Microbial Geochemistry Reflecting Sulfur, Iron, Manganese, and Calcium Sources in the San Diego River Watershed, Southern California USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Water Quality
2.3. Water Chemistry
2.4. Geology and Mineralogy
2.5. Microbiology
3. Results
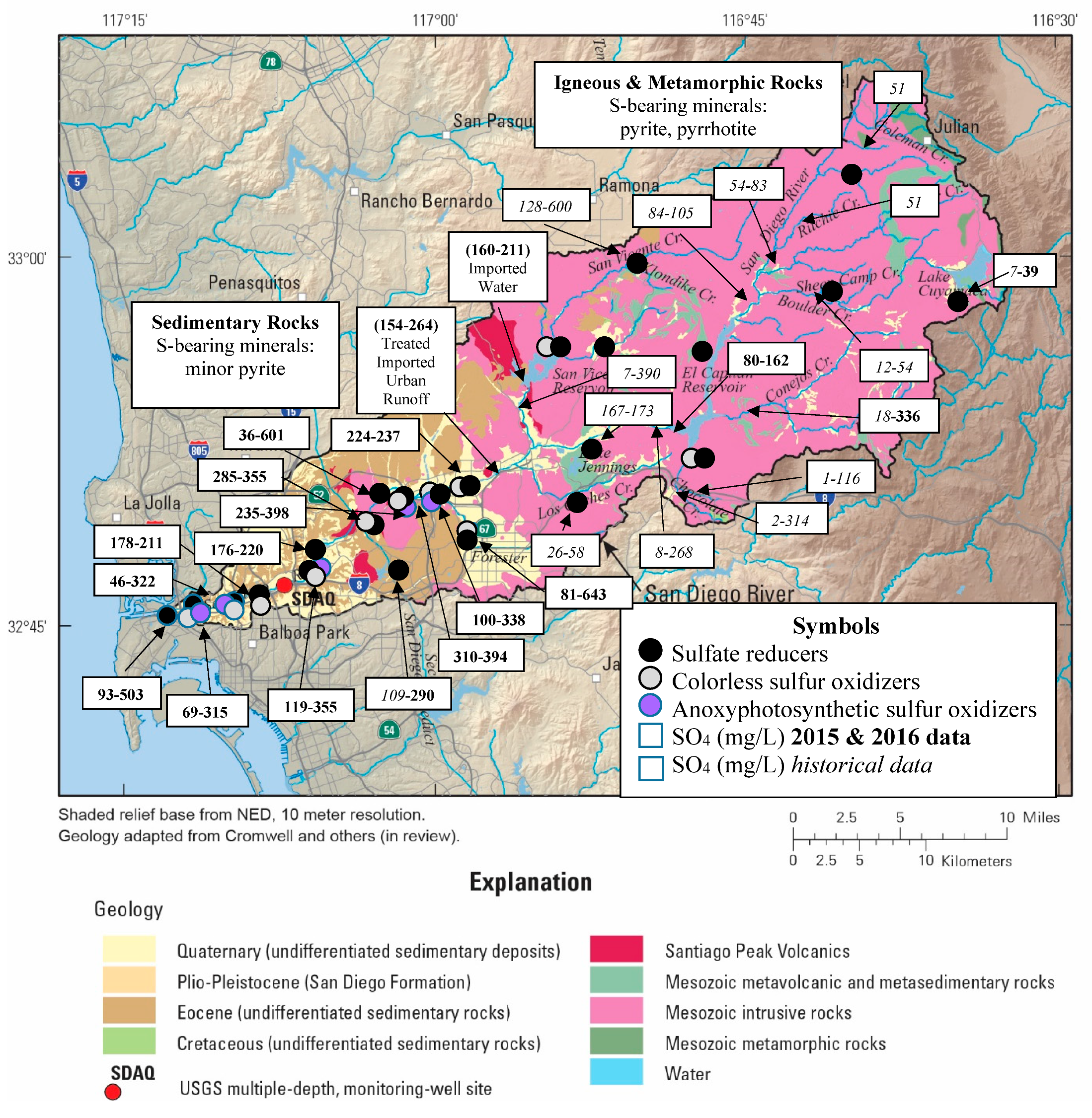
3.1. Sulfur Sources and Sinks
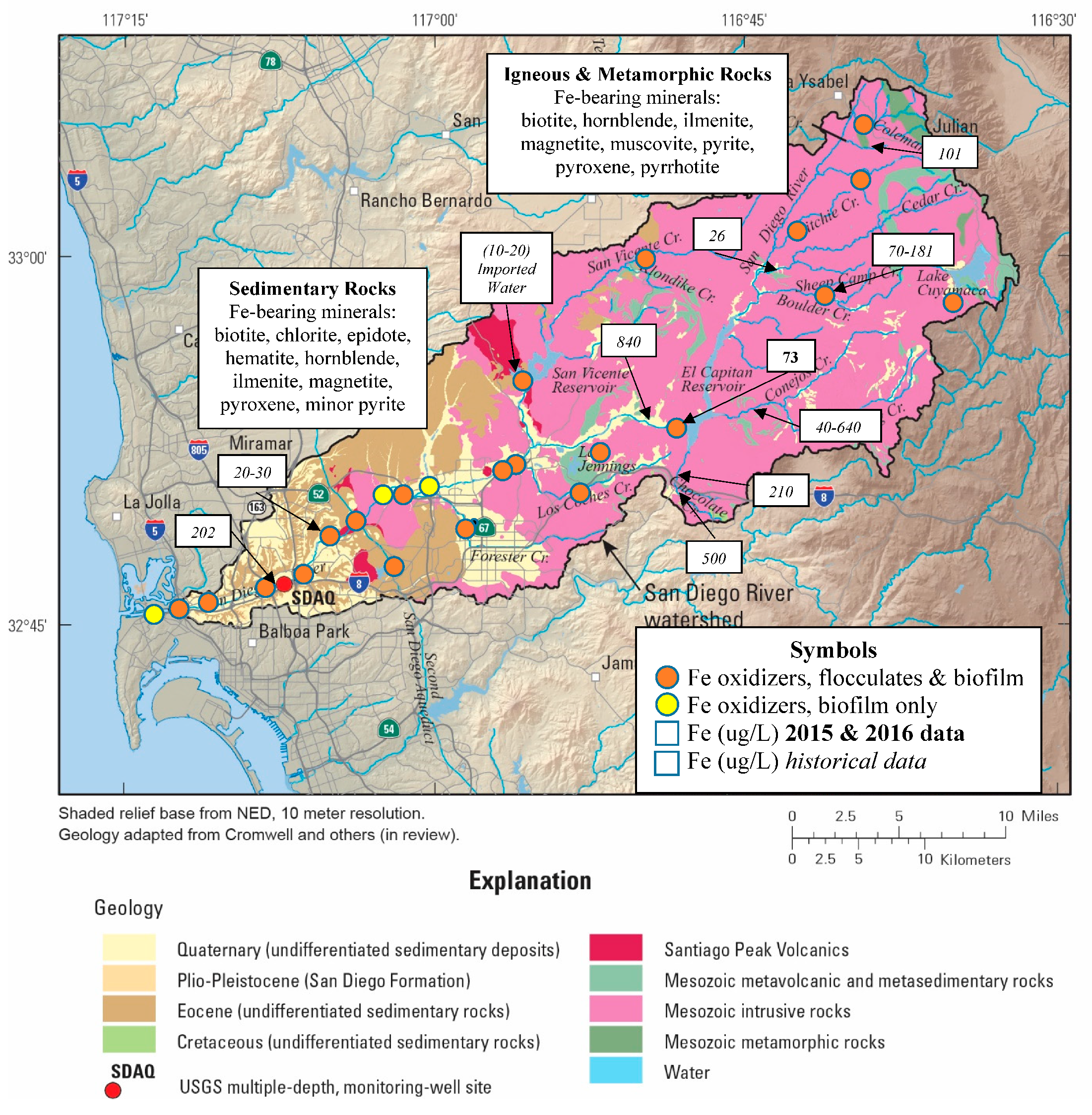
3.2. Iron Sources and Sinks
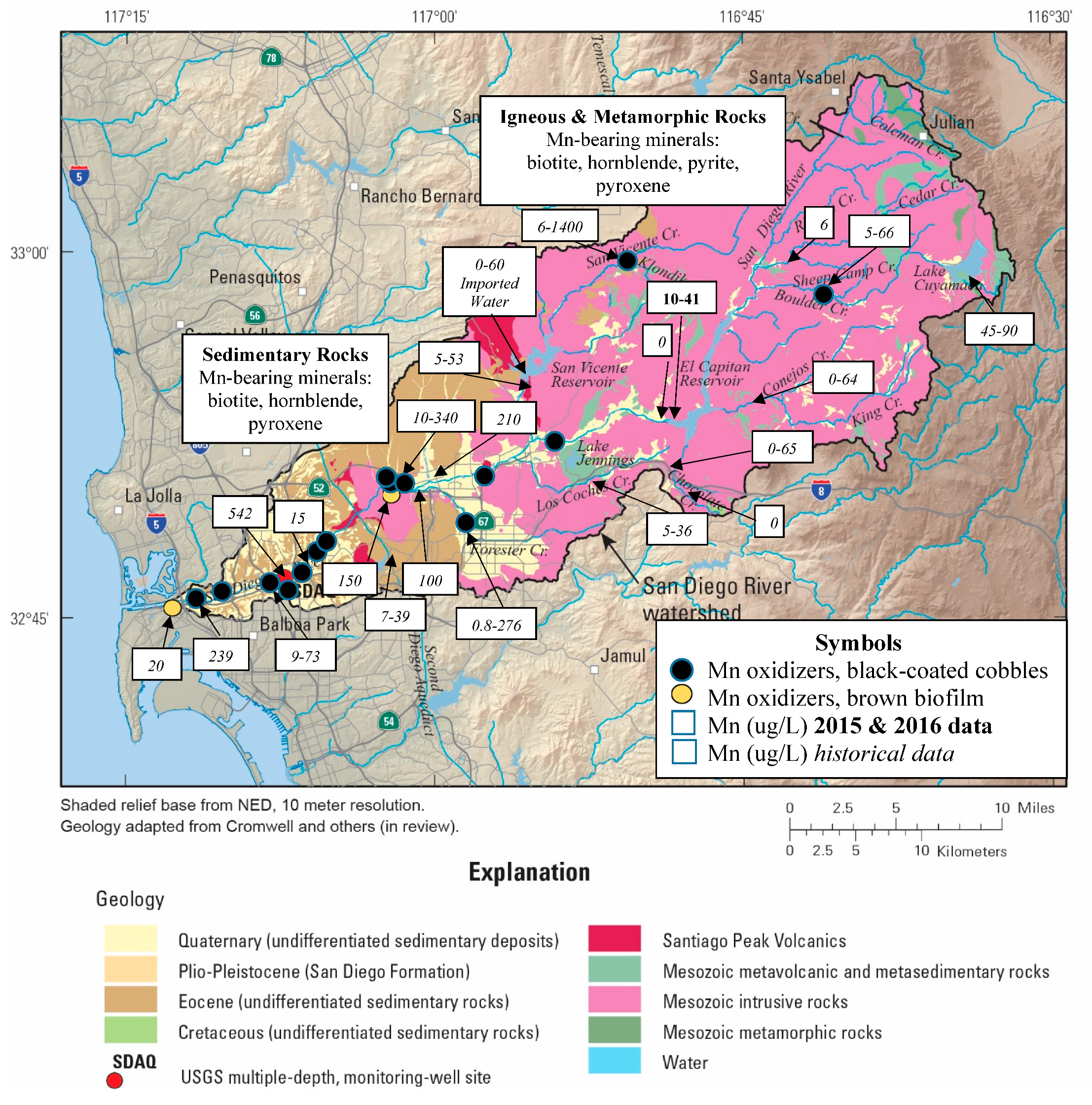
3.3. Manganese Sources and Sinks
3.4. Calcium Sources and Sinks
4. Discussion
4.1. Sulfur Dynamics
4.2. Iron Dynamics
4.3. Manganese Dynamics
4.4. Calcium Dynamics
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A. Microbiology, Water Chemistry, and Geology/Mineralogy of the San Diego River Watershed
Explanation of Abbreviations and Format Details in Appendix A
- (A)
- Distances for tributaries are measured where they enter the San Diego River.
- (B)
- Geology is reported from each sample locality; the mineralogy reflects the geology of a one-kilometer radius from the upstream side of the sample locality. Mineral and rock abbreviations are as follows: bio, biotite; calc, calcareous; chl, chlorite; cong, conglomerate; epi, epidote; ferrihy, ferrihydrite; hema, hematite; hbe, hornblende; ilm, ilmenite; musco, muscovite; py, pyrite; pyrrho, pyrrhotite; pyrox, pyroxene; volc, volcanic.
- (C)
- For water chemistry and microbiology, the numbers from 1–12 in parentheses are months (1 = Jan., 2 = Feb., etc.).
- (D)
- Fe and Mn are in ug/L; other chemical constituents are reported in mg/L.
- (E)
- FeOx refers to observations of iron oxide flocculates/precipitates that were not sampled. SOx refers to observations of white filaments and white biofilms that were not sampled.
- (F)
- References in [ ]
- (G)
- Minimum-maximum values in samples collected in the reporting interval 2015–2016 are in regular font; values from other years are in italics.
Upper Watershed
- Year Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Ann
- 2015 0.49 2.47 2.19 1.61 3.20 0.01 1.94 0.05 1.97 0.52 3.05 6.42 23.92
- 2016 7.95 0.66 1.98 1.47 0.88 0.00 0.00 0.00 1.20 0.31 3.57 7.88 25.90
| Km 83.68 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [39] | gabbro, tonalite | bio, hbe, ilm, mag | bio, hbe, pyrox | pyrox |
| Km 79.2 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [39] | schist, tonalite | py | bio, hbe, ilm, mag, musco, py | bio, hbe, py, pyrox | pyrox |
| Microbiology | no H2S↑ | FeOx(10) |
| Km 76.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [39] | schist, tonalite | py, pyrrho | bio, hbe, ilm, musco, py, pyrox, pyrrho | bio, hbe, py, pyrox | pyrox |
| Water Chemistry [22] | pH 7.3(5) DO 5.15 (5) | SO4 50.9(5) | Fe 101(5) |
| Km 74.0 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [39] | schist, tonalite | py, pyrrho | bio, hbe, ilm, musco, py, pyrox, pyrrho | bio, hbe, py, pyrox | pyrox |
| Water Chemistry | pH 6.91(3) | ||||
| Microbiology | H2S↑ (3,7,8,9,10) | FeOx(3,7,8,9,10) L. discophora biofilm(7,10) L. cholodnii(11) L. discophora(11) L. ochracea(11) Siderocapsa(11) |
| Km 68.4 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [39] | granodiorite, tonalite | py, pyrrho | bio, hbe, ilm, py, pyrox, pyrrho | bio, pyrox | pyrox |
| Water Chemistry [22] | SO4 51.2(5) | ||||
| Microbiology | no H2S↑ | L. discophora biofilm(2) FeOx(2) L. ochracea(2) |
| Km 66.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [37] | tonalite | py, pyrrho | bio, hbe, ilm, py, pyrox, pyrrho | bio, hbe, py, pyrox | pyrox |
| Water Chemistry [22,23] | pH 7.66(4)–8.43(4) DO 7.35(4)–8.38(4) | SO4 54(3)–83.4(3) | Fe 26.2(5) | Mn 5.96(5) |
| Km 65.3 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [37] | granodiorite, quartz diorite, tonalite | py, pyrrho | bio, hbe, ilm, py, pyrox, pyrrho | bio, hbe, py, pyrox | pyrox |
| Water Chemistry [22] | pH 7.75(4) DO 8.12(4) | SO4 12.3(4)–53.8(3) | Fe 70(5)–181(5) | Mn 5.14(2)–66.3(4) | |
| Microbiology | no H2S↑ | FeOx(7) | L. discophora coatings(7) |
| Km 65.3 | S | Fe | Mn | Ca | |
| Geology/Mineralogy [37] | tonalite, schist | py | bio, hbe, ilm, musco, py, pyrox | bio, hbe, py, pyrox | pyrox |
| Microbiology | H2S↑ (10) |
| Km 65.3 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [35] | schist, granodiorite, tonalite, quartz monzonite | py, pyrrho | bio, ilm, mag, musco, py, pyrox, pyrrho | bio, hbe, py, pyrox | pyrox |
| Water Chemistry [19,28] | pH 8.3(5) | SO4 7.2(5)–39(5) | Mn 45–90 | ||
| Microbiology | H2S↑ (2) | L.cholodnii(2) L. ochracea(2) cf. Siderocapsa (2) |
| Km 60.7 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [37] | tonalite | py, pyrrho | hbe, ilm, mag, py, pyrox, pyrrho | hbe, py, pyrox | pyrox |
| Water Chemistry [23] | pH 8.22(5) DO 7.23(3) | SO4 83.9(4)–105(3) |
| Km 49.1 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [40] | tonalite | py, pyrrho | bio, hbe, ilm, mag, py, pyrox, pyrrho | bio, hbe, py, pyrox | calc-silicate, pyrox |
| Water Chemistry [26] | pH 7.77(1)–8.45(7) | SO4 79.8(1)–162(7) | Fe 72.6(4) | Mn 10.4(4)–40.8(4) | Ca 31.1(1)–36(4) |
| Microbiology | H2S↑ (3,9) Beggiatoa(9) FeS2 framboids(3) | L. discophora biofilm(3,9) FeOx(3,9) L. discophora(3) L. ochracea(9) Siderocapsa(3) Siderococcus(3) Toxothrix trichogenes(3) |
| Km 51.5 | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [38] | tonalite, quartz monzonite | py, pyrrho | bio, hbe, ilm, mag, py, pyrox, pyrrho | bio, hbe, py, pyrox | calc-silicate, pyrox |
| Water Chemistry [18,22,23] | pH 7.7(11)–8.0(5) DO 8.0(5) | SO4 1(11)–336(3) | Fe 0.64(11)–39.7(5) | Mn 0(11)–63.7 | Ca 11.6(11) |
| Km 51.0 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [36] | tonalite, gabbro | py, pyrrho | hbe, ilm, mag, py, pyrox, pyrrho | hbe, py, pyrox | pyrox |
| Water Chemistry [18,23] | pH 7.21(5)–8.67(5) DO 5.72(5)–13.81(5) | SO4 2.16(2)–314(5) | Fe 0.5(11) | Mn 0(11)–64.9(5) | Ca 11.2(11) |
| Microbiology | no H2S↑ |
| Km 51.0 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [36] | tonalite | py, pyrrho | hbe, ilm, mag, py, pyrox, pyrrho | hbe, py, pyrox | pyrox |
| Water Chemistry [23] | pH 7.3(11) | SO4 1(11)–116(3) | Fe 210(11) | Mn 0(11) | Ca 4.8(11) |
| Km 50.5 | Geology | S | Fe | Mn | Ca |
| Water Chemistry [18,19,20] | pH 7.6–8.9 | SO4 8(11)–268 | Fe 0.84(11) | Mn 0(11) | Ca 6(11) |
| Location not published | Geology | S | Fe | Mn | Ca |
| Water Chemistry [27] | SO4 178 | Fe 140 | Mn 3000 |
Middle Watershed
- Year Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Ann
- 2015 0.62 0.42 1.05 0.15 1.13 0.20 0.57 0.01 0.42 0.75 0.98 1.45 7.75
- 2016 4.81 0.12 0.96 0.69 0.67 0.00 0.00 0.00 0.49 0.15 1.03 3.76 12.68
| Km 44.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [38] | granite, gabbro, monzogranite | hbe, ilm, mag, pyrox | hbe, pyrox | pyrox | |
| Microbiology | H2S↑ (4) | L. discophora coatings(6) |
| Km 44.9 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [38] | tonalite, cong | py | bio, hbe, ilm, mag, py | bio, hbe, py | |
| Water Chemistry [22] | pH 7.92(3)–8.44(5) DO 2.53(9)–11.19(5) | SO4 128(3)–600(9) | Mn 6.16(5)–1400(6) | ||
| Microbiology | H2S↑ (9) | L. discophora biofilm(9) FeOx(9) |
| Km 44.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [40] | tonalite, granodiorite | hbe, ilm, mag, pyrox | hbe, pyrox | ||
| Water Chemistry [18,23,26,28] | pH 8.3(3,11) | SO4 160(3)–336(11) | Fe 0.1(3)–0.2(11) | Mn 0(3,11)–60 | Ca 9.2(3)–83.2(11) |
| Microbiology | H2S↑ (2) Beggiatoa(2) | L. discophora biofilm(2) FeOx(2) |
| Km 44.9 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [40] | tonalite, granodiorite | hbe, ilm, mag, pyrox | hbe, pyrox | ||
| Water Chemistry [21,22] | pH 7.1–9.1 | SO4 7–390 | Mn 4.7–52.7 | Ca 22–112 |
| Km 37.5 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [40] | monzogranite, tonalite, meta-andesite | py framboids-2 | bio, hbe, ilm, mag, musco, pyrox | bio, hbe, pyrox | |
| Water Chemistry [22] | SO4 167(8)–173(8) | ||||
| Microbiology | H2S↑ (2) | L. discophora biofilm(2) FeOx(2) red rods(2) L. ochracea(2) |
| Km 37.5 | Geology | S | Fe | Mn | Ca |
| Water Chemistry [10] | pH 7.1–8.7 | SO4 154–264 | Fe not detected at ppb | Mn not measured | Ca 70–78 |
| Km 37.5 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [36] | tonalite, granite, schist | py | bio, hbe, ilm, mag, musco, py | bio, hbe, py, pyrox | pyrox |
| Water Chemistry [22] | pH 7.81(5)–8.23(11) DO 5.4(5)–12.17(4) | SO4 26(4)–58.1(12) | Mn 5.49(9)–35.9(5) | ||
| Microbiology | H2S↑ (11) | L. discophora biofilm(11) FeOx(11) |
| Km 33.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [40] | tonalite, granodiorite | bio, hbe, ilm, mag, pyrox | bio, hbe, pyrox | ||
| Microbiology | H2S↑ (8) | L. discophora biofilm(3,8) FeOx(3) |
| Km 31.2 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [40] | Quaternary river sediment | ||||
| Water Chemistry [30] | pH 7.14(3)–8.37(2) DO 0.97(6)–5.88(10) | SO4 224(9)–237(8) | |||
| Microbiology | H2S↑ (2,3,5,9,11) Thiothrix(10) | L. discophora biofilm(2,10) FeOx(2,3) L. ochracea(3,10) | L. discophora coatings(5,6) |
| Km 29.4 | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [40,41] | sandstone, claystone, Quaternary river sediment | minor py | bio, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche, marl(6,8,9,10) |
| Water Chemistry [29,30] | pH 7.03(4)–8.87(11) DO 0.08(11)–4.72(6) | SO4 100(9)–338(5) | Mn 100(12) | ||
| Microbiology | H2S↑ (1,2,5,6,9,10,11,12) SOx(3,11) Beggiatoa(7,8,10,11) Thiothrix(11) Chromatium(8,9) | L. discophora biofilm(9) | Oscillatoria(7) cf. Pseud-anaebaena(6) |
| Km 28.8 | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [34,40,41] | sandstone, cong, tonalite, granodiorite | minor py | bio, hbe, ilm, mag, pyrox, minor py | bio, hbe, pyrox | marl(10), concrete channel |
| Water Chemistry [19,22,30] | pH 7.50(8)–8.82(5) DO 5.91(10)–17.4(6) | SO4 81(3)–643(7) | Mn 0.84(6)–276(7) | Ca 91(10) | |
| Microbiology | H2S↑ (1,10,12) Beggiatoa (10,11,12) Thiothrix(12) vibrios(2) | FeOx(10) L. discophora biofilm(12) L. discophora(11,12) red rods(2) | L. discophora coatings(6,10) |
| Km 28.3 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34] | monzogranite, granodiorite, Quaternary river sediment | bio, hbe, ilm, mag, pyrox | bio, hbe, pyrox | ||
| Water Chemistry [29,30] | pH 7.68(6)–8.54(2) DO 5.16(11)–10.93(5) | SO4 310(6)–394(7) | Mn 210(12) | ||
| Microbiology | H2S↑ (1,2,3,4) | L. discophora biofilm(1,3) |
| Km 26.2 | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [34,41] | sandstone, claystone, monzogranite, volc cobbles | minor py | bio, hbe. hema, pyrox, minor py | bio, hbe, pyrox | caliche, feldspar |
| Water Chemistry [30] | pH 6.97(1)–8.73(12) DO 2.88(8)–11.48(5) | SO4 235(5)–398(8) | |||
| Microbiology | H2S↑ (2,3,4,5,6,9,10,11,12) SOx(10) Beggiatoa(9,10) Chromatium(5) | L. discophora biofilm(2,3,5,6,9,10) FeOx(5,6) | L. discophora coatings (3,6,7,8,9,10,11,12); gone (12) EPS induced oxide along filaments (6) Brown biofilm(9) |
| Km not published | Geology | S | Fe | Mn | Ca |
| Water Chemistry [20] | pH 7.1(5,12)–7.9(12) | SO4 120(6)–200(5,12) | Fe 20(12)–50(12) | Mn 8(5)–210(6) | Ca 42(5)–390(5,12) |
| Km 24.9 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [33,34,41] | sandstone, claystone, monzogranite, meta-andesite | minor py | bio, chl, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche, feldspar |
| Water Chemistry [29] | pH 8.5(7) DO 2(7) | Mn 150(12) | |||
| Microbiology | L. discophora biofilm(9) | L. discophora coatings(9) Brown biofilm(9) |
| 24.1 km | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [33,34,41] | sandstone, claystone, monzogranite, meta-andesite | minor py | bio, chl, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche, feldspar |
| Water Chemistry [19,29,30] | pH 7.33(4)–8.56(11) DO 2.29(9)–9.43(11) | SO4 36(9)–601(3) | Fe 20(3,12)–30(6) | Mn 10(6)–340(12) | |
| Microbiology | H2S↑ (1,2,3,4,5,6,10,11,12) | L. discophora biofilm(1,2,3,5,9,10,11) FeOx(1,2,9,10) L. discophora(10) L. ochracea(10) Siderocapsa(2,3,5,6,10) | L. discophora coatings(3,9) brown biofilm (8,9) |
| Km 21.2 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [33,34,41] | sandstone, claystone, cong, meta-andesite, monzogranite, volc cobbles | minor py | bio, chl, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche |
| Water Chemistry [30] | pH 7.04(7)–8.31(5) DO 0.36(8)–11.74(5) | SO4 155(9)–349(4) | |||
| Microbiology | H2S↑ (2,3,4,5,9,10.12) Beggiatoa(3) | L. discophora biofilm(2,3,4,11) FeOx(3,4) | L. discophora coatings(10,11) |
| Km 21.2 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,41] | sandstone, claystone, cong, meta-andesite, volc cobbles | minor py | bio, chl, hbe, hema, pyrox, minor py | bio, hbe, pyrox | calichetufa(1,2,3,6,9,10,12) |
| Water Chemistry [30] | pH 7.38(8)–8.20(2) DO 7.19(10)–13.55(6) | SO4 285(5)–355(6) | |||
| Microbiology | H2S↑ (3,10) Beggiatoa (7) Thiothrix (7) | L. ochracea(9) | L. discophora coatings(10) | cf. Gloeocapsa (1,9,12) Rivularia(6,7,9) |
Lower Watershed
- Year Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec Ann
- 2015 0.42 0.28 0.93 0.02 2.39 0.04 1.71 0.01 1.24 0.43 1.54 0.88 9.89
- 2016 3.21 0.05 0.76 0.55 0.44 0.00 0.00 0.00 0.32 0.07 0.61 4.22 10.23
- Site 14: SDR at southern end of Admiral Baker Golf Course, Zion Rd. [32.79304,-117.0998]. Sampled bimonthly.
| Km 15.4 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [34] | sandstone, claystone, cong, volc cobbles | bio, hbe, hema, pyrox | bio, hbe, pyrox | caliche, calc cement | |
| Water Chemistry [30] | pH 7.12(9)–8.43(12) DO 0.99(10)–11.14(5) | SO4 119(5)–355(7) S2− not detected (1,3,4,6,7,12)–0.97(8) | |||
| Microbiology | H2S↑ (6,10) Beggiatoa(6,10) Thiocystis(6) | L. discophora biofilm(9) | L. discophora coatings(6,9,10) |
| Km 15.2 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,41] | sandstone, claystone, cong, volc cobbles | minor py | bio, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche, calc cement |
| Water Chemistry | pH 7.17(9)–7.3(7) DO 0.42(9) | ||||
| Microbiology | H2S↑ (1,2,4,6,7,8,9,10) SOx(5,10) Beggiatoa(6,9,10) Thiothrix(6,7,9) Chromatium(10) Thiocystis (6,7) Thiospirillum (9,10) Chloroflexus(6,9,10) vibrios(1,6) | L. discophora biofilm(5,6,9,10) FeOx(1,9) Magnetotactics? (10) | L. discophora coatings(4,10,12) |
| Km 14.7 | Geology | S | Fe | Mn | Ca |
| Geology/Min-eralogy [34,41] | sandstone, claystone, mudstone, cong, volc cobbles | minor py | bio, hbe, hema, pyrox, minor py | bio, hbe, pyrox | caliche, mollusk fossils, calc cement |
| Water Chemistry [30] | pH 7.02(3)–8.12(12) DO 0.08(7)–6.02(1) | SO4 176(8)–220(7) | Fe 202(1) | Mn 14.6(1) | Ca 18.6(1) |
| Microbiology | H2S↑ (1,2,9,10,12) vibrios(1) |
| Km 14.5 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,42] | sandstone, cong, mudstone, meta-andesite | minor py | bio, chl, minor py | bio | caliche, calc cement, mollusk fossils |
| Water Chemistry [19,22] | SO4 109(12)–290(8) | Mn 7.11(9)–38.9(9) | |||
| Microbiology | H2S↑ (2) | FeOx(2) L. discophora biofilm(2) Gallionella ferruginea(2) L. ochracea(2) Siderocapsa(2) |
| Km 14.5 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,42] | sandstone, cong, mudstone, meta-andesite, volc cobbles | minor py | bio, chl, ferrihy, hema, minor py | bio | caliche, marl(10), mollusk fossils, calc cement |
| Water Chemistry [22] | pH 7.84(10)–8.02(10) DO 6.66(10)–9.5(10) | SO4 257(10)–377(2) | Mn 9.25(5)–72.9(2) | ||
| Microbiology | Beggiatoa(10) | FeOx(10) L. discophora biofilm(12) | L. discophora coatings(10) |
| Km 13.8 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,41,42] | sandstone, cong, mudstone, claystone, marl, Quaternary river sediment, volc cobbles | minor py | bio, epi, ferrihy, hbe, hema, ilm, mag, pyrox, minor py | bio, hbe, pyrox | caliche, marl, epi, mollusk fossils, calc cement |
| Water Chemistry [30] | pH 7.04(4)- 8.18(11) DO 0.38(10)–7.75(1) | SO4 178(7)–211(8) | Mn 542(4) | Ca 135(10) | |
| Microbiology | H2S↑ (1,2,4,6,9,12) Beggiatoa(9,10) Thiothrix(9,10) SOx(9,10) vibrios(1) | L. discophora biofilm(1,2) FeOx(2) L. ochracea(9) | L. discophora coatings(2,10) |
| Km 12.7 | Geology | S | Fe | Mn | Ca |
| Water Chemistry [15] | pH 7.0(8)–7.1(5) DO 0.5(5)–2.1(8) | SO4 224(8)–237(5) | Fe 524(8)–920(5) | Mn 2790(8)–3050(5) | Ca 219(5)–221(8) |
| Km 12.2 | Geology | S | Fe | Mn | Ca |
| Water Chemistry [15] | pH 7.0(6)–7.1(4) | SO4 203(4)–212(6) | Fe 4.11(4)–7.83(6) | Mn 1660(4)–2610(6) | Ca 160(4)–172(6) |
| Km 9.3 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,42] | sandstone, mudstone, cong, marl | minor py | bio, ferrihy, hbe, hema, ilm, mag, minor py | bio, hbe | caliche, marl. mollusk fossils, calc cement |
| Water Chemistry [30] | pH 7.12(3)–8.23(5) DO 0.57(10)–11.51(4) | SO4 46(11)–322(9) S2− not detected (1–7,9–12)–1.36(12) | |||
| Microbiology | H2S↑ (1,6,7,9,10) Beggiatoa(7,8,9,10) Thiothrix(8) Chromatium(7) vibrios(1) | L. discophora coatings(10,12) |
| Km 8.6 | Geology | S | Fe | Mn | Ca |
| Geology/Mineral-ogy [34,42] | sandstone, claystone, cong, volc cobbles | minor py | bio, epi, ferrihy, hema, hbe, ilm, mag, minor py | bio, hbe | caliche, calc mollusks, calc cement, epi |
| Microbiology | H2S↑ (1,8) Thiothrix(8) |
| Km 7.9 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34,42] | sandstone, claystone, cong, volc cobbles | minor py | bio, ferrihy, hema, minor py | bio | caliche, calc mollusks, calc cement, epi |
| Water Chemistry [30] | pH 7.12(3)–8.17(11) DO 0.18(9)–7.93(4) | SO4 69(7)–315(9) S2− not detected (1–7,9–12)–0.88(5) | |||
| Microbiology | H2S↑ (1,2,4,6,8,9,10,12) Beggiatoa(1–7,8–12) Chromatium(8) SOx(4,10) vibrios(1) | FeOx(1) |
| Km 5.4 | Geology | S | Fe | Mn | Ca |
| Geology/Miner-alogy [34,42] | artificial fill, sandstone, siltstone, claystone, cong | minor py | bio, ferrihy, hema, minor py | bio | mollusk & ostracod fossils, caliche, calc cement |
| Water Chemistry [22,30] | pH 7.12(3)–8.54(11) DO 0.62(10)–9.28(4) | SO4 82(9) –409(9)S2− not detected (1,3,4,6,7,9–12)–1.07(5) | Mn 239 | ||
| Microbiology | H2S↑ (1,2,4,6,7,8,9,10,12) Beggiatoa(6,8,9,10) Thiothrix(6,7,8,10) Chromatium(9) vibrios(1) | L. discophora biofilm(6) FeOx(6) | L. discophora coatings(6,8) |
| Km 4.6 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34] | artificial fill, sandstone | mollusk & ostracod fossils, marl(12) | |||
| Water Chemistry [29,30] | pH 7.40(4)–8.42(11) DO 1.85(7)–10.57(5) | SO4 93(9)–503(8) | Mn 20(12) | ||
| Microbiology | H2S↑ (1,2,9,12) vibrios(1) | L. discophora biofilm(10) | brown biofilm (10) |
| Km 2.2 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34] | artificial fill, sandstone | mollusk & ostracod fossils | |||
| Microbiology | H2S↑ (8) |
| Km 0.0 | Geology | S | Fe | Mn | Ca |
| Geology/Mineralogy [34] | artificial fill |
References
- San Diego River Conservancy, Environmental Initial Study 2009. Available online: sdrc.ca.gov/docs/SDRC_Initial_Study_MASTER_7.8.09_-_final.pdf (accessed on 13 October 2018).
- Western Regional Climate Center. Available online: https://wrcc.dri.edu/ (accessed on 13 October 2018).
- Kennedy, J.C.; (San Diego River Park Foundation, San Diego, CA, USA). Written communication, 2015.
- San Diego County, Groundwater Evaluation Technical Memorandum. Available online: https://www.sandiegocounty.gov/content/dam/sdc/pds/ProjectPlanning/El-Monte-Sand-Mining-And-Nature-Preserve/SDEIRPublicReview/Appendices/Appendix%20J6%20-%20Reclamation_Plan.pdf (accessed on 13 October 2018).
- Eidson, J.B.; (City of San Diego, Public Utilities Department, San Diego, CA, USA). El Capitan Reservoir Water Distribution, Water Systems Operations Memo. Written communication, 2018. [Google Scholar]
- San Diego County Water Authority b, Member Agency Map. Available online: https://www.sdcwa.org/annualreport/2014/member-agency-map (accessed on 13 October 2018).
- Lakeside Water District, 2016 and 2017, Newsletters. Available online: https://lakesidewater.org/2017%20Newsletter.pdf (accessed on 13 October 2018).
- San Diego County Water Authority a, Imported Supplies. Available online: https://www.sdcwa.org/imported-supplies (accessed on 13 October 2018).
- San Diego County Water Authority 2015 Urban Water Management Plan. Available online: https://www.sdcwa.org/sites/default/files/UWMP2015.pdf (accessed on 13 October 2018).
- City of San Diego, Annual Drinking Water Quality Reports. Available online: www.sandiego.gov/water/quality/reports/index.shtml (accessed on 13 October 2018).
- San Diego County Water Authority d, Annual Report 2008. Available online: https://www.sdcwa.org/sites/default/files/files/publications/annual_2008.pdf (accessed on 13 October 2018).
- U.S. Geological Survey National Water Information System. Available online: https://nwis.waterdata.usgs.gov/ (accessed on 13 October 2018).
- Irelan, B. Salinity of Surface Water in the Lower Colorado River—Salton Sea Area; Professional Paper 486-E; U.S. Geological Survey: Washington, DC, USA, 1971; 46p, ISBN 13:97812866781214032.
- Flint, L.E.; Flint, A.L.; Stolp, B.J.; Danskin, W.R. A basin-scale approach for assessing water resources in a semiarid environment: San Diego region, California and Mexico. Hydrol. Earth Syst. Sci. 2012, 16, 3817–3833. [Google Scholar] [CrossRef]
- Sengebush, R.M.; Heagle, D.J.; Jackson, R.E. The Late Quaternary history and groundwater quality of a coastal aquifer, San Diego, California. Envrion. Eng. Geosci. 2015, 21, 249–275. [Google Scholar] [CrossRef]
- Danskin, W.R. U.S. Geological Survey San Diego Hydrogeology Project. Available online: http://ca.water.usgs.gov/sandiego (accessed on 13 October 2018).
- San Diego River Park Foundation. Available online: http://sandiegoriver.org/online_info_center.html (accessed on 16 December 2018).
- Fast, A.W. Artificial Destratification of El Capitan Reservoir by Aeration, Part 1, Effects of Chemical and Physical Parameters; Fish Bulletin 141; California Dept. Fish and Game: Sacramento, CA, USA, 1968; 97p.
- Department of Water Resources, State of California. Vol. 5 Southern California. Bull. 130-71; Department Water Resources, State of California: Sacramento, CA, USA, 1972; 525p.
- Izbicki, J.A. Evaluation of the Mission, Santee, and Tijuana Hydrologic Subareas for Reclaimed-Water Use, San Diego County, California; Water-Resources Inv. Rpt. 85-4032; U.S. Geological Survey: Washington, DC, USA, 1985; 106p.
- California Environmental Data Exchange Network. Available online: www.ceden.org (accessed on 13 October 2018).
- Surface Water Ambient Monitoring Program (SWAMP), California Water Boards. Available online: https://www.waterboards.ca.gov/sandiego/water_issues/programs/swamp/docs/907sandiegorpt.pdf (accessed on 13 October 2018).
- City of San Diego, Public Utilities Department. Available online: https://www.sandiego.gov/public-utilities (accessed on 13 October 2018).
- Padre Dam Municipal Water District, Annual Water Quality Report. Available online: https://www.padredam.org/DocumentCenter/View/2351/PadreDamWQR2015?bidId= (accessed on 13 October 2018).
- Ramona Municipal Water District, Annual Water Quality Report. Available online: http://www.rmwd.org/images/Files/CCR-2016.pdf (accessed on 13 October 2018).
- Burger, T.B.; (Public Utilities Department, San Diego, CA, USA). Water Chemistry of El Capitan and San Vicente Reservoirs. Written communication, 2018. [Google Scholar]
- Smith, T.; Rasmus, J. Indirect Reuse with Multiple Benefits—The El Monte Valley Mining, Reclamation, and Groundwater Recharge Project. Managed Aquifer Recharge Symposium, Irvine, CA. 2011. Available online: http://www.nwri-usa.org/pdfs/SmithPresentationfinal.pdf (accessed on 13 October 2018).
- City of San Diego Water Department 2005 Watershed Sanitary Survey. Available online: https://www.sandiego.gov/sites/default/files/legacy/water/operations/environment/pdf/05wsfull.pdf (accessed on 21 November 2018).
- California Regional Water Quality Control Board San Diego. Available online: https://www.waterboards.ca.gov/water_issues/programs/tmdl/records/region_9/2003/ref1558.pdf (accessed on 21 November 2018).
- Fried, J. Analysis of Anionic Contributions to Total Dissolved Solids in the Lower San Diego River. Bachelor’s Thesis, San Diego State University, San Diego, CA, USA, 2015; 42p. [Google Scholar]
- Thorbjarnarson, K.W.; McCarlson, A.; Wood, A.; Durand, K.; Kauffman, L.; El-Najjar, N.; Hopper, G.; Daniels, S.; Wirster, C.; (San Diego State University, San Diego, CA, USA). Water quality investigation of Alvarado Creek, San Diego River Watershed, Geochemistry Class Poster. Written communication, 2015. [Google Scholar]
- Thorbjarnarson, K.W.; McManus, H.; Rice, J.; Carty, K.; Gonzales, J.; Garcia, B.; Hill, K.; Bartholomew, W.; Baigent, B.; Faye, A.; (San Diego State University, San Diego, CA, USA). Water quality investigation of Forester Creek, San Diego River Watershed, SDSU Geochemistry Class Poster. Written communication, 2015. [Google Scholar]
- Abbott, P.L. Geology Mission Trails Park; Mission Trails Regional Park Foundation: San Diego, CA, USA, 2017; 76p, ISBN 978-0-692-97712-5. [Google Scholar]
- Kennedy, M.P.; Peterson, G.L. Geology of the San Diego Metropolitan Area, California; Bulletin 200; California Division Mines Geology: Sacramento, CA, USA, 2001; 56p.
- Todd, V.R. Geologic Map of the Cuyamaca Peak 7.5’ Quadrangle, San Diego County, California; Open File Rept. OF-77-405; Map Scale 1:24,000; U.S. Geological Survey: Reston, VA, USA, 1977.
- Todd, V.R. Geologic Map of the Alpine 7.5’ Quadrangle, San Diego County, California; Open File Rept. OF-83-781; Map Scale 1:24,000; U.S. Geological Survey: Reston, VA, USA, 1980.
- Todd, V.R. Geologic Map of the Tule Springs 7.5’ Quadrangle, San Diego County, California; Open File Rept. OF-82-221; Map Scale 1:24,000; U.S. Geological Survey: Reston, VA, USA, 1982.
- Todd, V.R. Geologic Map of the El Cajon Mountain 7.5’ Quadrangle, San Diego County, California; Open File Rept. OF-83-781; Map Scale 1:24,000; U.S. Geological Survey: Reston, VA, USA, 1983.
- Todd, V.R. Geologic Map of the Santa Ysabel 7.5’ Quadrangle, San Diego County, California; Open File Rept.; Map Scale 1:24,000; U.S. Geological Survey: Reston, VA, USA, 2007.
- Todd, V.R.; Alvarez, R.M.; TechniGraphic Systems, Inc. Preliminary Geologic Map of the El Cajon 30’ × 60’ Quadrangle, Southern California; Open-File Rept. OF-2004-1361; Map Scale 1:100,000; U.S. Geological Survey: Reston, VA, USA, 2004.
- Fink, K.A. Petrology of the Eocene Friars Formation, El Cajon, Grossmont, and Tierrasanta Areas, Southwestern San Diego County, California. Bachelor’s Thesis, San Diego State University, San Diego, CA, USA, 1976. [Google Scholar]
- Kern, J.P. Paleoenvironment of new trace fossils from the Eocene Mission Valley Formation, California. J. Paleontol. 1978, 52, 188–194. [Google Scholar]
- National Geologic Map Database, U.S. Geological Survey. Available online: https://ngmdb.usgs.gov/mapview/ (accessed on 13 October 2018).
- Gastil, G.; Higley, R. Guide to San Diego Area Stratigraphy; San Diego State University: San Diego, CA, USA, 1977; 62p. [Google Scholar]
- Germinario, M.P. Depositional and Tectonic Environments of the Julian Schist, Julian, California. Master’s Thesis, San Diego State University, San Diego, CA, USA, 1982. [Google Scholar]
- Hertlein, L.G.; Grant, U.S., IV. The Geology and Paleontology of the Marine Pliocene of San Diego, California; Pt. 1, Geology. Memoir Volume 2; San Diego Society Natural History: San Diego, CA, USA, 1944; 72p. [Google Scholar]
- Schmidt, T.M.; Arieli, B.; Cohen, Y.; Padan, E.; Strohl, W.R. Sulfur metabolism in Beggiatoa alba. J. Bacteriol. 1987, 169, 5466–5472. [Google Scholar] [CrossRef] [PubMed]
- Todd, V.R.; Shaw, S.E.; Langenheim, V.E. Mineralogy and physical properties of plutonic and metamorphic rocks of the Peninsular Ranges batholith, San Diego County, California. In Peninsular Ranges Batholith, Baja California and Southern California; Morton, D.M., Miller, F.K., Eds.; Memoir 211; Geological Society America: Boulder, CO, USA, 2014; pp. 537–582. ISBN 9780813712116. [Google Scholar]
- Todd, V.R.; Hernandez, J.L.; Busch, L.L. The zoned Ramona plutonic complex: An Early Cretaceous mid-to upper-crustal intrusive sequence, Peninsular Ranges batholith, southern California. In Peninsular Ranges Batholith, Baja California and Southern California; Morton, D.M., Miller, F.K., Eds.; Memoir 211; Geological Society America: Boulder, CO, USA, 2014; pp. 583–608. ISBN 9780813712116. [Google Scholar]
- Van Gemerden, H.; Mas, J. Ecology of phototrophic sulfur bacteria. In Anoxygenic Photosynthetic Bacteria; Blankenship, R.E., Madigan, M.T., Bauer, C.E., Eds.; Kluwer: Dordrecht, The Netherlands, 1995; pp. 49–85. ISBN 13:9780792336815. [Google Scholar]
- Robbins, E.I.; LaBaugh, J.W.; Merk, D.A.; Parkhurst, R.S.; Puckett, L.J.; Rosenberry, D.O.; Schuster, P.F.; Shelito, P.A. Bacterial indicators of ground-water discharge—Iron seeps in the Shingobee River and Crow Wing watersheds, Northern Minnesota. In Hydrological and Biogeochemical Research in the Shingobee River Headwaters Area, North-Central Minnesota; Winter, T.C., Ed.; Water-Res. Inv. Rept. WRI 96-4215; U.S. Geological Survey: Denver, CO, USA, 1997; pp. 177–185. [Google Scholar]
- Fleischer, M. Glossary of Mineral Species; Mineral Record, Inc.: Bowie, MD, USA, 1975; 145p. [Google Scholar]
- Tebo, B.M.; Bargar, J.R.; Clement, B.B.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides, Properties and mechanisms of formation. Ann. Rev. Earth Planet. Sci. 2004, 32, 287–338. [Google Scholar] [CrossRef]
- Scheidegger, A.E. River Action. In Systematic Geomorphology; Springer: Vienna, AT, USA, 1987; Chapter 6; pp. 131–177. ISBN 13:9780387820019. [Google Scholar]
- Ford, T.D.; Pedley, H.M. A review of tufa and travertine deposits of the world. Earth-Sci. Rev. 1996, 41, 117–175. [Google Scholar] [CrossRef]
- Nordstrom, D.K.; Southam, G. Geomicrobiology of sulfide mineral oxidation, Chapter 11. In Geomicrobiology: Interactions between Microbes and Minerals; Banfield, J.F., Nelson, K.H., Eds.; V. 35, Reviews in Mineralogy; Mineralogical Society of America: Washington, DC, USA, 1997; pp. 361–390. ISBN 0-939950-45-6. [Google Scholar]
- Mielke, R.E.; Pace, D.L.; Porter, T.; Southam, G. A critical stage in the formation of acid mine drainage: Colonization of pyrite by Acidithiobacillus ferrooxidans under pH-neutral conditions. Geobiology 2003, 1, 81–90. [Google Scholar] [CrossRef]
- Rickard, D.; Luther, G.W., III. Chemistry of iron sulfides. Chem. Rev. 2007, 107, 514–562. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, O.; Van Gemerden, H.; Mas, J. Acclimation of the photosynthetic response of Chromatium vinosum to light-limiting conditions. Arch. Microbiol. 1998, 170, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, W.; Dam, B. Biochemistry and molecular biology of lithotrophic sulfur oxidation by taxonomically and ecologically diverse bacteria and archaea. FEMS Microbiol. Rev. 2009, 33, 999–1043. [Google Scholar] [CrossRef] [PubMed]
- Biggs, T.W.; Lai, C.-T.; (San Diego State University, San Diego, CA, USA). Personal communication, 2018.
- Kantachote, D.; Charernjiratrakul, W.; Noparatnaraporn, N.; Oda, K. Selection of sulfur oxidizing bacterium for sulfide removal in sulfate rich wastewater to enhance biogas production. Electron. J. Biotechnol. 2008, 11, 107–118. [Google Scholar] [CrossRef]
- Kobayashi, H.A.; Stenstrom, M.; Mah, R.A. Use of photosynthetic bacteria for hydrogen sulfide removal from anaerobic waste treatment effluent. Water Res. 1983, 17, 579–587. [Google Scholar] [CrossRef]
- Robbins, E.I.; Anderson, J.E.; Podwysocki, M.H.; Nord, G.L., Jr. Seasonal variations in spectral reflectance of microbial flocculates, precipitates, and oil-like films associated with neutral and acidic mine drainage. In Environmental Monitoring and Biodiagnostics of Hazardous Contaminants; Healy, M., Wise, D.L., Moo-Young, M., Eds.; Kluwer Academic Publishers: Boston, MA, USA, 2001; pp. 243–266. [Google Scholar]
- Chukrov, F.V.; Zvyagin, B.B.; Gorshkov, A.I.; Yermilova, L.P.; Balachova, V.V. Ferrihydrite. Int. Geol. Rev. 1974, 16, 1131–1143. [Google Scholar] [CrossRef]
- Ghiorse, W.C. Biology of iron-and manganese-depositing bacteria. Ann. Rev. Microbiol. 1984, 38, 515–550. [Google Scholar] [CrossRef] [PubMed]
- Grashoff, L. They Breathe Iron, Artistic and Scientific Encounters with an Ancient Life Form; Science & Art Press: Oberlin, OH, USA, 2014; 137p, ISBN 978-0-692-20586-0. [Google Scholar]
- Hem, J.D. Study and Interpretations of the Chemical Characteristics of Natural Water; Water-Supply Paper 2254; U.S. Geological Survey: Washington, DC, USA, 1989; 263p, ISBN 13:9789990638479.
- Robbins, E.I.; Corley, T.L. Microdynamics and seasonal changes in manganese oxide epiprecipitations in Pinal Creek, Arizona. Hydrobiologia 2005, 534, 165–180. [Google Scholar] [CrossRef]
- Robbins, E.I.; Brant, D.L.; Ziemkiewicz, P.F. Microbial, algal, and fungal strategies for manganese oxidation at a Shade Township Coal Mine, Somerset County, Penna. In Proceedings of the 16th Annual Meeting, American Society of Surface Mining and Reclamation, Scotsdale, AZ, USA, 13–19 August 1999; Volume 2, pp. 634–640. [Google Scholar]
- Sato, M.; Robbins, E.I. Recovery/Removal of Metallic Elements from Waste Water Using Ozone. U.S. Patent No. US 6,485,696 B1, 2003. [Google Scholar]
- Robbins, E.I.; D’Agostino, J.P.; Fanning, D.S.; Carter, V.; Van Hoven, R. Manganese nodules and microbial oxidation of manganese in the Huntley Meadows wetland, Virginia, USA. Catena Suppl. (Dutch Soils J.) 1992, 21, 1–23. [Google Scholar]
- Mindat.org. Buserite. Available online: https://www.mindat.org/min-9779.html (accessed on 13 October 2018).
- Weber, F.H. Geology and Mineral Resources of San Diego County, California; Map Scale 1:750,000; California Division of Mines & Geology: Sacramento, CA, USA, 1959.
- Kamennaya, N.A.; Ajo-Franklin, C.M.; Northen, T.; Jansson, C. Cyanobacteria as biocatalysts for carbonate mineralization. Minerals 2012, 2, 338–364. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robbins, E.I.; Quigley-Raymond, S.; Lai, M.; Fried, J. Microbial Geochemistry Reflecting Sulfur, Iron, Manganese, and Calcium Sources in the San Diego River Watershed, Southern California USA. Geosciences 2018, 8, 495. https://doi.org/10.3390/geosciences8120495
Robbins EI, Quigley-Raymond S, Lai M, Fried J. Microbial Geochemistry Reflecting Sulfur, Iron, Manganese, and Calcium Sources in the San Diego River Watershed, Southern California USA. Geosciences. 2018; 8(12):495. https://doi.org/10.3390/geosciences8120495
Chicago/Turabian StyleRobbins, Eleanora I., Shannon Quigley-Raymond, Ming Lai, and Janae Fried. 2018. "Microbial Geochemistry Reflecting Sulfur, Iron, Manganese, and Calcium Sources in the San Diego River Watershed, Southern California USA" Geosciences 8, no. 12: 495. https://doi.org/10.3390/geosciences8120495
APA StyleRobbins, E. I., Quigley-Raymond, S., Lai, M., & Fried, J. (2018). Microbial Geochemistry Reflecting Sulfur, Iron, Manganese, and Calcium Sources in the San Diego River Watershed, Southern California USA. Geosciences, 8(12), 495. https://doi.org/10.3390/geosciences8120495



