Avian Haemosporidian Infection in Wildlife Rehabilitation Centres of Portugal: Causes, Consequences, and Genetic Diversity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Sample Processing and Molecular Determination of Haemosporidian Infection
2.3. Statistical Analysis
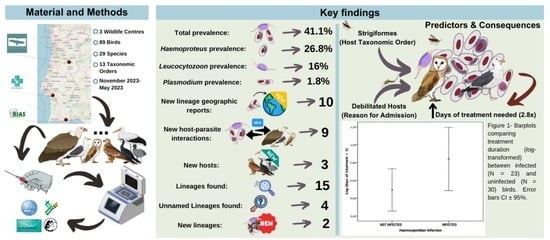
3. Results
3.1. Prevalence and Genetic Diversity of Haemosporidian Parasites
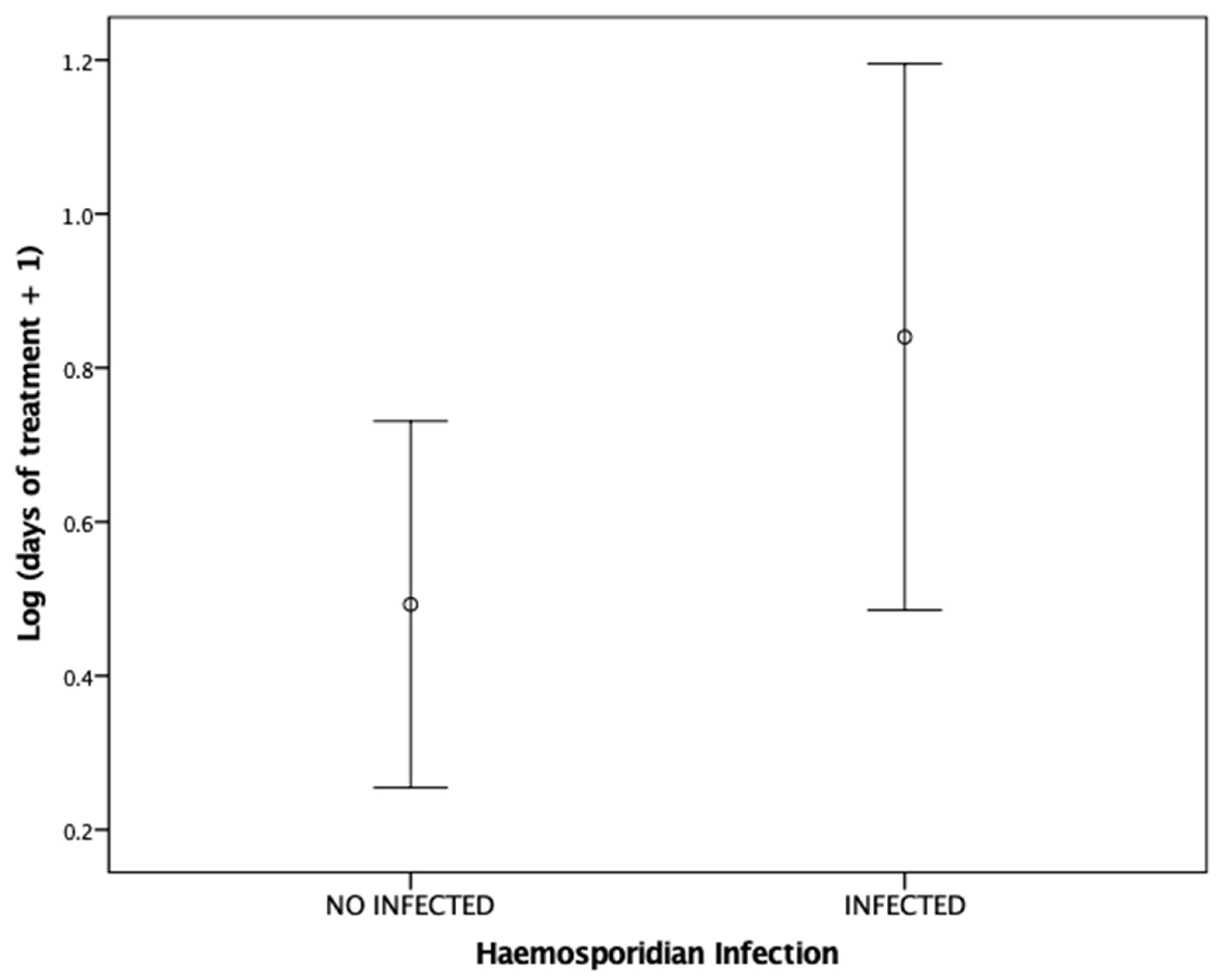
3.2. Factors Determining the Length of Medical Treatment
3.3. Factors Determining Haemosporidian Infection
4. Discussion
4.1. Prevalence and Genetic Diversity of Haemosporidian Parasites
4.2. Factors Determining the Length of Medical Treatment
4.3. Factors Determining Haemosporidian Infection
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Finn, C.; Grattarola, F.; Pincheira-Donoso, D. More losers than winners: Investigating Anthropocene defaunation through the diversity of population trends. Biol. Rev. 2023, 98, 1732–1748. [Google Scholar] [CrossRef] [PubMed]
- Almond, R.E.A.; Grooten, M.; Bignoli, J.D.; Petersen, T. Living Planet Report 2022—Building a Nature-Positive Society; WWF: Gland, Switzerland, 2022; ISBN 978-2-88085-316-7. [Google Scholar]
- Naumann, S.; Noebel, R.; Gaudillat, Z.; Stein, U.; Röschel, L.; Ittner, S.; Davis, M.; Staneva, A.; Rutherford, C.; Romão, C. Results from Reporting under the Nature Directives; European Environment Agency: Copenhagen, Denmark, 2020.
- BirdLife International. European Red List of Birds; Publications Office of the European Union: Luxembourg, 2021; ISBN 978-92-76-45973-6. [Google Scholar]
- Rigal, S.; Dakos, V.; Alonso, H.; Auniņš, A.; Benkő, Z.; Brotons, L.; Chodkiewicz, T.; Chylarecki, P.; de Carli, E.; Del Moral, J.C.; et al. Farmland practices are driving bird population decline across Europe. Proc. Natl. Acad. Sci. USA 2023, 120, e2216573120. [Google Scholar] [CrossRef] [PubMed]
- Matias, R.; Alfrey, P.; Costa, H.; Jara, J.; Moore, C.C.; Santos, J.L.; Tipper, R. Additions and changes to the systematic list of the birds of Mainland Portugal: First update. Anu. Ornitol. 2011, 8, 105–117. [Google Scholar]
- Almeida, J.; Godinho, C.; Leitão, D.; Lopes, R.J. Lista Vermelha das Aves de Portugal Continental; SPEA, ICNF, LabOR/UÉ, CIBIO/BIOPOLIS: Lisbon, Portugal, 2022. (In Portuguese) [Google Scholar]
- Cabral, M.J.; Almeida, J.; Almeida, P.R.; Dellinger, T.; Ferrand de Almeida, N.; Oliveira, M.E.; Palmeirim, J.M.; Queiroz, A.I.; Rogado, L.; Santos-Reis, M. Livro Vermelho dos Vertebrados de Portugal; ICNF: Lisboa, Portugal, 2005. (In Portuguese) [Google Scholar]
- Halupka, L.; Arlt, D.; Tolvanen, J.; Millon, A.; Bize, P.; Adamík, P.; Albert, P.; Arendt, W.J.; Artemyev, A.V.; Baglione, V.; et al. The Effect of Climate Change on Avian Offspring Production: A Global Meta-Analysis. Proc. Natl. Acad. Sci. USA 2023, 120, e2208389120. [Google Scholar] [CrossRef] [PubMed]
- Betts, M.G.; Yang, Z.; Hadley, A.S.; Smith, A.C.; Rousseau, J.S.; Northrup, J.M.; Nocera, J.J.; Gorelick, N.; Gerber, B.D. Forest Degradation Drives Widespread Avian Habitat and Population Declines. Nat. Ecol. Evol. 2022, 6, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Lawson, B.; Toms, M.P.; Peck, K.M.; Kirkwood, J.K.; Chantrey, J.; Clatworthy, I.R.; Evans, A.D.; Hughes, L.A.; Hutchinson, O.C.; et al. Emerging infectious disease leads to rapid population declines of common British birds. PLoS ONE 2010, 5, e12215. [Google Scholar] [CrossRef] [PubMed]
- Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; Terregino, C.; Guajardo, I.M.; Chuzhakina, K.; et al. Avian influenza overview. EFSA J. 2022, 20, e07597. [Google Scholar] [CrossRef] [PubMed]
- Bensch, S.; Hellgren, O.; Pérez-Tris, J. MalAvi: A public database of malaria parasites and related haemosporidians in avian hosts based on mitochondrial cytochrome b lineages. Mol. Ecol. Resour. 2009, 9, 1353–1358. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A. Recent advances in studies on avian malaria parasites. In Malaria Parasites; Okwa, O.O., Ed.; Intech: London, UK, 2012; pp. 135–158. Available online: http://www.intechopen.com/books/malaria-parasites (accessed on 1 April 2024).
- Ellis, V.A.; Fecchio, A.; Ricklefs, R.E. Haemosporidian parasites of Neotropical birds: Causes and consequences of infection. Auk 2020, 137, ukaa055. [Google Scholar] [CrossRef]
- Valkiūnas, G. Avian Malaria Parasites and Other Haemosporidia; CRC Press: Boca Raton, FL, USA, 2005; ISBN 0-415-30097-5. [Google Scholar]
- Ibáñez-Bernal, S.; Rivera-García, K.D.; Abella-Medrano, C.A. Introduction to the Taxonomy and General Biology of Diptera (Insecta) Involved in the Transmission of Avian Haemosporida. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Palinauskas, V.; la Puente, J.M.; Hernández-Soto, S.R.; Marzal, A. Experimental parasitology and ecoimmunology: Concepts and opportunities in avian haemosporidian studies. In Avian Malaria and Related Parasites in the Tropics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar]
- Merino, S.; Moreno, J.; Sanz, J.J.; Arriero, E. Are avian blood parasites pathogenic in the wild? A medication experiment in blue tits (Parus caeruleus). Proc. R. Soc. Lond. B Biol. Sci. 2000, 267, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Bensch, S.; Reviriego, M.; Balbontin, J.; de Lope, F. Effects of malaria double infection in birds: One plus one is not two. J. Evol. Biol. 2008, 21, 979–987. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; de Lope, F.; Navarro, C.; Møller, A.P. Malarial parasites decrease reproductive success: An experimental study in a passerine bird. Oecologia 2005, 142, 541–545. [Google Scholar] [CrossRef] [PubMed]
- Knowles, S.C.; Palinauskas, V.; Sheldon, B.C. Chronic malaria infections increase family inequalities and reduce parental fitness: Experimental evidence from a wild bird population. J. Evol. Biol. 2010, 23, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Bosholn, M.; Fecchio, A.; Silveira, P.; Braga, É.M.; Anciães, M. Effects of avian malaria on male behaviour and female visitation in lekking Blue-crowned Manakins. J. Avian Biol. 2016, 47, 457–465. [Google Scholar] [CrossRef]
- Marzal, A.; Balbontín, J.; Reviriego, M.; García-Longoria, L.; Relinque, C.; Hermosell, I.G.; Magallanes, S.; López-Calderón, C.; de Lope, F.; Møller, A.P. A longitudinal study of age-related changes in Haemoproteus infection in a passerine bird. Oikos 2016, 125, 1092–1099. [Google Scholar] [CrossRef]
- Lachish, S.; Knowles, S.C.L.; Alves, R.; Wood, M.J.; Sheldon, B.C. Fitness effects of endemic malaria infections in a wild bird population: The importance of ecological structure. J. Anim. Ecol. 2011, 80, 1196–1206. [Google Scholar] [CrossRef]
- Asghar, M.; Hasselquist, D.; Hansson, B.; Zehtindjiev, P.; Westerdahl, H.; Bensch, S. Chronic infection. Hidden costs of infection: Chronic malaria accelerates telomere degradation and senescence in wild birds. Science 2015, 347, 436–438. [Google Scholar] [CrossRef] [PubMed]
- Eastwood, J.R.; Peacock, L.; Hall, M.L.; Roast, M.; Murphy, S.A.; Gonçalves da Silva, A.; Peters, A. Persistent low avian malaria in a tropical species despite high community prevalence. Int. J. Parasitol. Parasites Wildl. 2019, 8, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; Valkiūnas, G.; Bolshakov, V.C.; Bensch, S. Effects of Plasmodium relictum (lineage P-SGS1) on experimentally infected passerine birds. Exp. Parasitol. 2008, 120, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Palinauskas, V.; Valkiūnas, G.; Bensch, S.; Bolshakov, V.C. Plasmodium relictum (lineage SGS1) and Plasmodium ashfordi (lineage GRW2): The effects of the co-infection on experimentally infected passerine birds. Exp. Parasitol. 2011, 127, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Tomás, G.; Merino, S.; Martínez, J.; Moreno, J.; Sanz, J.J. Stress protein levels and blood parasite infection in blue tits (Parus caeruleus): A medication field experiment. Ann. Zool. Fenn. 2005, 42, 45–56. [Google Scholar]
- Miller, E.A. Minimum Standards for Wildlife Rehabilitation, 4th ed.; NWRA & IWRC: St. Cloud, MN, USA, 2012; ISBN 978-1-931439-28-2. [Google Scholar]
- Molina-López, R.A.; Mañosa, S.; Torres-Riera, A.; Pomarol, M.; Darwich, L. Morbidity, outcomes and cost-benefit analysis of wildlife rehabilitation in Catalonia (Spain). PLoS ONE 2017, 12, e0181331. [Google Scholar] [CrossRef]
- Tribe, A.; Torregrosa, M.; Bouchon-Small, A. Wildlife rehabilitation in South East Queensland. In Proceedings of the Australian Wildlife Rehabilitation Conference, Hobart, Tasmania, 26–31 May 2014. [Google Scholar]
- Lukesova, G.; Voslarova, E.; Vecerek, V.; Vucinic, M. Causes of Admission, Length of Stay and Outcomes for Common Kestrels in Rehabilitation Centres in the Czech Republic. Sci. Rep. 2021, 11, 17269. [Google Scholar] [CrossRef] [PubMed]
- Gomes, J.; Leitão, M.; Louro, M.C.; Brandão, R.; Mateus, T.L. Avian Malaria in wild birds from a wildlife rehabilitation center in Central Portugal. Vet. Parasitol. Reg. Stud. Rep. 2023, 43, 100904. [Google Scholar] [CrossRef] [PubMed]
- Inumaru, M.; Murata, K.; Sato, Y. Prevalence of avian haemosporidia among injured wild birds in Tokyo and environs, Japan. Int. J. Parasitol. Parasites Wildl. 2017, 6, 299–309. [Google Scholar] [CrossRef]
- Jia, T.; Huang, X.; Valkiūnas, G.; Yang, M.; Zheng, C.; Pu, T.; Zhang, Y.; Dong, L.; Suo, X.; Zhang, C. Malaria Parasites and Related Haemosporidians Cause Mortality in Cranes: A Study on the Parasites Diversity, Prevalence and Distribution in Beijing Zoo. Malar. J. 2018, 17, 234. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Alarcon, D.; Marzal, A. Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Tully, T.N. Birds. In Manual of Exotic Pet Practice; Mitchell, M.A., Tully, T.N., Eds.; W.B. Saunders: Saint Louis, MO, USA, 2009; pp. 250–298. ISBN 978-1-4160-0119-5. [Google Scholar]
- Bensch, S.; Stjernman, M.; Hasselquist, D.; Ostman, O.; Hansson, B.; Westerdahl, H.; Pinheiro, R.T. Host specificity in avian blood parasites: A study of Plasmodium and Haemoproteus mitochondrial DNA amplified from birds. Proc. Biol. Sci. 2000, 267, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Hellgren, O.; Waldenström, J.; Bensch, S. A new PCR assay for simultaneous studies of Leucocytozoon, Plasmodium, and Haemoproteus from avian blood. J. Parasitol. 2004, 90, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, J.; Bensch, S.; Hasselquist, D.; Ostman, O. A new nested polymerase chain reaction method very efficient in detecting Plasmodium and Haemoproteus infections from avian blood. J. Parasitol. 2004, 90, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Hall, T. Symposium on RNA biology. III. RNA. Tool and Target. In Proceedings of the in Nucleic Acids Symposium Series, Research Triangle Park, NC, USA, 15–17 October 1999; pp. 1–218. [Google Scholar]
- Muriel, J.; Marzal, A.; Magallanes, S.; García-Longoria, L.; Suarez-Rubio, M.; Bates, P.J.J.; Lin, H.H.; Soe, A.N.; Oo, K.S.; Aye, A.A.; et al. Prevalence and Diversity of Avian Haemosporidians May Vary with Anthropogenic Disturbance in Tropical Habitats in Myanmar. Diversity 2021, 13, 111. [Google Scholar] [CrossRef]
- Garcia-Longoria, L.; Muriel, J.; Magallanes, S.; Villa-Galarce, Z.H.; Ricopa, L.; Inga-Díaz, W.G.; Fong, E.; Vecco, D.; Guerra-SaldaÑa, C.; Salas-Rengifo, T.; et al. Diversity and host assemblage of avian haemosporidians in different terrestrial ecoregions of Peru. Curr. Zool. 2022, 68, 27–40. [Google Scholar] [CrossRef]
- Billerman, S.M.; Keeney, B.K.; Rodewald, P.G.; Schulenberg, T.S. Birds of the World; Cornell Laboratory of Ornithology: Ithaca, NY, USA, 2022; Available online: https://birdsoftheworld-org.proxy.birdsoftheworld.org/bow/home (accessed on 15 April 2024).
- Paterson, J.E.; Carstairs, S.; Davy, C.M. Population-level effects of wildlife rehabilitation and release vary with life-history strategy. J. Nat. Conserv. 2021, 61, 125983. [Google Scholar] [CrossRef]
- Nourani, L.; Aliabadian, M.; Mirshamsi, O.; Djadid, N.D. Prevalence of co-infection and genetic diversity of avian haemosporidian parasites in two rehabilitation facilities in Iran: Implications for the conservation of captive raptors. BMC Ecol. Evol. 2022, 22, 114. [Google Scholar] [CrossRef] [PubMed]
- Pornpanom, P.; Fernandes Chagas, C.R.; Lertwatcharasarakul, P.; Kasorndorkbua, C.; Valkiūnas, G.; Salakij, C. Molecular Prevalence and Phylogenetic Relationship of Haemoproteus and Plasmodium Parasites of Owls in Thailand: Data from a Rehabilitation Centre. Int. J. Parasitol. Parasites Wildl. 2019, 9, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Pyke, G.H.; Szabo, J.K. Conservation and the 4 Rs, which are rescue, rehabilitation, release, and research. Conserv. Biol. 2018, 32, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Kwok, A.B.C.; Haering, R.; Travers, S.K.; Stathis, P. Trends in wildlife rehabilitation rescues and animal fate across a six-year period in New South Wales, Australia. PLoS ONE 2021, 16, e0257209. [Google Scholar] [CrossRef] [PubMed]
- Stauber, E. The value of wildlife rehabilitation-opportunities for medical training, research, education, conservation. Jpn. J. Zoo Wildl. Med. 2002, 7, 1–4. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Himmel, T.; Harl, J.; Dagys, M.; Valkiūnas, G.; Weissenböck, H. Exo-Erythrocytic Development of Avian Haemosporidian Parasites in European Owls. Animals 2022, 12, 2212. [Google Scholar] [CrossRef] [PubMed]
- Ber, J.L.; Goddard, J.; Nations, T.M.; Outlaw, D.C. Survey and Phylogenetic Analysis of Leucocytozoon (Apicomplexa: Haemosporida) Parasites in Mississippi Black Flies (Diptera: Simuliidae). J. Med. Entomol. 2022, 59, 1042–1046. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, R.; Bouwhuis, S.; Bichet, C.; Podlaszczuk, P.; Chyb, A.; Indykiewicz, P.; Dulisz, B.; Betleja, J.; Janiszewski, T.; Minias, P. Contrasting haemoparasite prevalence in larid species with divergent ecological niches and migration patterns. Parasitology 2022, 149, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Rodríguez, A.; de la Puente, J.; Onrubia, A.; Pérez-Tris, J. Molecular Characterization of Haemosporidian Parasites from Kites of the Genus Milvus (Aves: Accipitridae). Int. J. Parasitol. 2013, 43, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Harl, J.; Himmel, T.; Valkiūnas, G.; Ilgūnas, M.; Nedorost, N.; Matt, J.; Kübber-Heiss, A.; Alic, A.; Konicek, C.; Weissenböck, H. Avian Haemosporidian Parasites of Accipitriform Raptors. Malar. J. 2022, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, D.; Palinauskas, V.; Iezhova, T.A.; Bernotienė, R.; Ilgūnas, M.; Bukauskaitė, D.; Zehtindjiev, P.; Ilieva, M.; Shapoval, A.P.; Bolshakov, C.V.; et al. Plasmodium spp.: An experimental study on vertebrate host susceptibility to avian malaria. Exp. Parasitol. 2015, 148, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Ilgūnas, M.; Bukauskaitė, D.; Fragner, K.; Weissenböck, H.; Atkinson, C.T.; Iezhova, T.A. Characterization of Plasmodium relictum, a Cosmopolitan Agent of Avian Malaria. Malar. J. 2018, 17, 184. [Google Scholar] [CrossRef] [PubMed]
- Valkiūnas, G.; Atkinson, C.T. Introduction to Life Cycles, Taxonomy, Distribution, and Basic Research Techniques BT—Avian Malaria and Related Parasites in the Tropics: Ecology, Evolution and Systematics; Santiago-Alarcon, D., Marzal, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 45–80. ISBN 978-3-030-51633-8. [Google Scholar]
- Victoria. Code of Practice for the Welfare of Wildlife during Rehabilitation/Bureau of Animal Welfare; Department of Natural Resources and Environment. Bureau of Animal Welfare: Attwood, Australia, 2001; ISBN 0731147995.
- Cope, H.R.; McArthur, C.; Dickman, C.R.; Newsome, T.M.; Gray, R.; Herbert, C.A. A systematic review of factors affecting wildlife survival during rehabilitation and release. PLoS ONE 2022, 17, e0265514. [Google Scholar] [CrossRef] [PubMed]
- Ciloglu, A.; Yildirim, A.; Duzlu, O.; Onder, Z.; Dogan, Z.; Inci, A. Investigation of avian haemosporidian parasites from raptor birds in Turkey, with molecular characterisation and microscopic confirmation. Folia Parasitol. 2016, 63, 2016.023. [Google Scholar] [CrossRef] [PubMed]
- Marzal, A.; Magallanes, S.; Salas-Rengifo, T.; Muriel, J.; Navarro, C.; Vecco, D.; Guerra-Saldaña, C.; Mendo, L.; Paredes, V.; González-Blázquez, M.; et al. Prevalence and Diversity of Avian Malaria Parasites in Illegally Traded White-Winged Parakeets in Peruvian Amazonas. Anim. Conserv. 2024. [Google Scholar] [CrossRef]
- Englefield, B.; Candy, S.; Starling, M.; McGreevy, P. The Demography and Practice of Australians Caring for Native Wildlife and the Psychological, Physical and Financial Effects of Rescue, Rehabilitation and Release of Wildlife on the Welfare of Carers. Animals 2019, 9, 1127. [Google Scholar] [CrossRef] [PubMed]
- Cerda, J.R.; Webb, T.L. Wildlife conservation and preserving biodiversity: Impactful opportunities for veterinarians? J. Am. Vet. Med. Assoc. 2023, 261, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Kroch, E.A.; Duan, M.; Silow-Carrol, S.; Meyer, J.A. Hospital Performance Improvement: Trends in Quality and Efficiency. A Quantitative Analysis of Performance Improvement in U.S Hospitals; The Commonwealth Fund: New York, NY, USA, 2007. [Google Scholar]
- Willette, M.; Rosenhagen, N.; Buhl, G.; Innis, C.; Boehm, J. Interrupted Lives: Welfare Considerations in Wildlife Rehabilitation. Animals 2023, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Swanson, D.L. Substrate metabolism under cold stress in seasonally acclimatized dark-eyed juncos. Physiol. Zool. 1991, 64, 1578–1592. [Google Scholar] [CrossRef]
- Arens, J.R.; Cooper, S.J. Metabolic and Ventilatory Acclimatization to Cold Stress in House Sparrows (Passer domesticus). Physiol. Biochem. Zool. 2005, 78, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Pulgarín-R, P.C. Host species, and not environment, predicts variation in blood parasite prevalence, distribution, and diversity along a humidity gradient in northern South America. Ecol. Evol. 2017, 8, 3800–3814. [Google Scholar] [CrossRef] [PubMed]
- Beadell, J.S.; Gering, E.; Austin, J.; Dumbacher, J.P.; Peirce, M.A.; Pratt, T.K.; Atkinson, C.T.; Fleischer, R.C. Prevalence and differential host-specificity of two avian blood parasite genera in the Australo-Papuan region. Mol. Ecol. 2004, 13, 3829–3844. [Google Scholar] [CrossRef] [PubMed]
- Krone, O.; Priemer, J.; Streich, J.; Sommer, P.; Langgemach, T.; Lessow, O. Haemosporida of birds of prey and owls from Germany. Acta Protozool. 2001, 40, 281–290. [Google Scholar]
- Santos, N.G.; Pereira, M.C.; Melo, P.M.; Madeira de Carvalho, L.M. Pesquisa de hemoprotozoários em aves de rapina (ordens Falconiformes e Strigiformes) em centros de recuperação em Portugal. Rev. Port. Cienc. Vet. 2008, 103, 567–568. (In Portuguese) [Google Scholar]
- Baptista, A.C.; Pires, B.; Matos, R.; Melo, P.; Madeira de Carvalho, L.M. Blood parasites in birds of prey and the contribution of wild animal rehabilitation centres. In Proceedings of the 3rd International Congress of Veterinary Nursing, Elvas, Portugal, 8–10 October 2010. [Google Scholar]
- Gao, K.; Zhou, B.; Yang, L.-X.; Dong, L.; Huang, X.; Deng, W.-H. How Does Circadian Rhythm Shape Host-Parasite Associations? A Comparative Study on Infection Patterns in Diurnal and Nocturnal Raptors. Diversity 2021, 13, 338. [Google Scholar] [CrossRef]
- Marzal, A.; Magallanes, S.; Garcia-Longoria, L. Stimuli Followed by Avian Malaria Vectors in Host-Seeking Behaviour. Biology 2022, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Forrester, D.J.; Telford, S.R.; Foster, G.W.; Bennet, G.F. Blood parasites of Raptors in Florida. J. Rap. Res. 1994, 28, 226–231. [Google Scholar]
- Palinauskas, V.; Žiegytė, R.; Ilgūnas, M.; Iezhova, T.A.; Bernotienė, R.; Bolshakov, C.; Valkiūnas, G. Description of the First Cryptic Avian Malaria Parasite, Plasmodium homocircumflexum n. sp., with Experimental Data on Its Virulence and Development in Avian Hosts and Mosquitoes. Int. J. Parasitol. 2015, 45, 51–62. [Google Scholar] [CrossRef]
- Ilgūnas, M.; Bukauskaitė, D.; Palinauskas, V.; Iezhova, T.A.; Dinhopl, N.; Nedorost, N.; Weissenbacher-Lang, C.; Weissenböck, H.; Valkiūnas, G. Mortality and Pathology in Birds Due to Plasmodium (Giovannolaia) homocircumflexum Infection, with Emphasis on the Exoerythrocytic Development of Avian Malaria Parasites. Malar. J. 2016, 15, 256. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.S.; Giannitti, F.; Valkiūnas, G.; Tell, L.A.; Snipes, J.; Wright, S.; Cornel, A.J. A Method to Preserve Low Parasitaemia Plasmodium-Infected Avian Blood for Host and Vector Infectivity Assays. Malar. J. 2016, 15, 154. [Google Scholar] [CrossRef] [PubMed]
- Coon, C.A.C.; Garcia-Longoria, L.; Martin, L.B.; Magallanes, S.; de Lope, F.; Marzal, A. Malaria Infection Negatively Affects Feather Growth Rate in the House Sparrow Passer domesticus. J. Avian Biol. 2016, 47, 779–787. [Google Scholar] [CrossRef]
- Crommenacker, J.; Komdeur, J.; Richardson, D.S. Assessing the Cost of Helping: The Roles of Body Condition and Oxidative Balance in the Seychelles Warbler (Acrocephalus sechellensis). PLoS ONE 2011, 6, e26423. [Google Scholar] [CrossRef] [PubMed]
- Cornet, S.; Bichet, C.; Larcombe, S.; Faivre, B.; Sorci, G. Impact of Host Nutritional Status on Infection Dynamics and Parasite Virulence in a Bird-Malaria System. J. Anim. Ecol. 2014, 83, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Bueno, M.G.; Lopez, R.P.; de Menezes, R.M.; Costa-Nascimento, M.d.J.; Lima, G.F.; Araújo, R.A.; Guida, F.J.; Kirchgatter, K. Identification of Plasmodium relictum causing mortality in penguins (Spheniscus magellanicus) from São Paulo Zoo. Brazil. Vet. Parasitol. 2010, 173, 123–127. [Google Scholar] [CrossRef] [PubMed]
- García-Del-Río, M.; Sancho, R.; Martínez, J.; Merino, S. Blood parasite infections in Strigiformes and Psittaciformes species in captivity with a new record of potential fatal blood parasite transmission to parrots. J. Zoo Wildl. Med. 2021, 51, 799–813. [Google Scholar] [CrossRef] [PubMed]
- Merino, S.; Peirce, M.A.; Fernández, M.; Lanzarot, P. Redescription of Babesia moshkovskii (Schurenkova) from the griffon vulture Gyps fulvus (Hablizl). J. Nat. Hist. 2002, 36, 1635–1638. [Google Scholar] [CrossRef]

| Bird Species | Bird Order | Migratory | Uninfected | H | P | L | Total |
|---|---|---|---|---|---|---|---|
| Accipiter gentilis | Accipitriformes | R | 0 | 0 | 0 | 1 (0) | 1 (0) |
| Aegypius monachus | Accipitriformes | R | 3 (0) | 0 | 0 | 0 | 3 (0) |
| Alca torda | Charadriiformes | M | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Anas platyrhynchos | Anseriformes | R | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Ardea cinerea | Pelecaniformes | M | 0 | 0 | 0 | 1 (1) | 1 (1) |
| Asio flammeus | Strigiformes | M | 1 (0) | 0 | 0 | 0 | 1 (0) |
| Bubo bubo | Strigiformes | R | 0 | 1 $ (1 $) | 0 | 4 (3) | 4 (3) |
| Bubulcus ibis | Pelecaniformes | R | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Burhinus oedicnemus | Charadriiformes | R | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Buteo buteo | Accipitriformes | R | 1 (0) | 0 | 0 | 1 (1) | 2 (1) |
| Ciconia ciconia | Ciconiiformes | R | 10 (2) | 0 | 0 | 0 | 10 (2) |
| Falco tinnunculus | Falconiformes | R | 2 (0) | 0 | 0 | 0 | 2 (0) |
| Fratercula arctica | Charadriiformes | M | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Gallinula chloropus | Gruiformes | R | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Garrulus glandarius | Passeriformes | R | 0 | 1 $ (0) | 0 | 1 (0) | 1 (0) |
| Gyps fulvus | Accipitriformes | R | 2 (2) | 0 | 0 | 0 | 2 (2) |
| Larus fuscus * | Charadriiformes | M | 6 (4) | 8 (8) | 0 | 0 | 14 (12) |
| Larus michahelis * | Charadriiformes | R | 17 (10) | 5 (4) | 1 (1) | 0 | 23 (15) |
| Larus sp. | Charadriiformes | U | 2 (2) | 0 | 0 | 0 | 2 (2) |
| Milvus migrans | Accipitriformes | M | 1 (0) | 0 | 0 | 0 | 1 (0) |
| Milvus milvus | Accipitriformes | R | 3 (2) | 0 | 0 | 0 | 3 (2) |
| Morus bassanus | Suliformes | M | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Phalacrocorax carbo | Suliformes | M | 1 (1) | 0 | 0 | 0 | 1 (1) |
| Streptopelia decaocto * | Columbiformes | R | 0 | 0 | 0 | 1 (1) | 1 (1) |
| Strix aluco | Strigiformes | R | 1 (0) | 1 $ (1 $) | 0 | 3 (3) | 4 (3) |
| Sturnus unicolor | Passeriformes | R | 1 (0) | 0 | 0 | 0 | 1 (0) |
| Tachybaptus rufficolis | Podicipediformes | R | 1 (0) | 0 | 0 | 0 | 1 (0) |
| Tyto alba | Strigiformes | R | 2 (1) | 0 | 0 | 0 | 2 (1) |
| Upupa epops | Bucerotiformes | R | 2 (2) | 0 | 0 | 0 | 2 (2) |
| Total | 63 (33) | 16 (14) | 1 (1) | 12 (9) | 89 (56) |
| Variable | Type III SS | d.f. | F | p |
|---|---|---|---|---|
| Body condition | 0.017 | 1 | 0.038 | 0.846 |
| Haemosporidian infection | 2.172 | 1 | 4.747 | 0.034 |
| Rehabilitation centre | 0.010 | 1 | 0.021 | 0.884 |
| Season | 6.112 | 1 | 13.358 | 0.001 |
| Variable | B | S. E. | Wald | d.f. | p | Exp (B) |
|---|---|---|---|---|---|---|
| Avian taxonomic order | 0.180 | 0.091 | 0.943 | 1 | 0.047 | 1.197 |
| Reason for admission | −1.256 | 0.524 | 5.741 | 1 | 0.017 | 0.285 |
| Constant | 0.442 | 0.868 | 0.259 | 1 | 0.611 | 1.555 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.T.; de Carvalho, L.M.; Ferreira, M.R.; Nunes, C.; Casero, M.; Marzal, A. Avian Haemosporidian Infection in Wildlife Rehabilitation Centres of Portugal: Causes, Consequences, and Genetic Diversity. Animals 2024, 14, 1216. https://doi.org/10.3390/ani14081216
Cruz JT, de Carvalho LM, Ferreira MR, Nunes C, Casero M, Marzal A. Avian Haemosporidian Infection in Wildlife Rehabilitation Centres of Portugal: Causes, Consequences, and Genetic Diversity. Animals. 2024; 14(8):1216. https://doi.org/10.3390/ani14081216
Chicago/Turabian StyleCruz, João T., Luís Madeira de Carvalho, Mariana Ribeiro Ferreira, Carolina Nunes, María Casero, and Alfonso Marzal. 2024. "Avian Haemosporidian Infection in Wildlife Rehabilitation Centres of Portugal: Causes, Consequences, and Genetic Diversity" Animals 14, no. 8: 1216. https://doi.org/10.3390/ani14081216