Spectral Profiling (Fourier Transform Infrared Spectroscopy) and Machine Learning for the Recognition of Milk from Different Bovine Breeds
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples Collection
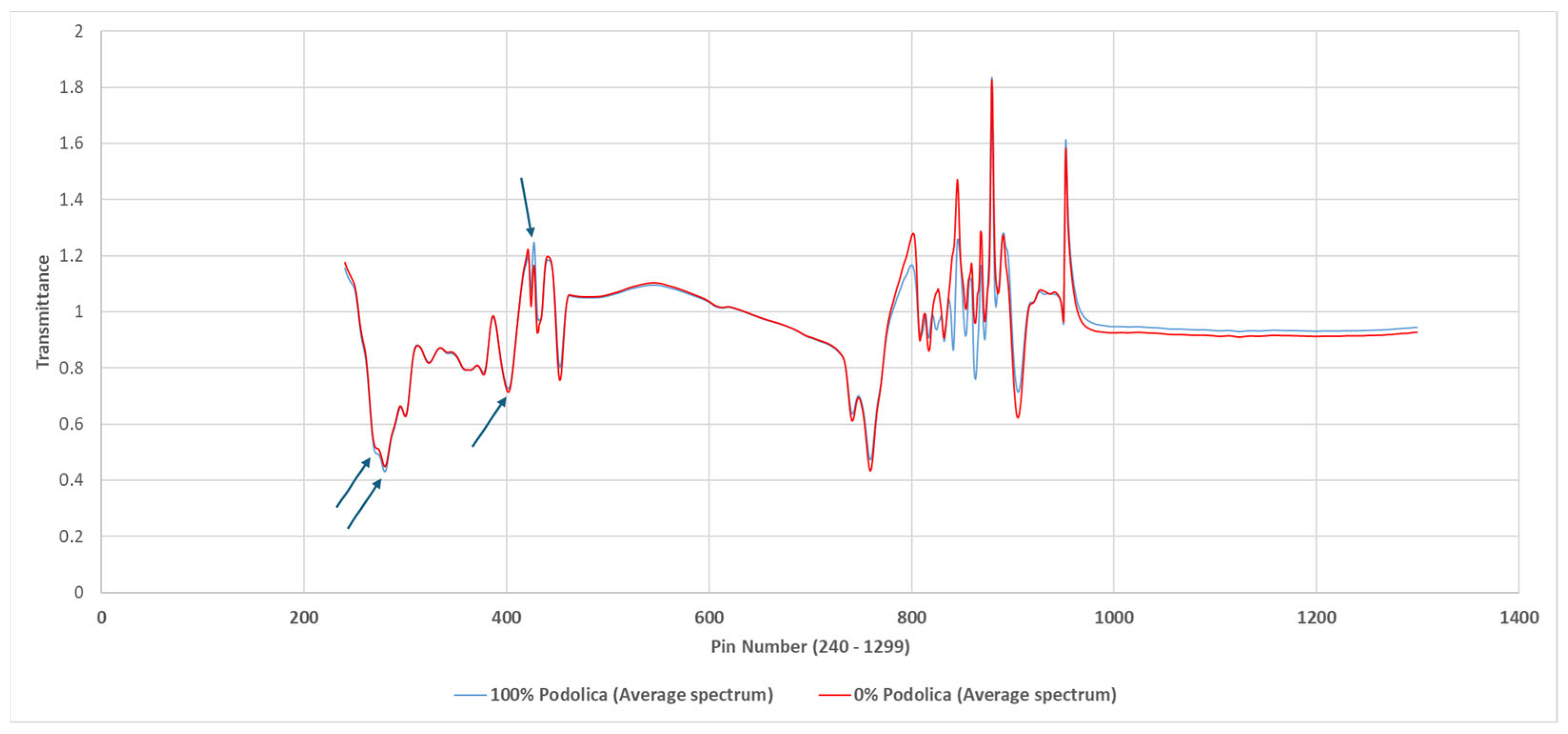
2.2. FTIR Analysis
2.3. Statistical Analysis
3. Results
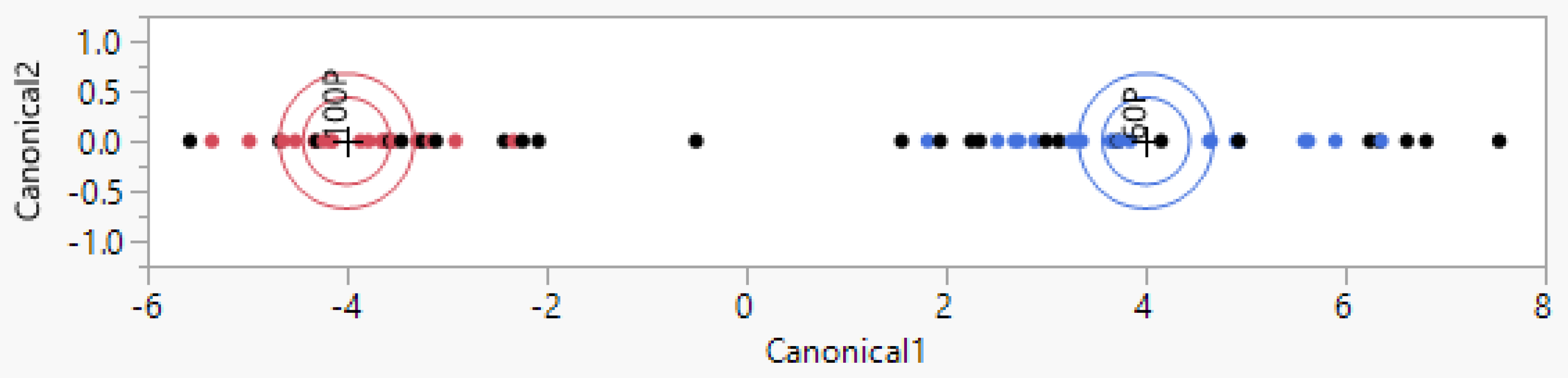
3.1. Pure Podolica Milk Recognition (Different Farms)
3.2. Pure Podolica Milk Recognition (Same Farm)
3.3. Recognition of Podolica Milk Contaminated with the Milk of Other Breeds (Different Farms)
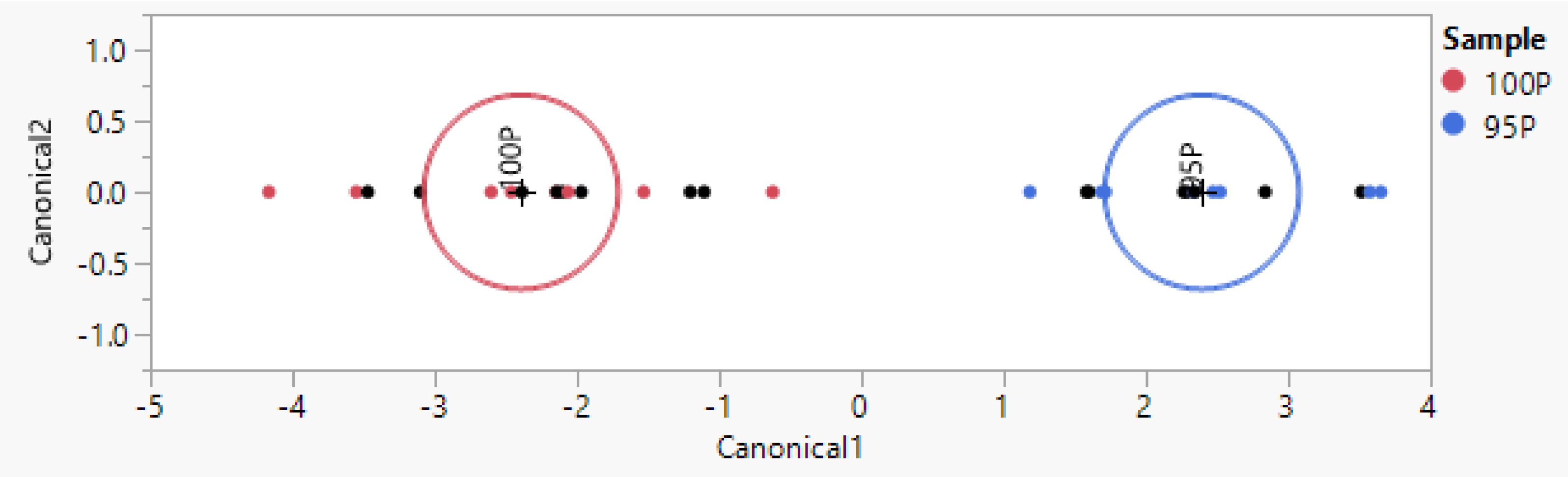
3.4. Recognition of Podolica Milk Contaminated with Milk from Other Breeds (Same Farm)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Costagliola, A.; Wojcik, S.; Pagano, T.B.; De Biase, D.; Russo, V.; Iovane, V.; Grieco, E.; Papparella, S.; Paciello, O. Age-related changes in skeletal muscle of cattle. Vet. Pathol. 2016, 53, 436–446. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, P.; Lancioni, H.; Ceccobelli, S.; Colli, L.; Cardinali, I.; Karsli, T.; Capodiferro, M.R.; Sahin, E.; Ferretti, L.; Ajmone Marsan, P. Mitochondrial DNA variants of Podolian cattle breeds testify for a dual maternal origin. PLoS ONE 2018, 13, e0192567. [Google Scholar] [CrossRef] [PubMed]
- Moioli, B.; Napolitano, F.; Catillo, G. Genetic diversity between Piedmontese, Maremmana, and Podolica cattle breeds. J. Hered. 2004, 95, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Spina, A.A.; Ceniti, C.; Piras, C.; Tilocca, B.; Britti, D.; Morittu, V.M. Mid-Infrared (MIR) Spectroscopy for the quantitative detection of cow’s milk in buffalo milk. J. Anim. Sci. Technol. 2022, 64, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Arifah, M.F.; Nisa, K.; Windarsih, A.; Rohman, A. The Application of FTIR Spectroscopy and Chemometrics for the Authentication Analysis of Horse Milk. Int. J. Food Sci. 2022, 2022, 7643959. [Google Scholar] [CrossRef] [PubMed]
- Balan, B.; Dhaulaniya, A.S.; Jamwal, R.; Yadav, A.; Kelly, S.; Cannavan, A.; Singh, D.K. Rapid detection and quantification of sucrose adulteration in cow milk using Attenuated total reflectance-Fourier transform infrared spectroscopy coupled with multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118628. [Google Scholar] [CrossRef] [PubMed]
- Coitinho, T.B.; Cassoli, L.D.; Cerqueira, P.H.R.; da Silva, H.K.; Coitinho, J.B.; Machado, P.F. Adulteration identification in raw milk using Fourier transform infrared spectroscopy. J. Food Sci. Technol. 2017, 54, 2394–2402. [Google Scholar] [CrossRef]
- Eid, S.M.; El-Shamy, S.; Farag, M.A. Identification of milk quality and adulteration by surface-enhanced infrared absorption spectroscopy coupled to artificial neural networks using citrate-capped silver nanoislands. Microchim. Acta 2022, 189, 301. [Google Scholar] [CrossRef]
- Hosseini, E.; Ghasemi, J.B.; Daraei, B.; Asadi, G.; Adib, N. Application of genetic algorithm and multivariate methods for the detection and measurement of milk-surfactant adulteration by attenuated total reflection and near-infrared spectroscopy. J. Sci. Food Agric. 2021, 101, 2696–2703. [Google Scholar] [CrossRef]
- Neto, H.A.; Tavares, W.L.F.; Ribeiro, D.C.S.Z.; Alves, R.C.O.; Fonseca, L.M.; Campos, S.V.A. On the utilization of deep and ensemble learning to detect milk adulteration. BioData Min. 2019, 12, 13. [Google Scholar] [CrossRef]
- Spieß, L.; de Peinder, P.; van den Bijgaart, H. Advances in Atypical FT-IR Milk Screening: Combining Untargeted Spectra Screening and Cluster Algorithms. Foods 2021, 10, 1111. [Google Scholar] [CrossRef] [PubMed]
- Tarapoulouzi, M.; Kokkinofta, R.; Theocharis, C.R. Chemometric analysis combined with FTIR spectroscopy of milk and Halloumi cheese samples according to species’ origin. Food Sci. Nutr. 2020, 8, 3262–3273. [Google Scholar] [CrossRef] [PubMed]
- Tiplady, K.M.; Lopdell, T.J.; Littlejohn, M.D.; Garrick, D.J. The evolving role of Fourier-transform mid-infrared spectroscopy in genetic improvement of dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 39. [Google Scholar] [CrossRef] [PubMed]
- Bahadi, M.; Ismail, A.A.; Vasseur, E. Fourier Transform Infrared Spectroscopy as a Tool to Study Milk Composition Changes in Dairy Cows Attributed to Housing Modifications to Improve Animal Welfare. Foods 2021, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Dadousis, C.; Cipolat-Gotet, C.; Stocco, G.; Ferragina, A.; Dettori, M.L.; Pazzola, M.; do Nascimento Rangel, A.H.; Vacca, G.M. Goat farm variability affects milk Fourier-transform infrared spectra used for predicting coagulation properties. J. Dairy Sci. 2021, 104, 3927–3935. [Google Scholar] [CrossRef]
- Cirak, O.; Icyer, N.C.; Durak, M.Z. Rapid detection of adulteration of milks from different species using Fourier Transform Infrared Spectroscopy (FTIR). J. Dairy Res. 2018, 85, 222–225. [Google Scholar] [CrossRef]
- Jaiswal, P.; Jha, S.N.; Borah, A.; Gautam, A.; Grewal, M.K.; Jindal, G. Detection and quantification of soymilk in cow-buffalo milk using Attenuated Total Reflectance Fourier Transform Infrared spectroscopy (ATR-FTIR). Food Chem. 2015, 168, 41–47. [Google Scholar] [CrossRef]
- Amalfitano, N.; Stocco, G.; Maurmayr, A.; Pegolo, S.; Cecchinato, A.; Bittante, G. Quantitative and qualitative detailed milk protein profiles of 6 cattle breeds: Sources of variation and contribution of protein genetic variants. J. Dairy Sci. 2020, 103, 11190–11208. [Google Scholar] [CrossRef]
- Hope, A.F. Laboratory Handbook on Bovine Mastitis. Aust. Vet. J. 2000, 78, 488. [Google Scholar] [CrossRef]
- Piras, C.; Hale, O.; Reynolds, C.K.; Jones, B.; Taylor, N.; Morris, M.; Cramer, R. LAP-MALDI MS coupled with machine learning: An ambient mass spectrometry approach for high-throughput diagnostics. Chem. Sci. 2022, 13, 1746–1758. [Google Scholar] [CrossRef]
- Quinto, M.; Sevi, A.; Di Caterina, R.; Albenzio, M.; Muscio, A.; Rotunno, T. Quality of milk and Caciocavallo cheese from farms rearing Podolica and Italian Friesian cows. Ital. J. Food Sci. 2003, 15, 485–498. [Google Scholar]
- Nicolaou, N.; Xu, Y.; Goodacre, R. Fourier transform infrared spectroscopy and multivariate analysis for the detection and quantification of different milk species. J. Dairy Sci. 2010, 93, 5651–5660. [Google Scholar] [CrossRef] [PubMed]
- Rachah, A.; Reksen, O.; Tafintseva, V.; Stehr, F.J.M.; Rukke, E.-O.; Prestløkken, E.; Martin, A.; Kohler, A.; Afseth, N.K. Exploring dry-film ftir spectroscopy to characterize milk composition and subclinical ketosis throughout a cow’s lactation. Foods 2021, 10, 2033. [Google Scholar] [CrossRef] [PubMed]
- Salleh, N.A.; Selamat, J.; Meng, G.Y.; Abas, F.; Jambari, N.N.; Khatib, A. Fourier transform infrared spectroscopy and multivariate analysis of milk from different goat breeds. Int. J. Food Prop. 2019, 22, 1673–1683. [Google Scholar] [CrossRef]
- Soyeurt, H.; Misztal, I.; Gengler, N. Genetic variability of milk components based on mid-infrared spectral data. J. Dairy Sci. 2010, 93, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hulzebosch, A.; Bovenhuis, H. Genetic and environmental variation in bovine milk infrared spectra. J. Dairy Sci. 2016, 99, 6793–6803. [Google Scholar] [CrossRef] [PubMed]
- Zaalberg, R.M.; Janss, L.; Buitenhuis, A.J. Genome-wide association study on Fourier transform infrared milk spectra for two Danish dairy cattle breeds. BMC Genet. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Bovenhuis, H. Genome-wide association study for milk infrared wavenumbers. J. Dairy Sci. 2018, 101, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- Knutsen, T.M.; Olsen, H.G.; Ketto, I.A.; Sundsaasen, K.K.; Kohler, A.; Tafintseva, V.; Svendsen, M.; Kent, M.P.; Lien, S. Genetic variants associated with two major bovine milk fatty acids offer opportunities to breed for altered milk fat composition. Genet. Sel. Evol. 2022, 54, 35. [Google Scholar] [CrossRef]
- Bevin, C.J.; Dambergs, R.G.; Fergusson, A.J.; Cozzolino, D. Varietal discrimination of Australian wines by means of mid-infrared spectroscopy and multivariate analysis. Anal. Chim. Acta 2008, 621, 19–23. [Google Scholar] [CrossRef]
- Pinto, P.A.; Anconi, A.C.S.A.; de Abreu, L.R.; Magalhães, E.J.; Nunes, C.A. Strategies to determine lactose in cow milk by mid infrared spectroscopy. J. Food Compos. Anal. 2021, 104, 104176. [Google Scholar] [CrossRef]
- Anjos, V. Near and Mid Infrared Spectroscopy to Assess Milk Products Quality: A Review of Recent Applications. J. Dairy Res. Technol. 2020, 3, 014. [Google Scholar] [CrossRef] [PubMed]


| Podolica | Frisona | Pezzata Rossa | Bruna Alpina | |
|---|---|---|---|---|
| Fat (g/100 g) | 2.92 ± 0.34 | 3.64 ± 0.39 | 3.83 ± 0.25 | 4.04 ± 0.24 |
| Protein (g/100 g) | 3.34 ± 0.21 | 3.07 ± 0.14 | 3.29 ± 0.05 | 3.35 ± 0.25 |
| Lactose (g/100 g) | 4.93 ± 0.10 | 4.75 ± 0.11 | 4.92 ± 0.19 | 4.73 ± 0.14 |
| Casein (g/100 g) | 2.71 ± 0.09 | 2.54 ± 0.26 | 2.68 ± 0.13 | 2.64 ± 0.17 |
| Test Set | |||||
|---|---|---|---|---|---|
| Actual | Predicted Count | Actual | Predicted Count | ||
| Class | 0% Podolica | 100% Podolica | Class | 0% Podolica | 100% Podolica |
| 0% Podolica | 19 | 0 | 0% Podolica | 13 | 2 |
| 100% Podolica | 0 | 19 | 100% Podolica | 0 | 15 |
| Sensitivity for 0% Podolica | 100% | Sensitivity for 0% Podolica | 86.7% | ||
| Specificity for 0% Podolica | 100% | Specificity for 0% Podolica | 100% | ||
| Test Set | |||||
|---|---|---|---|---|---|
| Actual | Predicted Count | Actual | Predicted Count | ||
| Class | 0% Podolica | 100% Podolica | Class | 0% Podolica | 100% Podolica |
| 0% Podolica | 8 | 0 | 0% Podolica | 8 | 0 |
| 100% Podolica | 0 | 8 | 100% Podolica | 0 | 8 |
| Sensitivity for 0% Podolica | 100% | Sensitivity for 0% Podolica | 100% | ||
| Specificity for 0% Podolica | 100% | Specificity for 0% Podolica | 100% | ||
| Test Set | |||||
|---|---|---|---|---|---|
| Actual | Predicted Count | Actual | Predicted Count | ||
| Class | 100% Podolica | 60% Podolica | Class | 100% Podolica | 60% Podolica |
| 100% Podolica | 20 | 0 | 100% Podolica | 14 | 0 |
| 60% Podolica | 0 | 20 | 60% Podolica | 0 | 14 |
| Sensitivity for 60% Podolica | 100% | Sensitivity for 60% Podolica | 100% | ||
| Specificity for 60% Podolica | 100% | Specificity for 60% Podolica | 100% | ||
| Test Set | |||||
|---|---|---|---|---|---|
| Actual | Predicted Count | Actual | Predicted Count | ||
| Class | 100% Podolica | 95% Podolica | Class | 100% Podolica | 95% Podolica |
| 100% Podolica | 8 | 0 | 100% Podolica | 8 | 0 |
| 95% Podolica | 0 | 8 | 95% Podolica | 0 | 8 |
| Sensitivity for 95% Podolica | 100% | Sensitivity for 95% Podolica | 100% | ||
| Specificity for 95% Podolica | 100% | Specificity for 95% Podolica | 100% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spina, A.A.; Ceniti, C.; De Fazio, R.; Oppedisano, F.; Palma, E.; Gugliandolo, E.; Crupi, R.; Raza, S.H.A.; Britti, D.; Piras, C.; et al. Spectral Profiling (Fourier Transform Infrared Spectroscopy) and Machine Learning for the Recognition of Milk from Different Bovine Breeds. Animals 2024, 14, 1271. https://doi.org/10.3390/ani14091271
Spina AA, Ceniti C, De Fazio R, Oppedisano F, Palma E, Gugliandolo E, Crupi R, Raza SHA, Britti D, Piras C, et al. Spectral Profiling (Fourier Transform Infrared Spectroscopy) and Machine Learning for the Recognition of Milk from Different Bovine Breeds. Animals. 2024; 14(9):1271. https://doi.org/10.3390/ani14091271
Chicago/Turabian StyleSpina, Anna Antonella, Carlotta Ceniti, Rosario De Fazio, Francesca Oppedisano, Ernesto Palma, Enrico Gugliandolo, Rosalia Crupi, Sayed Haidar Abbas Raza, Domenico Britti, Cristian Piras, and et al. 2024. "Spectral Profiling (Fourier Transform Infrared Spectroscopy) and Machine Learning for the Recognition of Milk from Different Bovine Breeds" Animals 14, no. 9: 1271. https://doi.org/10.3390/ani14091271






