Biogeochemical Characterization of Metal Behavior from Novel Mussel Shell Bioreactor Sludge Residues
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Laboratory Incubations
2.2. Microsensors and Diffusive Flux Calculations
2.3. Geochemical Phase Description
2.3.1. Scanning Electron Microscopy (SEM) and Particle Count Analysis
2.3.2. Selective Solid Phase Extractions
2.4. Microbial Community Analyses
2.4.1. DNA Extraction and Sequencing Preparations
2.4.2. Community Structure Analysis
3. Results and Discussion
3.1. Oxygen and Hydrogen Sulfide Flux
3.2. Geochemical Phase Classification Using Two Methods to Quantify Microbial and Atmospheric Effects
3.2.1. Biomineralization (Sulfide) Characterization in Oxic and Anoxic Environments Using Scanning Electron Microscopy (SEM)
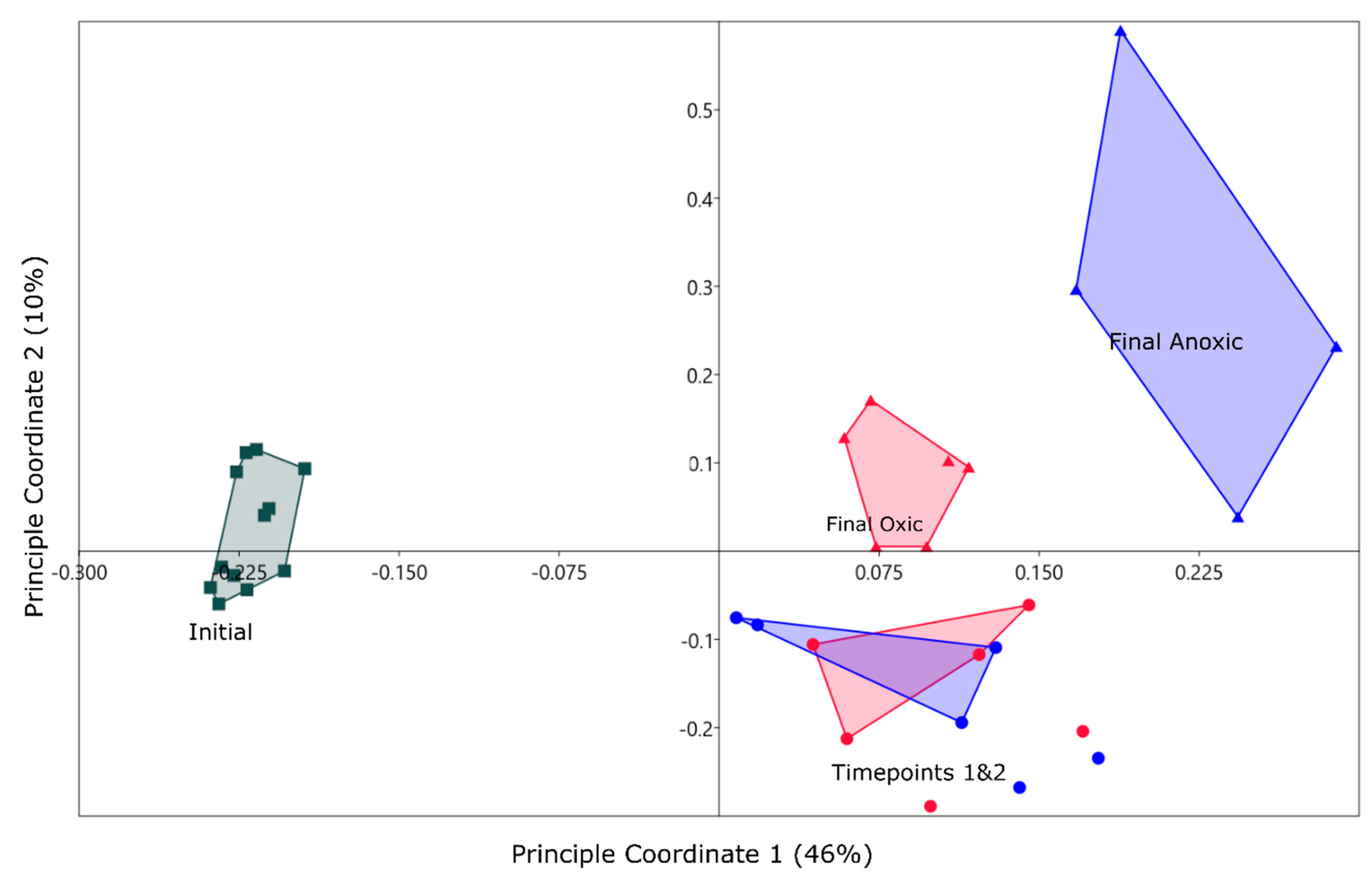
3.2.2. Solid Phase Characterization and Metal Behavior, Using Principal Component Analyses (PCA)
3.3. Community Structure Shifts as a Function of Anoxic and Oxic Incubation Environments
3.4. Biogeochemical Connections of AMD Sludge, and Associated Risks
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bridge, G. CONTESTED TERRAIN: Mining and the Environment. Annu. Rev. Environ. Resour. 2004, 29, 205–259. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Nordstrom, D.K. The rate of ferrous iron oxidation in a stream receiving acid mine effluent. Sel. Pap. Hydrol. Sci. 1985, 1985, 113–119. [Google Scholar]
- Mayes, W.M.; Johnston, D.; Potter, H.A.B.; Jarvis, A.P. A national strategy for identification, prioritisation and management of pollution from abandoned non-coal mine sites in England and Wales. I.: Methodology development and initial results. Sci. Total Environ. 2009, 407, 5435–5447. [Google Scholar] [CrossRef] [PubMed]
- Armitage, P.D.; Bowes, M.J.; Vincent, H.M. Long-term changes in macroinvertebrate communities of a heavy metal polluted stream: The river Nent (Cumbria, UK) after 28 years. River Res. Appl. 2007, 23, 997–1015. [Google Scholar] [CrossRef]
- Han, Y.-S.; Youm, S.-J.; Oh, C.; Cho, Y.-C.; Ahn, J.S. Geochemical and eco-toxicological characteristics of stream water and its sediments affected by acid mine drainage. Catena 2017, 148, 52–59. [Google Scholar] [CrossRef]
- Singer, P.C.; Stumm, W. Acidic Mine Drainage: The Rate-Determining Step. Source Sci. New Ser. 1970, 167, 1121–1123. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.-G.; Sand, W. Evaluation of the efficiency of measures for sulphidic mine waste mitigation. Appl. Microbiol. Biotechnol. 1998, 49, 698–701. [Google Scholar] [CrossRef]
- DiLoreto, Z.A.; Weber, P.A.; Olds, W.; Pope, J.; Trumm, D.; Chaganti, S.R.; Heath, D.D.; Weisener, C.G. Novel cost effective full scale mussel shell bioreactors for metal removal and acid neutralization. J. Environ. Manag. 2016. [Google Scholar] [CrossRef]
- Zinck, J.; Griffith, W. CANMET Mining and Mineral Sciences Laboratories. Mine Environ. Neutral Drain. 2005, Report 3.43.1, 1–60. [Google Scholar]
- Demers, I.; Mbonimpa, M.; Benzaazoua, M.; Bouda, M.; Awoh, S.; Lortie, S.; Gagnon, M. Use of acid mine drainage treatment sludge by combination with a natural soil as an oxygen barrier cover for mine waste reclamation: Laboratory column tests and intermediate scale field tests. Miner. Eng. 2017, 107, 43–52. [Google Scholar] [CrossRef]
- Macías, F.; Pérez-López, R.; Caraballo, M.A.; Cánovas, C.R.; Nieto, J.M. Management strategies and valorization for waste sludge from active treatment of extremely metal-polluted acid mine drainage: A contribution for sustainable mining. J. Clean. Prod. 2017, 141, 1057–1066. [Google Scholar] [CrossRef]
- DiLoreto, Z.A.; Weber, P.A.; Weisener, C.G. Solid phase characterization and metal deportment in a mussel shell bioreactor for the treatment of AMD, Stockton Coal Mine, New Zealand. Appl. Geochem. 2016, 67, 133–143. [Google Scholar] [CrossRef]
- Trumm, D.; Ball, J.; Pope, J.; Weisener, C. Passive Treatment of ARD Using Mussel Shells—Part III: Technology Improvement and Future Direction. In Proceedings of the 10th International Conference on Acid Rock Drain, IMWA Conference, Santiago, Chile, 21–24 April 2015; pp. 1–9. [Google Scholar]
- McCauley, C.; O’Sullivan, A.; Weber, P.; Trumm, D. Variability of Stockton Coal Mine drainage chemistry and its treatment potential with biogeochemical reactors. N. Z. J. Geol. Geophys. 2010, 53, 211–226. [Google Scholar] [CrossRef] [Green Version]
- Pope, J.; Weber, P.; Mackenzie, A.; Newman, N.; Rait, R. Correlation of acid base accounting characteristics with the Geology of commonly mined coal measures, West Coast and Southland, New Zealand. N. Z. J. Geol. Geophys. 2010, 53, 153–166. [Google Scholar] [CrossRef] [Green Version]
- Weisener, C.; Weber, P. Preferential oxidation of pyrite as a function of morphology and relict texture. N. Z. J. Geol. Geophys. 2010, 53, 167–176. [Google Scholar] [CrossRef] [Green Version]
- McCauley, C.A.; O’Sullivan, A.D.; Milke, M.W.; Weber, P.A.; Trumm, D.A. Sulfate and metal removal in bioreactors treating acid mine drainage dominated with iron and aluminum. Water Res. 2009, 43, 961–970. [Google Scholar] [CrossRef] [PubMed]
- McCauley, C.; O’Sullivan, A.D.; Weber, P.; Trumm, D. Variability of Stockton Mine Acid Mine Drainage and Its Treatment Using Waste Substrates in Biogeochemical Reactors. Available online: https://ir.canterbury.ac.nz/handle/10092/4533 (accessed on 18 January 2019).
- Chen, M.; Walshe, G.; Chi Fru, E.; Ciborowski, J.J.H.; Weisener, C.G. Microcosm assessment of the biogeochemical development of sulfur and oxygen in oil sands fluid fine tailings. Appl. Geochem. 2013, 37, 1–11. [Google Scholar] [CrossRef]
- Reid, T.; Boudens, R.; Ciborowski, J.J.H.; Weisener, C.G. Physicochemical gradients, diffusive flux, and sediment oxygen demand within oil sands tailings materials from Alberta, Canada. Appl. Geochem. 2016, 75, 90–99. [Google Scholar] [CrossRef]
- Boudens, R.; Reid, T.; VanMensel, D.; Prakasan, M.R.S.; Ciborowski, J.J.H.; Weisener, C.G. Bio-physicochemical effects of gamma irradiation treatment for naphthenic acids in oil sands fluid fine tailings. Sci. Total Environ. 2016, 539, 114–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revsbech, N.P. Diffusion characteristics of microbial communities determined by use of oxygen microsensors. J. Microbiol. Methods 1989, 9, 111–122. [Google Scholar] [CrossRef]
- Unisense A/S. Website 2017. Available online: http://.unisense.com/ (accessed on 30 December 2018).
- Revsbech, N.P.; Nielsen, L.P.; Ramsing, N.B. A novel microsensor for determination of apparent diffusivity in sediments. Limnol. Oceanogr. 1998, 43, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Diloreto, Z.A. Scholarship at UWindsor Biogeochemical Investigations of a Full Scale Mussel Shell Bioreactor for the Treatment of Acid Mine Drainage (AMD), the Stockton Mine, New Zealand. Master’s Thesis, University of Windsor, Windsor, ON, Canada, 2016. [Google Scholar]
- Ribeta, I.; Ptacek, C.J.; Blowes, D.W.; Jambor, J.L. The potential for metal release by reductive dissolution of weathered mine tailings. J. Contam. Hydrol. 1995, 17, 239–273. [Google Scholar] [CrossRef]
- Fangueiro, D.; Bermond, A.; Santos, E.; Carapuça, H.; Duarte, A. Heavy metal mobility assessment in sediments based on a kinetic approach of the EDTA extraction: Search for optimal experimental conditions. Anal. Chim. Acta 2002, 459, 245–256. [Google Scholar] [CrossRef]
- Heron, G.; Christensen, T.H.; Tjell, J.C. Oxidation Capacity of Aquifer Sediments. Environ. Sci. Technol. 1994, 28, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Amirbahman, A.; Schönenberger, R.; Johnson, C.A.; Sigg, L. Aqueous- and Solid-Phase Biogeochemistry of a Calcareous Aquifer System Downgradient from a Municipal Solid Waste Landfill (Winterthur, Switzerland). Environ. Sci. Technol. 1998, 32, 1933–1940. [Google Scholar] [CrossRef]
- Falk, N.; Chaganti, S.R.; Weisener, C.G. Evaluating the microbial community and gene regulation involved in crystallization kinetics of ZnS formation in reduced environments. Geochim. Cosmochim. Acta 2018, 220, 201–216. [Google Scholar] [CrossRef] [Green Version]
- Parada, A.E.; Needham, D.M.; Fuhrman, J.A. Every base matters: Assessing small subunit rRNA primers for marine microbiomes with mock communities, time series and global field samples. Environ. Microbiol. 2016, 18, 1403–1414. [Google Scholar] [CrossRef]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Xu, Z.Z.; Peddada, S.; Amir, A.; Bittinger, K.; Gonzalez, A.; Lozupone, C.; Zaneveld, J.R.; Vázquez-Baeza, Y.; Birmingham, A.; et al. Normalization and microbial differential abundance strategies depend upon data characteristics. Microbiome 2017, 5, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lloyd, M.; Ghelardi, R.J. A Table for Calculating the ‘Equitability’ Component of Species Diversity. J. Anim. Ecol. 1964, 33, 217. [Google Scholar] [CrossRef]
- Chao, A. Nonparametric Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Thorbergsdóttir, I.M.; Reynir Gíslason, S.; Ingvason, H.R.; Einarsson, Á. Benthic oxygen flux in the highly productive subarctic Lake Myvatn, Iceland: In situ benthic flux chamber study. Aquat. Ecol. 2004, 38, 177–189. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L. Formation processes of framboidal pyrite. Geochim. Cosmochim. Acta 1997, 61, 323–339. [Google Scholar] [CrossRef]
- Frankel, R.B. Biologically Induced Mineralization by Bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef] [Green Version]
- Gadd, G.M. Metals, minerals and microbes: Geomicrobiology and bioremediation. Microbiology 2010, 156, 609–643. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Tazaki, K.; Fyfe, W.S. Iron oxides in acid mine drainage environments and their association with bacteria. Chem. Geol. 1989, 74, 321–330. [Google Scholar] [CrossRef]
- Gadde, R.R.; Laitinen, H.A. Studies of Heavy Metal Adsorption by Hydrous Iron and Manganese Oxides. Anal. Chem. 1974, 46, 2022–2026. [Google Scholar] [CrossRef]
- Lee, G.; Bigham, J.M.; Faure, G. Removal of trace metals by coprecipitation with Fe, Al and Mn from natural waters contaminated with acid mine drainage in the Ducktown Mining District, Tennessee. Appl. Geochem. 2002, 17, 569–581. [Google Scholar] [CrossRef]
- Tessier, A.; Rapin, F.; Carignan, R. Trace metals in oxic lake sediments: Possible adsorption onto iron oxyhydroxides. Geochim. Cosmochim. Acta 1985, 49, 183–194. [Google Scholar] [CrossRef]
- Liao, P.; Li, W.; Jiang, Y.; Wu, J.; Yuan, S.; Fortner, J.D. Formation, Aggregation, and Deposition Dynamics of NOM-Iron Colloids at Anoxic–Oxic Interfaces. Environ. Sci. Technol. 2017, 51, 12235–12245. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.A.; Norris, P.R. Acidimicrobium ferrooxidans gen. nov., sp. nov.: Mixed-culture ferrous iron oxidation with Sulfobacillus species. Microbiology 1996, 142, 785–790. [Google Scholar] [CrossRef]
- Schwertmann, U. Solubility and dissolution of iron oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Demers, I.; Benzaazoua, M.; Mbonimpa, M.; Bouda, M.; Bois, D.; Gagnon, M. Valorisation of acid mine drainage treatment sludge as remediation component to control acid generation from mine wastes, part 1: Material characterization and laboratory kinetic testing. Miner. Eng. 2015, 76, 109–116. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Sanz, J.L.; Bijmans, M.F.M.; Stams, A.J.M. Sulfate reduction at low pH to remediate acid mine drainage. J. Hazard. Mater. 2014, 269, 98–109. [Google Scholar] [CrossRef]
- Méndez-García, C.; Peláez, A.I.; Mesa, V.; Sánchez, J.; Golyshina, O.V.; Ferrer, M. Microbial diversity and metabolic networks in acid mine drainage habitats. Front. Microbiol. 2015, 6, 475. [Google Scholar]
- Schippers, A.; Breuker, A.; Blazejak, A.; Bosecker, K.; Kock, D.; Wright, T.L. The biogeochemistry and microbiology of sulfidic mine waste and bioleaching dumps and heaps, and novel Fe(II)-oxidizing bacteria. Hydrometallurgy 2010, 104, 342–350. [Google Scholar] [CrossRef]
- Florentino, A.P.; Weijma, J.; Stams, A.J.M.; Sánchez-Andrea, I. Sulfur Reduction in Acid Rock Drainage Environments. Environ. Sci. Technol. 2015, 49, 11746–11755. [Google Scholar] [CrossRef] [Green Version]
- Barton, L.L.; Hamilton, A. The Sulphate-Reducing Bacteria; Cambridge University Press: New York, NY, USA, 2007; Volume 1542, ISBN 9780511541490. [Google Scholar]
- Lee, Y.-J.; Romanek, C.S.; Wiegel, J. Desulfosporosinus youngiae sp. nov., a spore-forming, sulfate-reducing bacterium isolated from a constructed wetland treating acid mine drainage. Int. J. Syst. Evol. Microbiol. 2009, 59, 2743–2746. [Google Scholar] [CrossRef]
- Bruneel, O.; Mghazli, N.; Hakkou, R.; Dahmani, I.; Filali Maltouf, A.; Sbabou, L. In-depth characterization of bacterial and archaeal communities present in the abandoned Kettara pyrrhotite mine tailings (Morocco). Extremophiles 2017, 21, 671–685. [Google Scholar] [CrossRef] [PubMed]
- Wakeman, K.D.; Erving, L.; Riekkola-Vanhanen, M.L.; Puhakka, J.A. Silage supports sulfate reduction in the treatment of metals- and sulfate-containing waste waters. Water Res. 2010, 44, 4932–4939. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.; Faleiro, M.L.; Silva, G.; Chaves, S.; Tenreiro, R.; Costa, M.C. Dynamics of bacterial community in up-flow anaerobic packed bed system for acid mine drainage treatment using wine wastes as carbon source. Int. Biodeterior. Biodegrad. 2011, 65, 78–84. [Google Scholar] [CrossRef]





| Target | Extractant | Citation |
|---|---|---|
| Water-soluble | N purged milli Q water | Ribeta et al. (1995) [27] |
| Bio-available | 0.005 EDTA adjusted to pH 6 | Fangueio at al. (2001) [28] |
| Metals weakly bonded to oxide phases | 0.5 M HCl | Heron et al. (1994) [29] |
| Amorphous oxyhydroxide (reducible) | 0.12 M sodium ascorbate; 0.17 M sodium citrate; 0.6 M NaHCO3, adjusted to pH 8 | Amirbahman (1998) [30] |
| Strong acid extractable | 5 M HCl | Heron at al. (1994) [29] |
| Chemical Component | Month | Oxic | Anoxic | ||
|---|---|---|---|---|---|
| Biotic | Abiotic | Biotic | Abiotic | ||
| oxygen flux 1 | 1 | 1.83 | 2.79 | ND | ND |
| 5 | 1.21 | 6.23 | ND | ND | |
| H2S flux 1 | 1 | ND | ND | 210 | ND |
| 5 | ND | ND | 60.9 | ND | |
| O2 (mg/L) | 1 | 2.6 | 5.5 | 0 | 0 |
| 5 | 3.01 | 7.1 | 0 | 0 | |
| HS (µmo/L) | 1 | 0 | 0 | 8.7 | 5.96 |
| 5 | 0 | 0 | 15 | ND | |
| Redox Potential | 1 | 262 | 230 | 10 | 80 |
| 5 | 229 | 300 | −80 | −100 | |
| pH | 1 | 3.27 | 4.1 | 5.89 | 5.92 |
| 5 | 3.24 | 3.74 | 5.19 | 4.87 | |
| Months | Chao | Shannon | Observed Rarefied OTUs | |
|---|---|---|---|---|
| Initial | 2065 ± 200 | 7.5 ± 0.2 | 930 ± 50 | |
| anoxic | 1 | 2199 ± 100 | 7.71 ± 0.03 | 960 ± 10 |
| 3 | 2184 ± 300 | 7.4 ± 0.5 | 920 ± 100 | |
| 5 | 1479 ± 60 | 7.5 ± 0.4 | 810 ± 100 | |
| oxic | 1 | 2618 ± 100 | 8.2 ± 0.1 | 1130 ± 40 |
| 3 | 2243 ± 200 | 7.7 ± 0.1 | 960 ± 40 | |
| 5 | 1732 ± 200 | 7.6 ± 0.3 | 850 ± 100 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Butler, S.C.; Pope, J.; Chaganti, S.R.; Heath, D.D.; Weisener, C.G. Biogeochemical Characterization of Metal Behavior from Novel Mussel Shell Bioreactor Sludge Residues. Geosciences 2019, 9, 50. https://doi.org/10.3390/geosciences9010050
Butler SC, Pope J, Chaganti SR, Heath DD, Weisener CG. Biogeochemical Characterization of Metal Behavior from Novel Mussel Shell Bioreactor Sludge Residues. Geosciences. 2019; 9(1):50. https://doi.org/10.3390/geosciences9010050
Chicago/Turabian StyleButler, Sara C., James Pope, Subba Rao Chaganti, Daniel D. Heath, and Christopher G. Weisener. 2019. "Biogeochemical Characterization of Metal Behavior from Novel Mussel Shell Bioreactor Sludge Residues" Geosciences 9, no. 1: 50. https://doi.org/10.3390/geosciences9010050




