.; Klasen, J.; Gloess, A.N.; Klopprogge, B.; Hausmann, O.; Boos, N.; Wuertz, K. Curcuma DMSO extracts and curcumin exhibit an anti-inflammatory and anti-catabolic effect on human intervertebral disc cells, possibly by influencing TLR2 expression and JNK activity. J. Inflamm. 2012, 9, 29. [Google Scholar]
- Wang, J.; Ghosh, S.; Ghosh, S. Curcumin improves intestinal barrier function: Modulation of intracellular signaling, and organization of tight junctions. Am. J. Physiol. Cell Physiol. 2017, 312, C438–C445. [Google Scholar] [PubMed]
- Wang, H.; Xu, R.; Zhang, H.; Su, Y.; Zhu, W. Swine gut microbiota and its interaction with host nutrient metabolism. Anim. Nutr. 2020, 6, 410–420. [Google Scholar]
- Pastor Fernández, I.; Pegg, E.; Macdonald, S.E.; Fiona, M.T.; Damer, P.B.; Virginia, M. Laboratory Growth and Genetic Manipulation of Eimeria tenella. Curr. Prot. Microbiol. 2019, 53, e81. [Google Scholar]
- Shapiro, D.; Kapourchali, F.R.; Santilli, A.; Han, Y.; Cresci, G.A.M. Targeting the Gut Microbiota and Host Immunity with a Bacilli-Species Probiotic during Antibiotic Exposure in Mice. Microorganisms 2022, 10, 1178. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, S.; Berlec, A.; Štrukelj, B. The Influence of Probiotics on the Firmicutes/Bacteroidetes Ratio in the Treatment of Obesity and Inflammatory Bowel Disease. Microorganisms 2020, 8, 1715. [Google Scholar] [CrossRef]
- Keren, N.; Konikoff, F.M.; Paitan, Y. Interactions between the intestinal microbiota and bile acids in gallstones patients. Environ. Microbiol. Rep. 2015, 7, 874–880. [Google Scholar]
- Wu, Y.; Wang, B.; Tang, L.; Zhou, Y.; Wang, Q.; Gong, L.; Ni, J.; Li, W. Probiotic Bacillus Alleviates Oxidative Stress-Induced Liver Injury by Modulating Gut-Liver Axis in a Rat Model. Antioxidants 2022, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, F.; Margarucci, S.; Galderisi, U.; Crispi, S.; Peluso, G. Curcumin, Gut Microbiota, and Neuroprotection. Nutrients 2019, 11, 2426. [Google Scholar] [CrossRef] [PubMed]

| Ingredients | Content (%) | Calculated Nutrient Levels | |
|---|---|---|---|
| Corn | 60.50 | Metabolizable energy (MJ/kg) | 12.95 |
| Soybean meal (46% CP) | 30.00 | Crude protein (%) | 19.17 |
| Fish meal (67% CP) | 1.80 | Ca (%) | 0.90 |
| Soybean oil | 3.50 | Available phosphorous | 0.65 |
| Limestone | 1.60 | SID methionine (%) | 0.40 |
| Dicalcium phosphate | 1.45 | SID lysine (%) | 1.00 |
| NaCl | 0.36 | SID threonine (%) | 0.72 |
| DL-methionine | 0.06 | Methionine + Cystine (%) | 0.76 |
| L-lysine hydrochloride (98.5%) | 0.03 | ||
| Mineral premix b | 0.50 | ||
| Vitamin premix a | 0.20 | ||
| Total | 100 |
| Target | Primer Sequence (5′-3′) | Accession on. | Product Size (bp) |
|---|---|---|---|
| Gapdh | F: GTGAAAGTCGGAGTCAACGG R: CGTTCTCAGCCTTGACAGTG | NM_001289745.3 | 184 |
| IL-1β | F: GCATCAAGGGCTACAAGCTC R: GTCCAGGCGGTAGAAGATGA | XM_015297469.1 | 134 |
| IL-2 | F: TCGAGCTCTACACACCAACT R: CTTGCATTCACTTCCGGTGT | M_015276098.2 | 197 |
| IL-17 | F: GAGCCAGAGAGCCTCTTCAA R: TGTGGTCCTCATCGATCCTG | NM_204460.1 | 181 |
| TNF-alpha | F: CTGATGGCGTGAAGAAGGTC R: GAAGAGTTCATTCGCGGCTT | NM_205149.1 | 95 |
| Claudin-1 | F: TACAGCCCTTGGCCAATACA R: CCAAGAAACAACCACCAGCA | NM_001013611.2 | 171 |
| Occludin | F: CCTCATCGTCATCCTGCTCT R: GGTCCCAGTAGATGTTGGCT | XM_025144248.1 | 95 |
| ZO-1 | F: GAGCTCACAAGCTACGCAAA R: ACTTGTAGCACCATCTGCCA | XM_015278981.2 | 161 |
| Items | CT | CTE | CUE | SEM | p-Value | |
|---|---|---|---|---|---|---|
| 1–14 d | ADG (g/d) | 14.04 | 14.23 | 13.89 | 1.67 | 0.736 |
| ADFI (g/d) | 27.60 | 27.02 | 26.06 | 0.36 | 0.219 | |
| FCR | 1.96 | 1.90 | 1.88 | 0.02 | 0.385 | |
| 15–21 d | ADG (g/d) | 16.92 a | 10.72 c | 15.23 b | 0.86 | 0.001 |
| ADFI (g/d) | 34.99 a | 28.74 b | 35.00 a | 1.01 | 0.004 | |
| FCR | 2.12 b | 2.72 a | 2.30 ab | 1.06 | 0.044 | |
| 22–42 d | ADG (g/d) | 30.75 a | 26.10 c | 28.43 b | 0.63 | 0.002 |
| ADFI (g/d) | 75.60 | 69.80 | 74.50 | 1.08 | 0.051 | |
| FCR | 2.46 | 2.68 | 2.62 | 0.42 | 0.073 | |
| 1–42 d | ADG (g/d) | 20.57 a | 17.01 c | 19.18 b | 0.44 | <0.000 |
| ADFI (g/d) | 46.06 a | 41.85 b | 45.18 a | 0.57 | 0.001 | |
| FCR | 2.24 c | 2.46 a | 2.35 b | 0.02 | 0.03 |
| Items | CT | CTE | CUE | SEM | p-Value |
|---|---|---|---|---|---|
| Phylum level | |||||
| Firmicutes | 95.095 a | 50.259 b | 42.251 b | 7.554 | 0.001 |
| Proteobacteria | 1.021 b | 29.105 a | 26.342 a | 4.557 | 0.008 |
| Bacteroidetes | 2.337 b | 19.599 ab | 31.009 a | 5.370 | 0.080 |
| Tenericutes | 1.234 a | 0.011 b | 0.062 b | 0.226 | 0.029 |
| Fusobacteria | 0.008 | 0.666 | 0.024 | 0.147 | 0.108 |
| Actinobacteria | 0.197 | 0.220 | 0.180 | 0.035 | 0.909 |
| Cyanobacteria | 0.018 | 0.025 | 0.013 | 0.004 | 0.535 |
| Acidobacteria | 0.020 | 0.012 | 0.011 | 0.003 | 0.487 |
| Verrucomicrobia | 0.014 | 0.005 | 0.007 | 0.003 | 0.476 |
| Chloroflexi | 0.009 | 0.009 | 0.002 | 0.002 | 0.279 |
| Others | 0.047 | 0.090 | 0.100 | 0.023 | 0.634 |
| Genus level | |||||
| Bacteroides | 0.007 b | 0.282 a | 0.258 a | 0.045 | 0.009 |
| Shigella | 0.016 b | 0.177 ab | 0.288 a | 0.052 | 0.096 |
| Faecalibacterium | 0.003 | 0.188 | 0.243 | 0.056 | 0.199 |
| Enterococcus | 0.360 a | 0.003 b | 0.001 b | 0.055 | 0.001 |
| Oscillospira | 0.012 b | 0.172 a | 0.042 ab | 0.029 | 0.045 |
| Butyricicoccus | 0.007 | 0.052 | 0.051 | 0.016 | 0.437 |
| Subdoligranulum | 0.047 | 0.001 | 0.005 | 0.009 | 0.085 |
| Lactobacillus | 0.031 | 0.002 | 0.010 | 0.009 | 0.396 |
| Blautia | 0.037 a | 0.000 b | 0.001 b | 0.006 | 0.015 |
| Ruminococcus | 0.018 | 0.005 | 0.005 | 0.003 | 0.100 |
| Others | 0.461 a | 0.118 b | 0.095 b | 0.053 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, L.; Yu, L.; Li, S.; Zhu, N.; You, J. Curcumin Supplementation Improves Growth Performance and Anticoccidial Index by Improving the Antioxidant Capacity, Inhibiting Inflammatory Responses, and Maintaining Intestinal Barrier Function in Eimeria tenella-Infected Broilers. Animals 2024, 14, 1223. https://doi.org/10.3390/ani14081223
Chen Y, Liu L, Yu L, Li S, Zhu N, You J. Curcumin Supplementation Improves Growth Performance and Anticoccidial Index by Improving the Antioxidant Capacity, Inhibiting Inflammatory Responses, and Maintaining Intestinal Barrier Function in Eimeria tenella-Infected Broilers. Animals. 2024; 14(8):1223. https://doi.org/10.3390/ani14081223
Chicago/Turabian StyleChen, Yan, Liheng Liu, Longfei Yu, Shuo Li, Nianhua Zhu, and Jinming You. 2024. "Curcumin Supplementation Improves Growth Performance and Anticoccidial Index by Improving the Antioxidant Capacity, Inhibiting Inflammatory Responses, and Maintaining Intestinal Barrier Function in Eimeria tenella-Infected Broilers" Animals 14, no. 8: 1223. https://doi.org/10.3390/ani14081223




