Effects of Supplementing Tributyrin on Meat Quality Characteristics of Foreshank Muscle of Weaned Small-Tailed Han Sheep Lambs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Statement
2.2. Lambs and the Experimental Design
2.3. Samples Collection
2.4. Chemical Analysis
2.5. Determinations of Muscle pH, Color, Water-Holding Capacity, and Texture
2.6. Analysis of AAs Content
2.7. Analysis of FAs Composition
2.8. Determination of Nucleotides Content
2.9. Calculation and Statistical Analysis
3. Results
3.1. Effects of TB on Muscle Chemicals, pH, Color, Water-Holding Capacity, and Texture
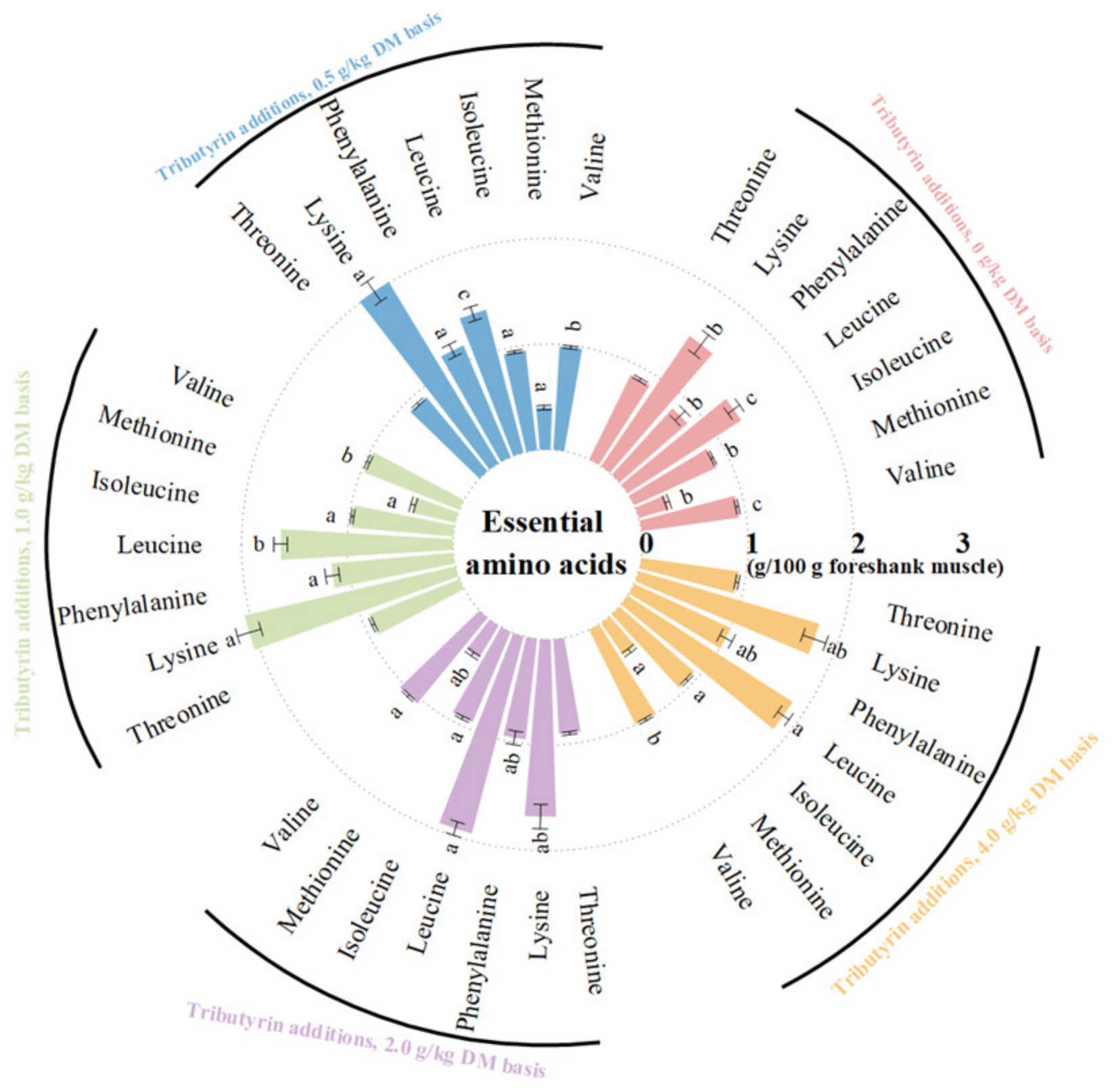
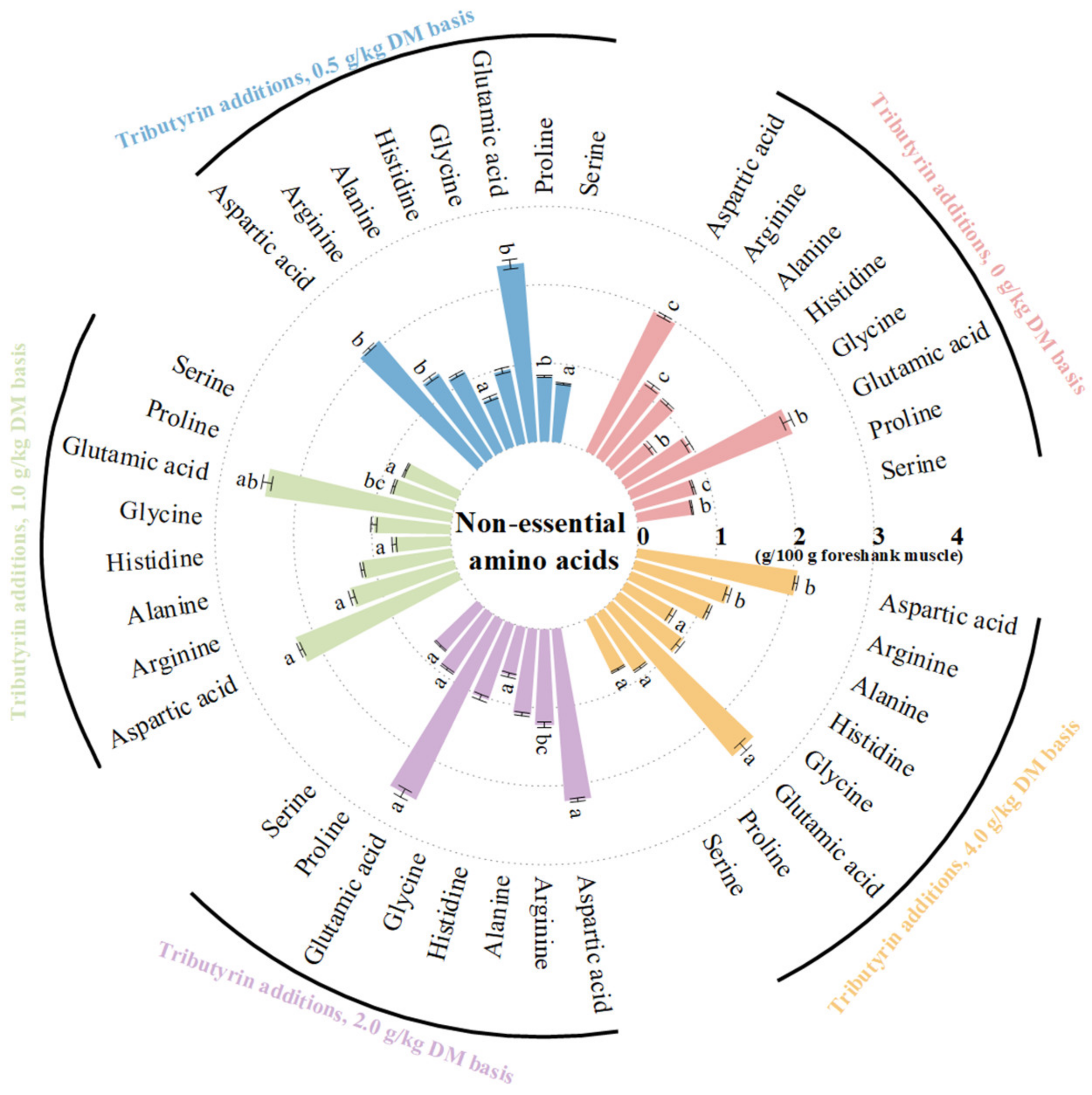
3.2. Effects of TB on AAs Content of Foreshank Muscle
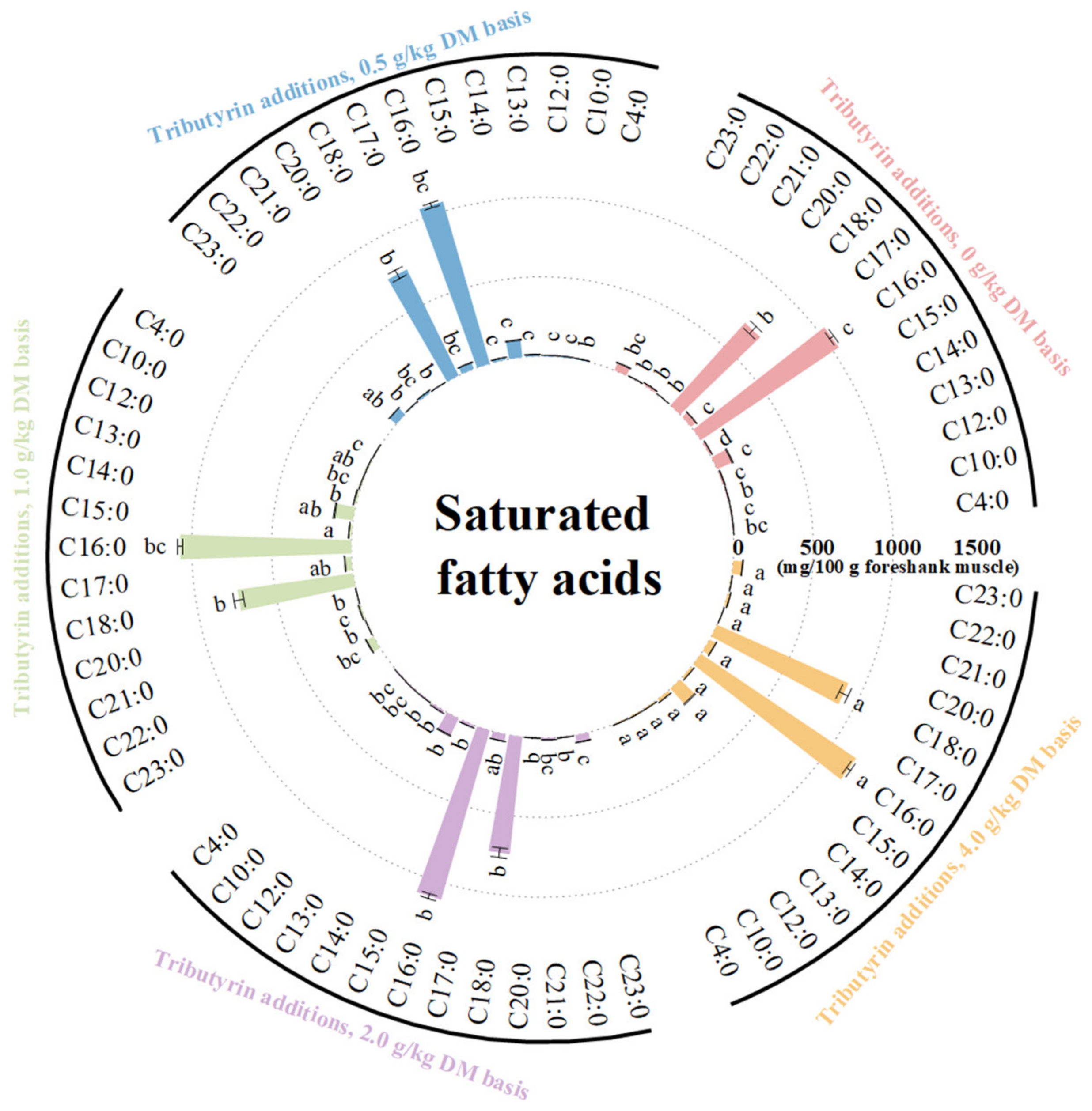
3.3. Effects of TB on FA Content of Foreshank Muscle
3.4. Effects of TB on Nucleotides Content
4. Discussion
4.1. Effects of TB on Intramuscular Fat Accumulation in Foreshank Muscle
4.2. Effects of TB on Nutritional Composition, pH, Color, Water-Holding Capacity, and Texture in Foreshank Muscle
4.3. Effects of TB on AAs and Nucleotides Content in Foreshank Muscle
4.4. Effects of TB on FAs Level in Foreshank Muscle
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahsani, M.R.; Mohammadabadi, M.R.; Shamsaddini, M.B. Clostridium perfringens isolate typing by multiplex PCR. J. Venom. Anim. Toxins Incl. Trop. Dis. 2010, 16, 573–578. [Google Scholar] [CrossRef]
- Mohamadipoor, L.; Mohammadabadi, M.; Amiri, Z.; Babenko, O.; Stavetska, R.; Kalashnik, O.; Kucher, D.; Kochuk-Yashchenko, O.; Asadollahpour, H. Signature selection analysis reveals candidate genes associated with production traits in Iranian sheep breeds. BMC Vet. Res. 2021, 17, 369. [Google Scholar] [CrossRef] [PubMed]
- Jafari Ahmadabadi, S.A.A.; Askari-Hemmat, H.; Mohammadabadi, M.; Asadi, M.; Mansouri, M. The effect of Cannabis seed on DLK1 gene expression in heart tissue of Kermani lambs. Agri. Bio. J. 2023, 15, 217–234. [Google Scholar]
- Li, F.K.; Yang, Y.; Wang, H.; Pan, Z.Y.; Lv, S.J. Behavioral and physiological difference of small-tailed Han sheep at different estrus stage after delivery. J. Vet. Behav. 2023, 60, 44–50. [Google Scholar] [CrossRef]
- Shi, J.P.; Wang, X.Y.; Song, Y.L.; Liu, T.; Cheng, S.R.; Zhang, Q.W. Excavation of genes related to the mining of growth, development, and meat quality of two crossbred sheep populations based on comparative transcriptomes. Animals 2021, 11, 1492. [Google Scholar] [CrossRef] [PubMed]
- Scollan, N.D.; Price, E.M.; Morgan, S.A.; Huws, S.A.; Shingfield, K.J. Can we improve the nutritional quality of meat? Proc. Nutr. Soc. 2017, 76, 603–618. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating meat quality and composition. Proc. Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Geay, Y.; Bauchart, D.; Hocquette, J.F.; Culioli, J. Effect of nutritional factors on biochemical, structural and metabolic characteristics of muscles in ruminants, consequences on dietetic value and sensorial qualities of meat. Reprod. Nutr. Dev. 2001, 41, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wu, J.; Wu, Z.; Alugongo, G.M.; Khan, M.Z.; Li, J.H.; Xiao, J.X.; He, Z.Y.; Ma, Y.L.; Lo, S.L.; et al. Tributyrin administration improves intestinal development and health in pre-weaned dairy calves fed milk replacer. Anim. Nutr. 2022, 10, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.W.; Wang, X.E.; Wang, W.; An, R.; Wang, Y.X.; Ren, Q.C.; Xuan, J.J. Benefits of tributyrin on growth performance, gastrointestinal tract development, ruminal bacteria and volatile fatty acid formation of weaned Small-Tailed Han lambs. Anim. Nutr. 2023, 15, 187–196. [Google Scholar] [CrossRef]
- Ren, Q.C.; Xuan, J.J.; Wang, L.K.; Zhan, Q.W.; Yin, D.Z.; Hu, Z.Z.; Yang, H.J.; Zhang, W.; Jiang, L.S. Effects of tributyrin supplementation on ruminal microbial protein yield, fermentation characteristics and nutrients degradability in adult Small Tail ewes. Anim. Sci. J. 2018, 89, 1271–1279. [Google Scholar] [CrossRef]
- Ren, Q.C.; Xuan, J.J.; Wang, L.K.; Hu, Z.Z.; Yang, H.J.; Zhang, W.; Jiang, L.S. Effects of tributyrin supplementation on in vitro culture fermentation and methanogenesis and in vivo dietary nitrogen, calcium and phosphorus losses in Small Tail ewes. Anim. Feed Sci. Tech. 2018, 243, 64–71. [Google Scholar] [CrossRef]
- Ren, Q.C.; Xuan, J.J.; Hu, Z.Z.; Wang, L.K.; Zhan, Q.W.; Dai, S.F.; Li, S.H.; Yang, H.J.; Zhang, W.; Jiang, L.S. Effects of tributyrin supplementation on short-chain fatty acid concentration, fibrolytic enzyme activity, nutrient digestibility and methanogenesis in adult Small Tail ewes. J. Agri. Sci. Camb. 2018, 156, 465–470. [Google Scholar] [CrossRef]
- Song, Z.H.; Xuan, J.J.; Ren, Q.C. Effects of Dietary tributyrin supplementation on in vitro fermentation characteristics of ruminal microbes. J. Anhui Sci. Technol. Univ. 2020, 34, 6–12. [Google Scholar]
- NY/T1564-2007. Available online: https://www.51zbz.net/biaozhun/139114.html (accessed on 28 March 2024).
- AOAC. Official Methods of Analysis, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- NRC. Nutrient Requirements of Sheep, 6th ed.; National Academies Press: Washington, DC, USA, 1985. [Google Scholar]
- Ren, Q.C.; Xuan, J.J.; Yan, X.C.; Hu, Z.Z.; Wang, F. Effects of dietary supplementation of guanidino acetic acid on growth performance, thigh meat quality and development of small intestine in Partridge-Shank broilers. J. Agri. Sci. Camb. 2019, 156, 1130–1137. [Google Scholar] [CrossRef]
- GB 5009.124-2016. Available online: https://www.chinesestandard.net/Related.aspx/GB5009.124-2016 (accessed on 28 March 2024).
- GB 5009.168-2016. Available online: https://www.chinesestandard.net/PDF.aspx/GB5009.168-2016 (accessed on 28 March 2024).
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef] [PubMed]
- GB 5413.40-2016. Available online: https://www.chinesestandard.net/PDF.aspx/GB5413.40-2016 (accessed on 28 March 2024).
- Xiong, J.; Qiu, H.; Bi, Y.; Zhou, H.L.; Guo, S.; Ding, B. Effects of dietary supplementation with tributyrin and coated sodium butyrate on intestinal morphology, disaccharidase activity and intramuscular fat of lipopolysaccharide-challenged broilers. Braz. J. Poult. Sci. 2018, 20, 707–716. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Prykhodko, O.; Hållenius, F.F.; Nyman, M. Effects of monobutyrin and tributyrin on liver lipid profile, caecal microbiota composition and SCFA in high-fat diet-fed rats. J. Nutr. Sci. 2017, 6, e51. [Google Scholar] [CrossRef] [PubMed]
- Gu, T.T.; Duan, M.C.; Liu, J.Y.; Chen, L.; Tian, Y.; Xu, W.W.; Zeng, T.; Lu, L. Effects of tributyrin supplementation on liver fat deposition, lipid levels and lipid metabolism-related gene expression in broiler chickens. Genes 2022, 13, 2219. [Google Scholar] [CrossRef]
- Hoenderop, J.G.; Nilius, B.; Bindels, R.J. Calcium absorption across epithelia. Physiol. Rev. 2005, 85, 373–422. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Liu, C.; Yang, Z.H.; Su, R.N.; Chen, X.Y.; Hou, Y.R.; Hu, G.H.; Yao, D.; Zhao, L.H.; Su, L.; et al. Effects of oxidative stability variation on lamb meat quality and flavor during postmortem aging. J. Food Sci. 2022, 87, 2578–2594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.P.; Zhang, H.Y.; Bai, S.P.; Zeng, Q.F.; Su, Z.W.; Zhuo, Y.; Mao, X.B.; Yin, H.D.; Feng, B.; Liu, J.B.; et al. Dietary tributyrin improves reproductive performance, antioxidant capacity, and ovary function of broiler breeders. Poult. Sci. 2021, 100, 101429. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.B.; Yin, F.G.; Yang, L.; Li, B.C.; Lei, G.; Wang, C.; Yin, Y.L.; Liu, D. Dietary tributyrin intervention improves the carcass traits, organ indices, and blood biomarker profiles in broilers under the isocaloric diets administration. Poult. Sci. 2022, 101, 102061. [Google Scholar] [CrossRef]
- Yu, J.Y.; Liu, G.S.; Zhang, J.J.; Zhang, C.; Fan, N.Y.; Xu, Y.Q.; Guo, J.J.; Yuan, J.T. Correlation among serum biochemical indices and slaughter traits, texture characteristics and water-holding capacity of Tan sheep. Ital. J. Anim. Sci. 2021, 20, 1781–1790. [Google Scholar] [CrossRef]
- Clark, J.H.; Klusmeyer, T.H.; Cameron, M.R. Microbial protein synthesis and flows of nitrogen fractions to the duodenum of dairy cows. J. Dairy Sci. 1992, 75, 2304–2323. [Google Scholar] [CrossRef] [PubMed]
- Nolte, J.E. Essential Amino Acid Requirements for Growth in Woolled Sheep. Ph.D. Thesis, University of Stellenbosch, Stellenbosch, South Africa, 2006. [Google Scholar]
- Firkins, J.L. Maximizing microbial protein synthesis in the rumen. J. Nutr. 1996, 126 (Suppl. S4), 1347S–1354S. [Google Scholar] [CrossRef] [PubMed]
- Sok, M.; Ouellet, D.R.; Firkins, J.L.; Pellerin, D.; Lapierre, H. Amino acid composition of rumen bacteria and protozoa in cattle. J. Dairy Sci. 2017, 100, 5241–5249. [Google Scholar] [CrossRef] [PubMed]
- FAO. Energy and Protein Requirements; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Geneva, Switzerland; FAO Food and Nutrition: Rome, Italy, 1973; Volume 12.
- Nakatani, Y.; Fujita, T.; Sawa, S.; Otani, T.; Hori, Y.; Takagahara, I. Changes in ATP-related compounds of beef and rabbit muscles and a new index of freshness of muscle. Agric. Biol. Chem. 1986, 50, 1751–1856. [Google Scholar]
- Zhang, L.L.; Hao, Z.L.; Zhao, C.; Zhang, Y.Y.; Li, J.; Sun, B.G.; Tang, Y.Z.; Yao, M.X. Taste compounds, affecting factors, and methods used to evaluate chicken soup: A review. Food Sci. Nutr. 2021, 9, 5833–5853. [Google Scholar] [CrossRef] [PubMed]
- Li, X.J.; Egervari, G.; Wang, Y.G.; Berger, S.L.; Lu, Z.M. Regulation of chromatin and gene expression by metabolic enzymes and metabolites. Nat. Rev. Mol. Cell Bio. 2018, 19, 563–578. [Google Scholar] [CrossRef] [PubMed]
- Van der Knaap, J.A.; Verrijzer, C.P. Undercover: Gene control by metabolites and metabolic enzymes. Genes Dev. 2016, 30, 2345–2369. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Guo, C.Z.; Wang, L.; Chen, F.; Dong, X.W.; Li, X.M.; Ni, K.K.; Yang, F.Y. Effects of paper mulberry silage on the growth performance, rumen microbiota and muscle fatty acid composition in hu lambs. Fermentation 2021, 7, 286. [Google Scholar] [CrossRef]
- Zhang, X.; Han, L.J.; Hou, S.Z.; Raza, S.H.A.; Gui, L.S.; Sun, S.N.; Wang, Z.Y.; Yang, B.C.; Yuan, Z.Z.; Simal-Gandara, J.; et al. Metabolomics approach reveals high energy diet improves the quality and enhances the flavor of black Tibetan sheep meat by altering the composition of rumen microbiota. Front. Nutr. 2022, 9, 915558. [Google Scholar] [CrossRef] [PubMed]
- Potu, R.; AbuGhazaleh, A.; Hastings, D.; Jones, K.; Ibrahim, S. The effect of lipid supplements on ruminal bacteria in continuous culture fermenters varies with the fatty acid composition. J. Microbiol. 2011, 49, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, K.; Hao, X.; Xin, H. The relationships between odd-and branched-chain fatty acids to ruminal fermentation parameters and bacterial populations with different dietary ratios of forage and concentrate. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; Chaudhary, L.C.; McKain, N.; McEwan, N.R.; Richardson, A.J.; Vercoe, P.E.; Walker, N.D.; Paillard, D. Clostridium proteoclasticum: A ruminal bacterium that forms stearic acid from linoleic acid. FEMS Microbiol. Lett. 2006, 265, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Boeckaert, C.; Vlaeminck, B.; Fievez, V.; Maignien, L.; Dijkstra, J.; Boon, N. Accumulation of trans-C18:1 fatty acids in the rumen after dietary algal supplementation is associated with changes in the Butyrivibrio community. Appl. Environ. Microb. 2008, 74, 6923–6930. [Google Scholar] [CrossRef] [PubMed]
- Jeyanathan, J.; Escobar, M.; Wallace, R.J.; Fievez, V.; Vlaeminck, B. Biohydrogenation of 22:6n-3 by Butyrivibrio proteoclasticus p18. BMC Microbiol. 2016, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Z.; Liu, Y.; Lai, Y.J.S.; Barbosa, T.S.; Chandra, R.; Parameswaran, P.; Rittmann, B.E. Electro-selective fermentation enhances lipid extraction and biohydrogenation of Scenedesmus acutus biomass. Algal Res. 2019, 38, 101397. [Google Scholar] [CrossRef]
- Sakurama, H.; Kishino, S.; Mihara, K.; Ando, A.; Kita, K.; Takahashi, S.; Shimizu, S.; Ogawa, J. Biohydrogenation of C20 polyunsaturated fatty acids by anaerobic bacteria. J. Lipid Res. 2014, 55, 1855–1863. [Google Scholar] [CrossRef] [PubMed]


| Items | Content |
|---|---|
| Ingredients | |
| Maize | 250 |
| Soybean meal | 110 |
| Ensiled total corn stover | 350 |
| Peanut straw | 200 |
| Garlic by-products | 50.0 |
| Premix 1 | 40.0 |
| Nutrients | |
| Metabolizable energy 2, MJ/kg | 12.3 |
| Crude protein | 181 |
| Ether extract | 31.0 |
| NDF | 373 |
| ADF | 242 |
| Non-fiber carbohydrate 3 | 341 |
| Ash | 74.0 |
| Ca | 7.0 |
| Total P | 4.0 |
| Items | Tributyrin Additions, g/kg DM Basis | SEM | p-Values 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | Contrast | Linear | Quadratic | ||
| Dry matter (g/100 g) | 25.5 | 26.3 | 26.9 | 26.7 | 25.3 | 0.96 | 0.476 | 0.980 | 0.961 |
| Protein (g/100 g) | 18.8 | 19.5 | 19.7 | 19.6 | 18.1 | 0.99 | 0.703 | 0.665 | 0.845 |
| Ether extract (g/100 g) | 5.94 b | 6.20 ab | 6.67 a | 6.53 ab | 6.61 ab | 0.214 | 0.029 | 0.021 | 0.372 |
| Ash (mg/100 g) | 715 | 625 | 699 | 745 | 676 | 69.8 | 0.292 | 0.510 | 0.779 |
| Calcium (mg/100 g) | 4.98 b | 6.61 ab | 6.63 ab | 8.15 a | 6.62 ab | 0.786 | 0.030 | 0.035 | 0.254 |
| Phosphorus (mg/100 g) | 48.1 b | 59.0 b | 78.1 a | 62.5 b | 58.5 b | 4.98 | 0.007 | 0.137 | 0.041 |
| Intermuscular fat length (μm) | 37.2 b | 42.7 ab | 44.4 ab | 49.1 a | 41.9 ab | 2.69 | 0.022 | 0.045 | 0.342 |
| Intermuscular fat width (μm) | 12.6 b | 15.0 ab | 12.4 b | 17.8 a | 15.1 ab | 1.56 | 0.176 | 0.131 | 0.033 |
| Items | Tributyrin Additions, g/kg DM Basis | SEM | p-Values 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | Contrast | Linear | Quadratic | ||
| pH2 | 6.60 b | 6.84 ab | 7.02 a | 6.72 b | 7.09 a | 0.085 | 0.001 | 0.002 | 0.033 |
| L* (lightness) | 36.4 a | 32.7 b | 32.1 b | 31.1 b | 31.4 b | 1.00 | <0.001 | <0.001 | 0.532 |
| a* (redness) | 14.6 c | 18.1 b | 20.6 a | 17.6 b | 16.9 bc | 0.84 | <0.001 | 0.130 | 0.043 |
| b* (yellowness) | 3.36 | 3.43 | 3.74 | 2.97 | 3.09 | 0.263 | 0.856 | 0.235 | 0.142 |
| Drip loss24h (g/100 g) | 7.91 a | 4.48 b | 6.03 ab | 6.47 ab | 5.47 ab | 0.919 | 0.029 | 0.325 | 0.045 |
| Cooking loss (g/100 g) | 31.6 a | 28.8 ab | 23.1 c | 24.5 c | 25.9 bc | 1.33 | <0.001 | <0.001 | 0.119 |
| Shear force (N) | 25.5 a | 21.1 ab | 19.7 b | 17.6 b | 19.2 b | 1.66 | 0.001 | 0.004 | 0.555 |
| Items | Tributyrin Additions, g/kg DM Basis | SEM | p-Values 1 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 2.0 | 4.0 | Contrast | Linear | Quadratic | ||
| Hardness (g) | 228 a | 215 b | 193 c | 183 d | 185 cd | 3.3 | <0.001 | <0.001 | 0.527 |
| Cohesiveness | 0.59 a | 0.57 a | 0.53 b | 0.50 bc | 0.49 c | 0.010 | <0.001 | <0.001 | 0.741 |
| Springiness | 0.30 a | 0.26 b | 0.24 b | 0.20 c | 0.21 c | 0.009 | <0.001 | <0.001 | 0.164 |
| Gumminess (g) | 34.2 a | 31.2 b | 25.8 c | 23.0 d | 23.1 d | 0.78 | <0.001 | <0.001 | 0.495 |
| Chewiness (g) | 42.0 a | 33.7 b | 25.2 c | 18.7 d | 20.0 d | 1.65 | <0.001 | <0.001 | 0.762 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.-E.; Li, Z.-W.; Liu, L.-L.; Ren, Q.-C. Effects of Supplementing Tributyrin on Meat Quality Characteristics of Foreshank Muscle of Weaned Small-Tailed Han Sheep Lambs. Animals 2024, 14, 1235. https://doi.org/10.3390/ani14081235
Wang X-E, Li Z-W, Liu L-L, Ren Q-C. Effects of Supplementing Tributyrin on Meat Quality Characteristics of Foreshank Muscle of Weaned Small-Tailed Han Sheep Lambs. Animals. 2024; 14(8):1235. https://doi.org/10.3390/ani14081235
Chicago/Turabian StyleWang, Xue-Er, Zhi-Wei Li, Li-Lin Liu, and Qing-Chang Ren. 2024. "Effects of Supplementing Tributyrin on Meat Quality Characteristics of Foreshank Muscle of Weaned Small-Tailed Han Sheep Lambs" Animals 14, no. 8: 1235. https://doi.org/10.3390/ani14081235





