Hellenic Natural Zeolite as a Replacement of Sand in Mortar: Mineralogy Monitoring and Evaluation of Its Influence on Mechanical Properties
Abstract
:1. Introduction
2. Materials and Methods
| Mixture | Cement | Sand | Zeolite |
|---|---|---|---|
| REF1 | 26.7 | 73.3 | 0.0 |
| M1 | 26.7 | 55.0 | 18.3 |
| M2 | 26.7 | 36.7 | 36.7 |
| M3 | 26.7 | 18.3 | 55.0 |
| REF2 | 26.7 | 0.0 | 73.3 |
3. Results and Discussion
3.1. Chemical and Mineralogical Composition
| Starting material | SiO2 | TiO2 | Al2O3 | Fe2O3T | MnO | MgO | CaO | Na2O | K2O | P2O5 | SO3 | L.O.I. | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 27.79 | 0.33 | 8.36 | 3.70 | 0.06 | 1.94 | 49.36 | 0.80 | 1.56 | 0.14 | 1.32 | 4.57 | 99.93 |
| Sand | 74.37 | 0.35 | 10.26 | 2.36 | 0.06 | 1.13 | 3.18 | 2.74 | 2.29 | 0.09 | - | 2.48 | 99.31 |
| HNZ | 66.49 | 0.20 | 11.78 | 1.30 | 0.04 | 0.98 | 2.96 | 0.80 | 1.94 | 0.03 | - | 12.81 | 99.33 |
| Starting material | C2S | C3S | C4AF | C3A | Q | F | C | A | An | H | M | Cl | Am |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cement | 43 | 27 | 7 | 5 | 4 | - | - | - | 4 | - | 3 | - | 7 |
| Sand | - | - | - | - | 48 | 41 | 2 | 2 | - | - | 5 | 2 | - |
| HNZ | - | - | - | - | 2 | 6 | - | - | - | 89 | 1 | 2 | - |
| Sample | Days | C2S | C3S | C4AF | C3A | F | TC | HEU | Am | Ch | Q | Po | C | A |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| REF1 | 3 | +++ | bdl | bdl | bdl | +++ | + | na | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | +++ | + | na | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | +++ | + | na | + | + | +++ | ++ | + | ++ | |
| 90 | bdl | bdl | bdl | bdl | +++ | + | na | + | + | +++ | bdl | + | ++ | |
| M1 | 3 | +++ | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | ++ | + | ++ | |
| 90 | bdl | bdl | bdl | bdl | +++ | + | ++ | + | + | +++ | + | + | ++ | |
| M2 | 3 | +++ | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | +++ | + | ++ |
| 7 | ++ | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | +++ | + | ++ | |
| 28 | + | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | ++ | + | ++ | |
| 90 | + | bdl | bdl | bdl | ++ | + | ++ | + | + | +++ | + | + | ++ | |
| M3 | 3 | +++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | +++ | + | ++ |
| 7 | +++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | ++ | + | ++ | |
| 28 | ++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | ++ | + | ++ | |
| 90 | ++ | bdl | bdl | bdl | ++ | + | +++ | + | + | +++ | + | + | ++ | |
| REF2 | 3 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | ++ | + | ++ |
| 7 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | + | + | ++ | |
| 28 | +++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | + | + | ++ | |
| 90 | ++ | bdl | bdl | bdl | ++ | + | +++ | nd | nd | ++ | bdl | + | ++ |
3.2. Physico-Mechanical Characteristics
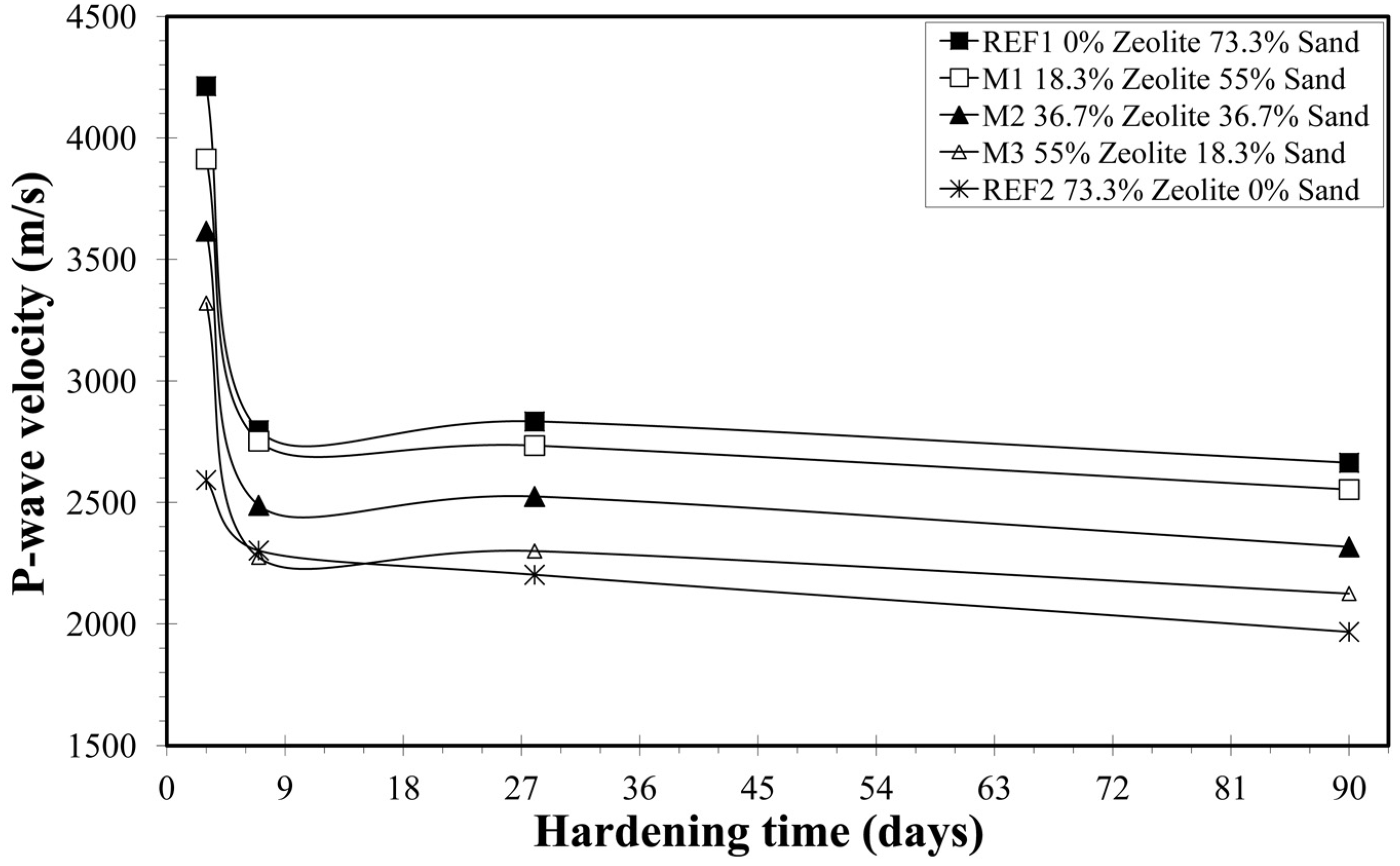
3.2.1. Primary Wave Velocity
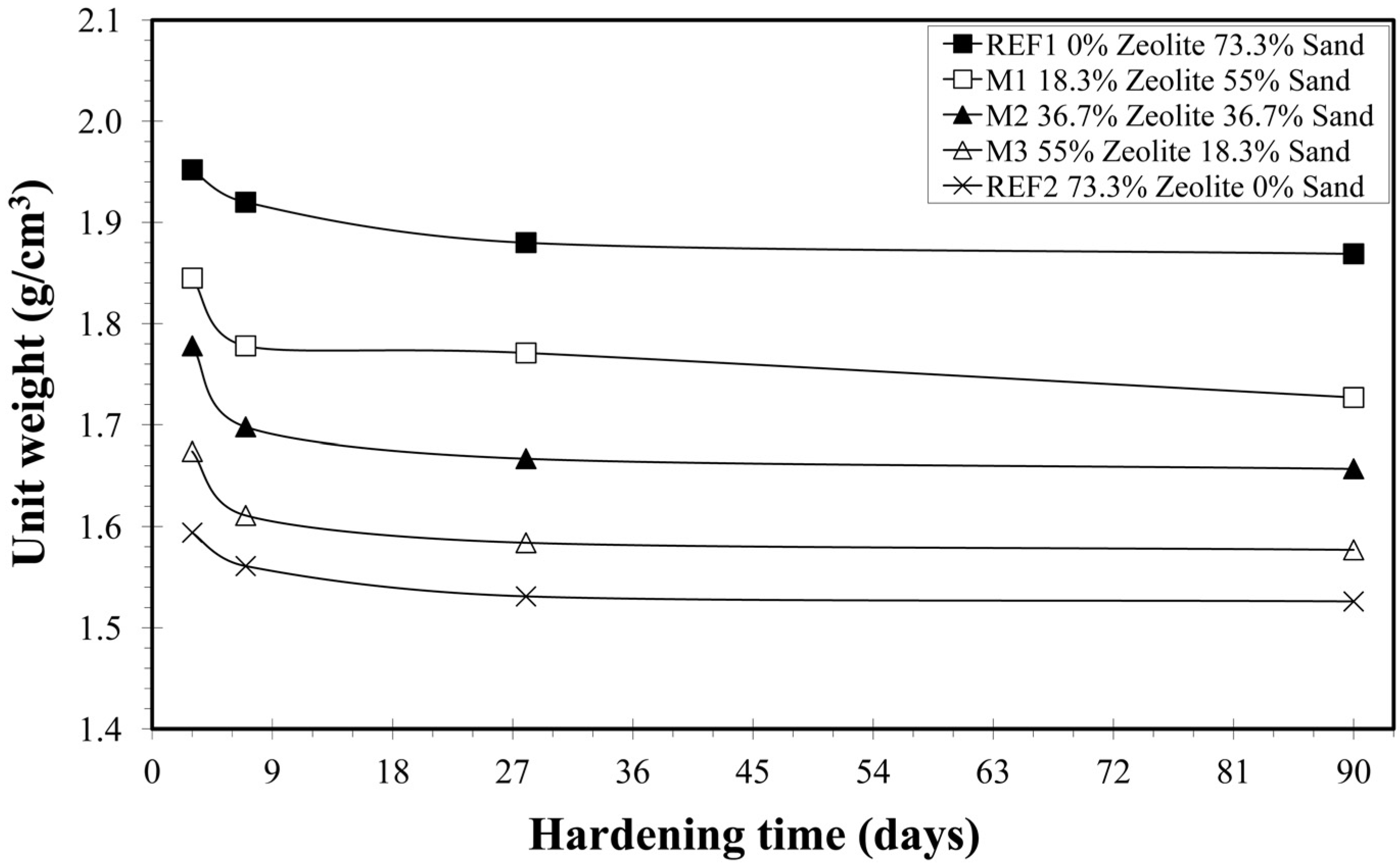
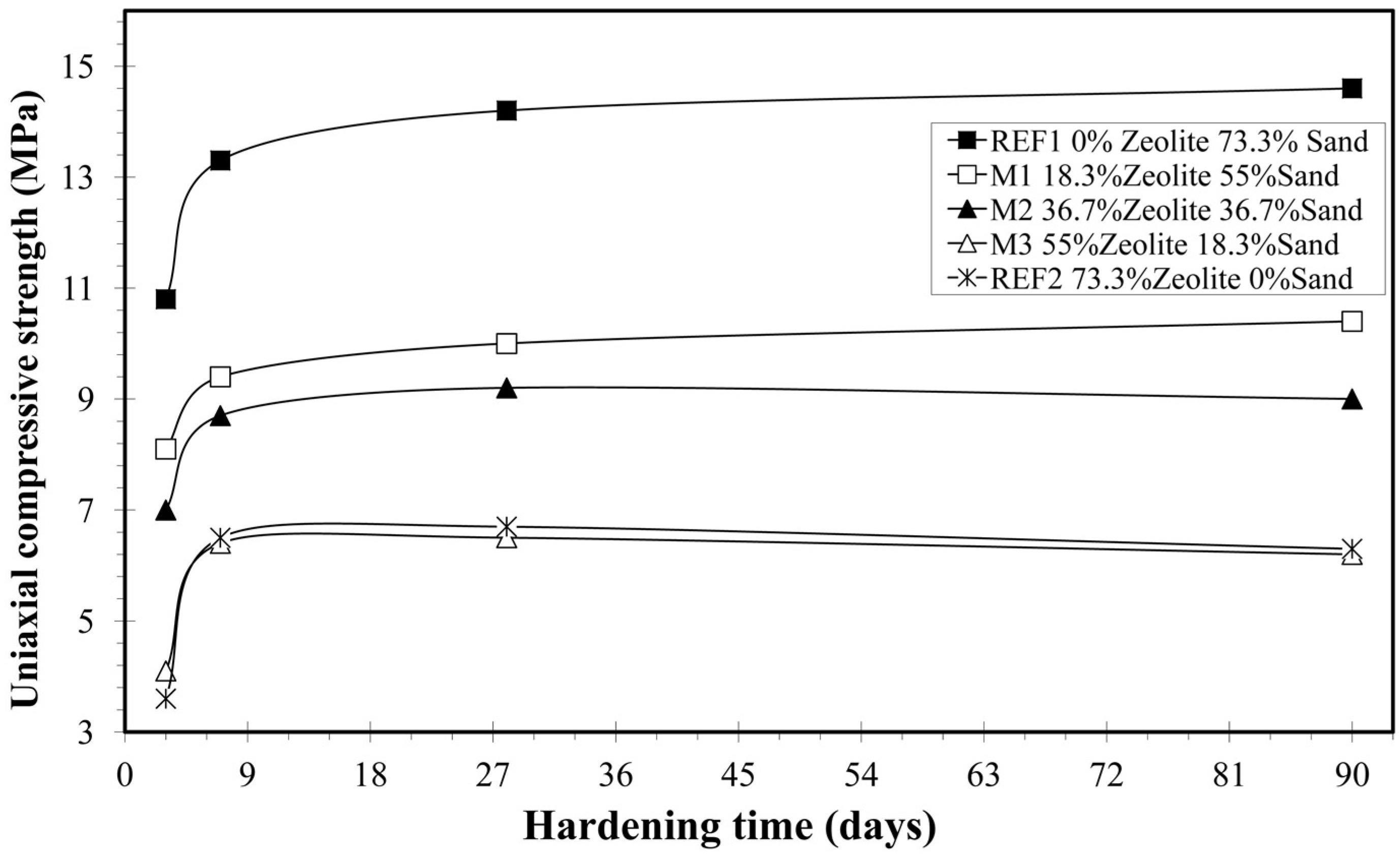
| Mixture | Days | Vp (m/s) | S.D. | Unit Weight (g/cm3) | S.D. | U.C.S. (MPa) | S.D. |
|---|---|---|---|---|---|---|---|
| REF1 | 3 | 4213 | 242 | 1.952 | 0.014 | 10.8 | 0.6 |
| 7 | 2797 | 69 | 1.920 | 0.035 | 13.3 | 1.7 | |
| 28 | 2833 | 66 | 1.880 | 0.019 | 14.2 | 1.0 | |
| 90 | 2663 | 62 | 1.869 | 0.015 | 14.6 | 0.6 | |
| Μ1 | 3 | 3913 | 258 | 1.845 | 0.018 | 8.1 | 0.4 |
| 7 | 2752 | 95 | 1.778 | 0.022 | 9.4 | 1.2 | |
| 28 | 2734 | 109 | 1.771 | 0.016 | 10.0 | 0.2 | |
| 90 | 2553 | 48 | 1.727 | 0.027 | 10.4 | 1.0 | |
| Μ2 | 3 | 3616 | 180 | 1.778 | 0.035 | 7.0 | 0.2 |
| 7 | 2488 | 45 | 1.698 | 0.016 | 8.7 | 0.1 | |
| 28 | 2524 | 137 | 1.667 | 0.007 | 9.2 | 0.7 | |
| 90 | 2317 | 130 | 1.657 | 0.011 | 9.0 | 0.9 | |
| Μ3 | 3 | 3320 | 176 | 1.674 | 0.008 | 4.1 | 0.5 |
| 7 | 2274 | 48 | 1.611 | 0.020 | 6.4 | 0.3 | |
| 28 | 2300 | 55 | 1.584 | 0.032 | 6.5 | 0.6 | |
| 90 | 2125 | 68 | 1.577 | 0.013 | 6.2 | 0.2 | |
| REF2 | 3 | 2589 | 114 | 1.594 | 0.008 | 3.6 | 0.8 |
| 7 | 2307 | 91 | 1.561 | 0.030 | 6.5 | 0.5 | |
| 28 | 2201 | 86 | 1.531 | 0.013 | 6.6 | 0.5 | |
| 90 | 1965 | 76 | 1.526 | 0.010 | 6.3 | 0.2 |
3.2.2. Unit Weight
3.2.3. Uniaxial Compressive Strength
4. Conclusions
Acknowledgments
References
- Lightweight Aggregates—Part 1: Lightweight Aggregates for Concrete, Mortar and Grout; SS-EN 13055-1; European Standards (EN): Pilsen, Czech Republic, 2002.
- Canpolat, F.; Yilmaz, K.; Kose, M.M.; Sumer, M.; Yurdusev, M.A. Use of zeolite, coal bottom ash and fly ash as replacement materials in cement production. Cem. Concr. Res. 2004, 34, 731–736. [Google Scholar] [CrossRef]
- De’ Gennaro, R.; Cappelletti, P.; Cerri, G.; de’ Gennaro, M.; Dondi, M.; Langella, A. Zeolitic tuffs as raw materials for lightweight aggregates. Appl. Clay Sci. 2004, 25, 71–81. [Google Scholar] [CrossRef] [Green Version]
- De’ Gennaro, R.; Langella, A.; D’Amore, M.; Dondi, M.; Colella, A.; Cappelletti, P.; de’ Gennaro, M. Use of zeolite-rich rocks and waste materials for the production of structural lightweight concretes. Appl. Clay Sci. 2008, 41, 61–72. [Google Scholar] [CrossRef]
- Feng, N.Q.; Peng, G.F. Applications of natural zeolite to construction and building materials in China. Constr. Build. Mater. 2005, 19, 579–584. [Google Scholar] [CrossRef]
- Filippidis, A.; Kantiranis, N. Experimental neutralization of lake and stream waters from N. Greece using domestic HEU-type rich natural zeolitic material. Desalination 2007, 213, 47–55. [Google Scholar] [CrossRef]
- Park, S.-K.; Kim, J.J.-H.; Nam, J.-W.; Phan, H.D.; Kim, J.-K. Development of anti-fungal mortar and concrete using zeolite and zeocarbon microcapsules. Cem. Concr. Compos. 2009, 31, 447–453. [Google Scholar] [CrossRef]
- Perraki, T.; Kontori, E.; Tsivilis, S.; Kakali, G. The effect of zeolite on the properties and hydration of blended cements. Cem. Concr. Compos. 2010, 32, 128–133. [Google Scholar] [CrossRef]
- Stamatakis, M.G.; Hall, A.; Hein, J.R. The zeolite deposits of Greece. Miner. Deposita. 1996, 31, 473–481. [Google Scholar] [CrossRef]
- Filippidis, A. Environmental industrial and agricultural applications of Hellenic natural zeolite. Hell. J. Geosci. 2010, 45, 91–100. [Google Scholar]
- Kantiranis, N.; Chrissafis, C.; Filippidis, A.; Paraskevopoulos, K.M. Thermal distinction of HEU-type mineral phases contained in Greek zeolite-rich volcaniclastic tuffs. Eur. J. Mineral. 2006, 18, 509–516. [Google Scholar] [CrossRef]
- Shi, C.; Day, R. Pozzolanic reaction in the presence of chemical activators: Part II—Reaction products and mechanism. Cem. Concr. Res. 2000, 30, 607–613. [Google Scholar] [CrossRef]
- Chan, S.; Ji, X. Comparative study of the initial surface absorption and chloride diffusion of high performance zeolite, silica fume and PFA concretes. Cem. Concr. Compos. 1999, 21, 293–300. [Google Scholar] [CrossRef]
- ASTM C109/C109Μ-02 Standard Test Method for Compressive Strength of Hydraulic Cement Mortars Using 2-in. or [50-mm] Cube Specimens. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002; Volume 04.01.
- ASTM C29/C29M-09 Standard Test Method for Bulk Density (“Unit Weight”) and Voids in Aggregate. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2009; Volume 04.02.
- NF B10-505-1973 Quarry Products. Limestones. Measurement of the Speed of Sound Propagation (Longitudinal Waves), AFNOR Group: Paris, France, 1973.
- Ramachandran, V.S.; Beaudoin, J.J. Concrete science. In Handbook of Analytical Techniques in Concrete Science and Technology: Principles, Techniques and Applications; William Andrew Publishing: Norwich, NY, USA, 2001; pp. 1–55. [Google Scholar]
- Olson, R.A.; Jennings, H.M. Estimation of C-S-H content in a blended cement paste using water adsorption. Cem. Concr. Res. 2001, 31, 351–356. [Google Scholar] [CrossRef]
- Matusinovic, T.; Kurajica, S.; Sipusic, J. The correlation between compressive strength and ultrasonic parameters of calcium aluminate cement materials. Cem. Concr. Res. 2004, 34, 1451–1457. [Google Scholar] [CrossRef]
- Hernandez, M.G.; Anaya, J.J.; Sanchez, T.; Segura, I. Porosity estimation of aged mortar using a micromechanical model. Ultrasonics 2006, 44, 1007–1011. [Google Scholar] [CrossRef]
- Wyllie, M.R.J.; Gregory, A.R.; Gardner, G.H.F. An experimental investigation of factors affecting elastic wave velocities in porous media. Geophysics 1958, 23, 459–493. [Google Scholar] [CrossRef]
- Janotka, I.; Mojumbar, S.C. Hydration of Portland cement, natural zeolite mortar in water and sulphate solution. Solid State Phenom. 2003, 90-91, 309–316. [Google Scholar] [CrossRef]
- Janotka, I. The influence of zeolitic cement and sand on resistance of mortar subjected to hydrochloric acid solution attack. Ceramics 1999, 43, 61–66. [Google Scholar]
- Short, A.; Kinniburgh, W. Lightweight Concrete, 3rd ed; Applied Science Publishers: London, UK, 1978. [Google Scholar]
- ASTM C 1329-04 Standard Specification for Mortar Cement. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2002; Volume 04.01.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Vogiatzis, D.; Kantiranis, N.; Filippidis, A.; Tzamos, E.; Sikalidis, C. Hellenic Natural Zeolite as a Replacement of Sand in Mortar: Mineralogy Monitoring and Evaluation of Its Influence on Mechanical Properties. Geosciences 2012, 2, 298-307. https://doi.org/10.3390/geosciences2040298
Vogiatzis D, Kantiranis N, Filippidis A, Tzamos E, Sikalidis C. Hellenic Natural Zeolite as a Replacement of Sand in Mortar: Mineralogy Monitoring and Evaluation of Its Influence on Mechanical Properties. Geosciences. 2012; 2(4):298-307. https://doi.org/10.3390/geosciences2040298
Chicago/Turabian StyleVogiatzis, Dimitrios, Nikolaos Kantiranis, Anestis Filippidis, Evaggelos Tzamos, and Costas Sikalidis. 2012. "Hellenic Natural Zeolite as a Replacement of Sand in Mortar: Mineralogy Monitoring and Evaluation of Its Influence on Mechanical Properties" Geosciences 2, no. 4: 298-307. https://doi.org/10.3390/geosciences2040298





