Evaporite Dissolution Rate through an on-site Experiment into Piezometric Tubes Applied to the Real Case-Study of Quinis (NE Italy)
Abstract
:1. Introduction
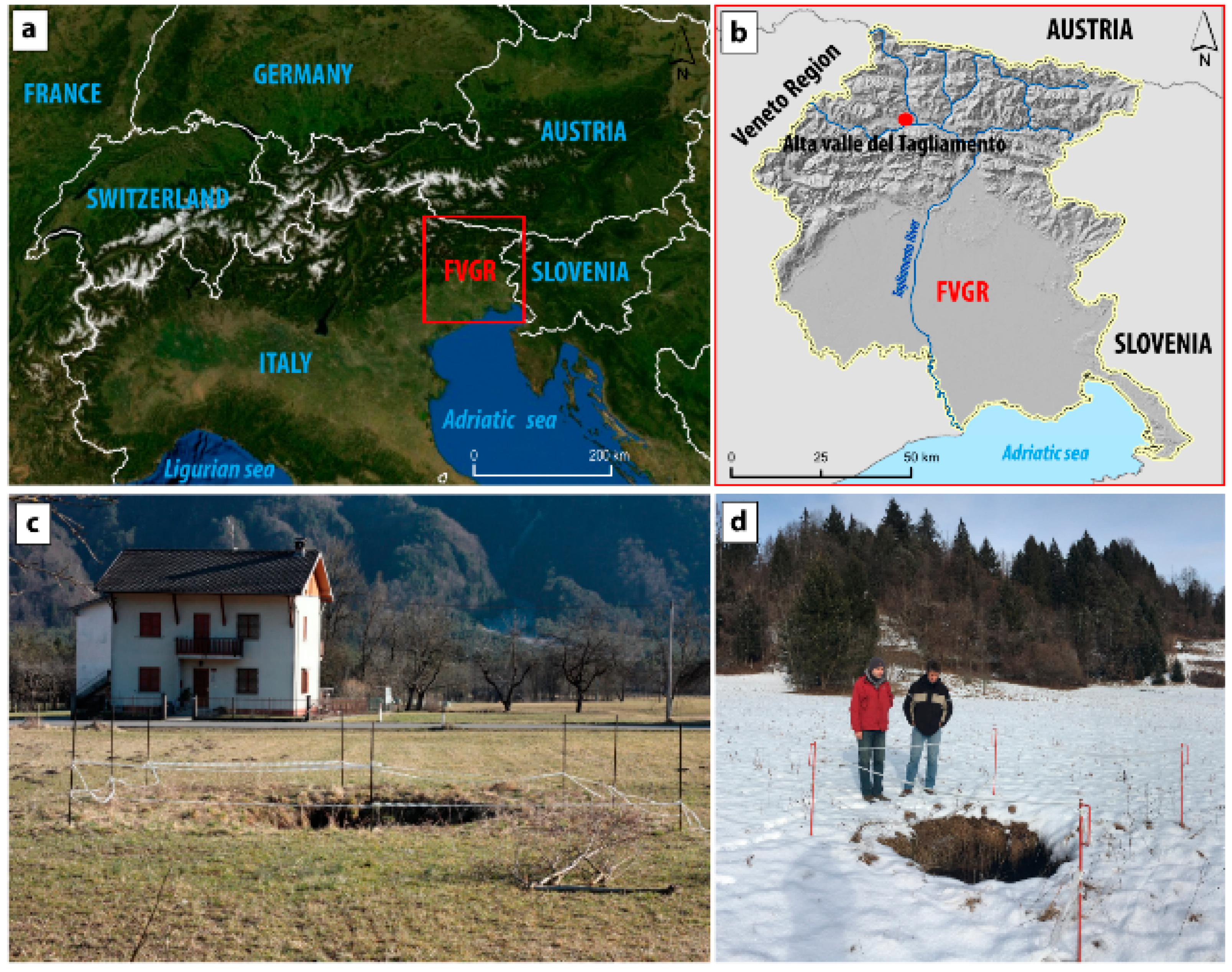
2. Study Area Characterization: Structural, Geological, Geomorphological and Hydrogeological Settings
3. Materials and Methods: Description of the Field Experiment
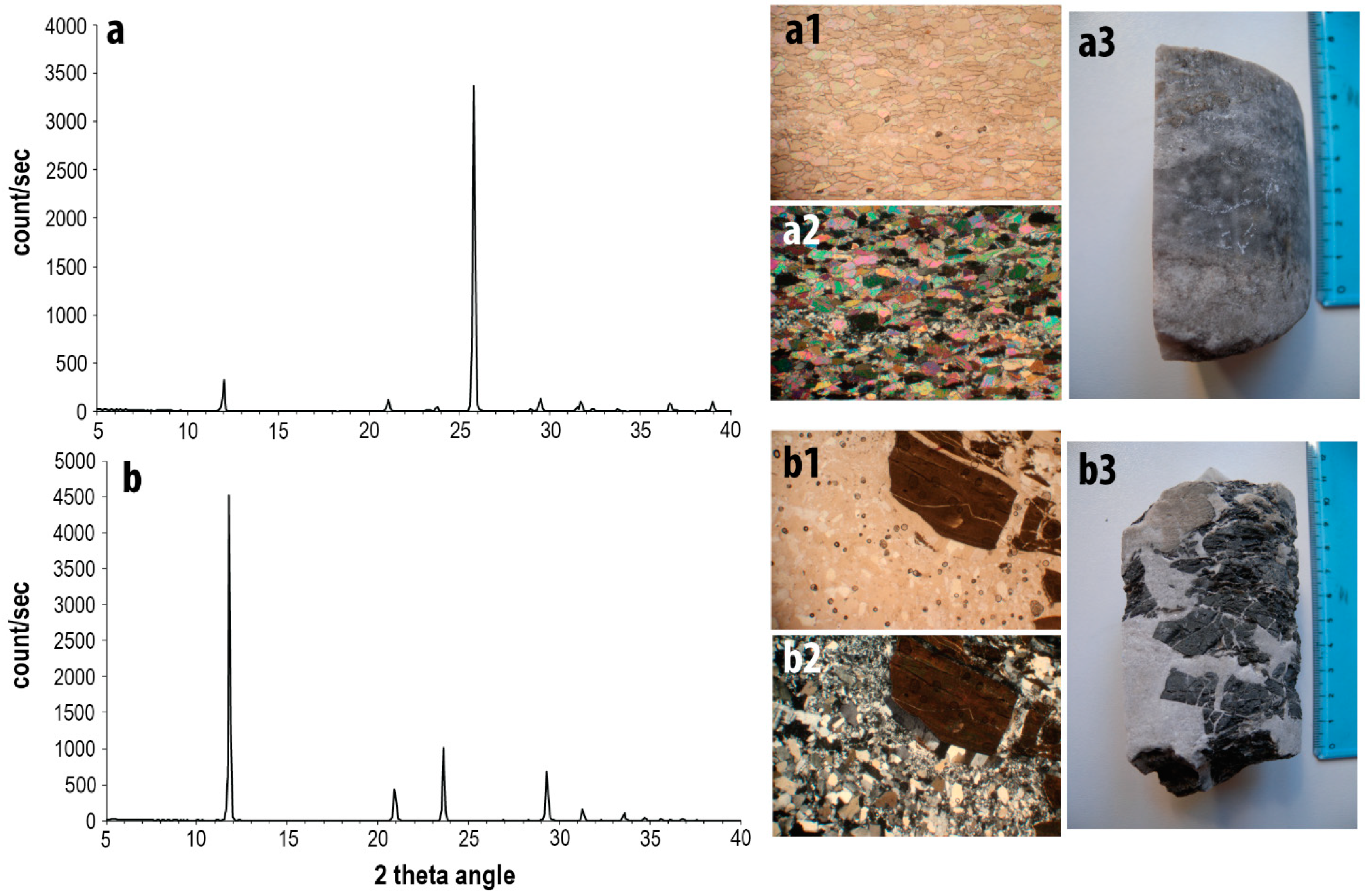
3.1. Evaporite-Rock Samples
3.2. Mineralogical Analyses
3.3. Hydrogeological Investigations: Data-Logger Measurements and Geochemical Analyses
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Beck, B.F. Soil piping and sinkhole failures. In Encyclopedia of Caves; White, W.B., Ed.; Elsevier: New York, NY, USA, 2004; pp. 523–528. [Google Scholar]
- Waltham, T.; Bell, F.; Culshaw, M. Sinkholes and Subsidence, Karst and Cavernous Rocks in Engineering and Construction; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2005. [Google Scholar]
- Gutiérrez, F.; Cooper, A.H.; Johnson, K.S. Identification, prediction and mitigation of sinkhole hazards in evaporite karst areas. Environ. Geol. 2008, 53, 1007–1022. [Google Scholar] [CrossRef]
- Gutiérrez, F.; Parise, M.; De Waele, J.; Jourde, H. A review on natural and human-induced geohazards and impacts in karst. Earth Sci. Rev. 2014, 138, 61–88. [Google Scholar] [CrossRef]
- Cooper, A.H.; Waltham, A.C. Subsidence caused by gypsum dissolution at Ripon, North Yorkshire. Q. J. Eng. Geol. 1999, 32, 305–310. [Google Scholar] [CrossRef] [Green Version]
- Parise, M. Karst geo-hazards: Causal factors and management issues. Acta Carsol. 2015, 44, 401–414. [Google Scholar] [CrossRef]
- Forti, P.; Sauro, U. The gypsum karst of Italy. Int. J. Speleol. 1996, 25, 239–250. [Google Scholar] [CrossRef]
- Sauro, U. Dolines and sinkholes: Aspects of evolution and problems of classification. Acta Carsol. 2003, 32, 41–52. [Google Scholar]
- Ford, D.C.; Williams, P.W. Karst Hydrogeology and Geomorphology; John Wiley and Sons: Chichester, UK, 2007; 562p. [Google Scholar]
- Delle Rose, M.; Federico, A.; Parise, M. Sinkhole genesis and evolution in Apulia, and their interrelations with the anthropogenic environment. Nat. Hazards Earth Syst. Sci. 2004, 4, 747–755. [Google Scholar] [CrossRef] [Green Version]
- Zini, L.; Calligaris, C.; Forte, E.; Petronio, L.; Zavagno, E.; Boccali, C.; Cucchi, F. A multidisciplinary approach in sinkhole analysis: The Quinis village case study (NE-Italy). Eng. Geol. 2015, 197, 132–144. [Google Scholar] [CrossRef]
- Vigna, B.; D’Angeli, I.M.; De Waele, J. Hydrogeological flow in gypsum areas: Some examples from northern Italy and main circulation models. Int. J. Speleol. 2017, 46, 205–217. [Google Scholar] [CrossRef]
- Lamont-Black, J.; Baker, A.; Younger, P.L.; Cooper, A.H. Utilising seasonal variations in hydrogeochemistry and excitation–emission fluorescence to develop a conceptual groundwater flowmodel with implications for subsidence hazards: An example from Co. Durham, UK. Environ. Geol. 2005, 48, 320–335. [Google Scholar] [CrossRef]
- Cooper, A.H.; Calow, R. Gypsum Geohazards: Their Impact on Development-Project; Technical Report, WC/97/17; British Geological Survey: Nottingham, UK, 1997. [Google Scholar]
- Wanfang, Z. The formation of sinkholes in karst mining areas in China and some methods of prevention. Environ. Geol. 1997, 31, 50–58. [Google Scholar] [CrossRef]
- Cucchi, F.; Forti, P.; Finocchiaro, F. Gypsum degradation in Italy with respect to climatic textural and erosional conditions. Geogr. Fis. Din. Quat. 1998, 3, 41–49. [Google Scholar]
- Furlani, S.; Cucchi, F.; Forti, F.; Rossi, A. Comparison between coastal and inland karst limestone lowering rates in the northeastern Adriatic Region (Italy and Croatia). Geomorphology 2009, 104, 73–81. [Google Scholar] [CrossRef]
- Klimchouk, A.; Cucchi, F.; Calaforra, J.M.; Aksem, S.; Finocchiaro, F.; Forti, P. Dissolution of gypsum from field observations. Int. J. Speleol. 1996, 25, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.H.; Calow, R.C. Avoiding Gypsum Geohazards: Guidance for Planning and Construction; Technical Report, WC/98/5; British Geological Survey: Nottingham, UK, 1998. [Google Scholar]
- Cooper, A.H. Gypsum dissolution geohazards at Ripon, North Yorkshire, UK. In Engineering Geology for Tomorrow’s Cities; The Geological Society of London: London, UK, 2006; pp. 1–14. [Google Scholar]
- Benson, R.C.; Kaufmann, R.D. Characterization of a highway sinkhole within the gypsum karst of Michigan. In Geotechnical and Environmental Applications of Karst Geology and Hydrology; Beck, B.F., Herring, J.G., Eds.; Balkema: Lisse, The Netherlands, 2001; pp. 103–112. [Google Scholar]
- Gutiérrez, F.; Cooper, A.H. Evaporite dissolution subsidence in the historical city of Calatayud, Spain: Damage appraisal and prevention. Nat. Hazards 2002, 25, 259–288. [Google Scholar] [CrossRef]
- Guerrero, J.; Gutiérrez, F.; Lucha, P. Paleosubsidence and active subsidence due to evaporite dissolution in Zaragoza city area (Huerva River valley, NE Spain). Processes, spatial distribution and protection measures for linear infrastructures. Eng. Geol. 2004, 72, 309–329. [Google Scholar] [CrossRef]
- Galve, J.P.; Gutiérrez, F.; Lucha, P.; Bonachea, J.; Remondo, J.; Cendrero, J.; Gutiérrez, M.; Gimeno, M.J.; Pardo, G.; Sánchez, J.A. Sinkholes in the salt-bearing evaporitic karst of the Ebro River valley upstream of Zaragoza city (NE Spain). Geomorphological mapping and analysis as a basis for risk management. Geomorphology 2009, 108, 145–158. [Google Scholar] [CrossRef]
- Iovine, G.; Parise, M.; Trocino, A. Breakdownmechanisms in gypsum caves of southern Italy, and the related effects at the surface. Z. Geomorphol. 2001, 54 (Suppl. 2), 153–178. [Google Scholar] [CrossRef]
- Calligaris, C.; Devoto, S.; Galve, J.P.; Zini, L.; Pérez-Peña, J.V. Integration of multi-criteria and nearest neighbour analysis with kernel density functions for improving sinkhole susceptibility models: The case study of Enemonzo (NE Italy). Int. J. Speleol. 2017, 42, 191–204. [Google Scholar] [CrossRef]
- Stawski, T.M.; van Driessche, A.E.; Ossorio, M. Formation of calcium sulfate through the aggregation of sub-3 nanometre primary species. Nat. Commun. 2016, 7, 11177. [Google Scholar] [CrossRef]
- Warren, J.K. Evaporites: Sediments, Resources and Hydrocarbons; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Zhu, X.M. Sedimentary Petrology; Petroleum Industry Press: Beijing, China, 2008. [Google Scholar]
- De Waele, J.; Piccini, L.; Columbu, A.; Madonia, G.; Vattano, M.; Calligaris, C.; D’Angeli, I.M.; Parise, M.; Chiesi, M.; Sivelli, M.; et al. Evaporite karst in Italy: A review. Int. J. Speleol. 2017, 46, 137–168. [Google Scholar] [CrossRef]
- Beydoun, Z.R. Arabian plate oil and gas: Why so rich and so prolific? Episodes 1998, 21, 74–81. [Google Scholar]
- Chen, Z.; Osadetz, K.G.; Li, M. Spatial characteristics of Middle Devonian oils and non-associated gases in the Rainbow area, northwest Alberta. Mar. Pet. Geol. 2005, 22, 391–401. [Google Scholar] [CrossRef]
- Klimchouk, A. The dissolution and conversion of gypsum and anhydrite. Int. J. Speleol. 1996, 25, 21–36. [Google Scholar] [CrossRef]
- Cucchi, F.; Forti, P. Dissoluzione sottocutanea nei gessi: Analisi e considerazioni. Atti Congresso Naz. Speleol. 1993, 1, 89–100. [Google Scholar]
- Cucchi, F.; Forti, P.; Ulcigrai, F. Znizevanje krskega povrsja zaradi korozije (Valori di abbassamento per dissoluzione di superfici carsiche). Acta Carsol. 1994, 23, 55–61. [Google Scholar]
- Dreybrodt, W.; Eisenlohr, L. Limestones dissolution rates in karst environments. In Speleogenesis: Evolutions in Karst Aquifers; Klimchouk, A.B., Ford, D.C., Palmer, A.N., Dreybrodt, W., Eds.; National Speleological Society: Huntsville, AL, USA, 2000; pp. 136–148. [Google Scholar]
- Jeschke, A.; Vosbeck, K.; Dreybrodt, W. Surface controlled dissolution rates of gypsum in aqueous solutions exhibit nonlinear dissolution kinetics. Geochim. Cosmochim. Acta 2001, 65, 27–34. [Google Scholar] [CrossRef]
- Gutiérrez, F. Sinkhole Hazards; Oxfrod Research Encyclopedia of Natural Hazard Science; Oxford University Press: Oxford, UK, 2016; pp. 1–92. [Google Scholar]
- Galdenzi, S. Corrosion of limestone tablets in sulfidic ground-water: Measurements and speleogenetic implications. Int. J. Speleol. 2012, 41, 149–159. [Google Scholar] [CrossRef]
- Cucchi, F.; Forti, F.; Marinetti, E. Surface degradation of carbonate rocks in the karst of Trieste (Classical Karst, Italy). In Karren Landforms; Fornós, J.-J., Ginés, A., Eds.; Universitat de les Illes Balears: Palma de Mallorca, Spain, 1996; pp. 41–51. [Google Scholar]
- Stephenson, W.J.; Finlayson, B.L. Measuring erosion with the micro-erosion meter—Contributions to understanding landform evolution. Earth Sci. Rev. 2009, 95, 53–62. [Google Scholar] [CrossRef]
- Sanna, L.; De Waele, J.; Calaforra, J.M.; Forti, P. Long-term erosion rate measurements in gypsum caves of Sorbas (SE Spain) by the Micro-Erosion Meter method. Geomorphology 2015, 228, 213–225. [Google Scholar] [CrossRef]
- Trudgill, S. Measurements of erosional weight loss of rock tablets. Br. Geomorphol. Res. Group Tech. Bull. 1975, 17, 13–19. [Google Scholar]
- Crowther, J. A comparison of the rock tablet andwater hardnessmethods for determining chemical erosion rates on karst surfaces. Annu. Geomorphol. 1983, 27, 55–64. [Google Scholar]
- Gams, I. International comparative measurements of surface solution by means of standard limestone tablets. Zb. Ivana Rakovca 1986, 26, 361–386. [Google Scholar]
- Tarhule-Lips, R.F.A.; Ford, D.C. Condensation corrosion in caves on Cayman Brac and Isla de Mona. J. Caves Karst Stud. 1998, 60, 84–95. [Google Scholar]
- Plan, L. Factors controlling carbonate dissolution rates quantified in a field test in the Austrian alps. Geomorphology 2005, 68, 201–212. [Google Scholar] [CrossRef]
- Gabrovšek, F. On concepts andmethods for the estimation of dissolutional denudation rates in karst areas. Geomorphology 2009, 106, 9–14. [Google Scholar] [CrossRef]
- Calligaris, C.; Devoto, S.; Zini, L. Evaporite sinkholes of the Friuli Venezia Giulia region (NE Italy). J. Maps 2017, 13, 406–414. [Google Scholar] [CrossRef]
- Venturini, C. Evoluzione Geologica Delle Alpi Carniche, un Viaggio Attraverso il Tempo; Edizioni del Museo Friulano di Storia Naturale: Comune di Udine, Italy, 2006; Volume 48, p. 208. [Google Scholar]
- Venturini, C.; Delzotto, S.; Pondrelli, M.; Fontana, C.; Pondrelli, M.; Longo Salvador, G.; Carulli, G.B.; Pisa, G.F. 031 Ampezzo (Carta Geologica d’Italia Alla Scala 1:50.000); Servizio Geologico Nazionale: Ispra, Italy, 2010. [Google Scholar]
- Carulli, G.B. Carta Geologica del Friuli Venezia Giulia, Scala 1:150000; Direzione Centrale Ambiente e Lavori Pubblici, Servizio Geologico: Firenze, Italy, 2006. [Google Scholar]
- Zini, L.; Calligaris, C.; Devoto, S.; Zavagno, E.; Forte, E.; Petronio, L.; Boccali, C.; Cucchi, F. Fenomeni di sprofondamento nella piana di Enemonzo (UD). Mem. Descr. Carta Geol. D’Italia 2015, XCIX, 101–110. [Google Scholar]
- Marinelli, O. Fenomeni di tipo carsico nei terrazzi alluvionali della Valle del Tagliamento. Mem. Della Soc. Geogr. Ital. 1898, 8, 415–419. [Google Scholar]
- Gortani, M. Le doline alluvionali. Nat. Mont. 1965, 3, 120–128. [Google Scholar]
- Cameron, F.K.; Breazeale, J.F. Solubility of calcium sulfate in aqueous solutions of sulphuric acid. J. Phys. Chem. 1903, 7, 571–577. [Google Scholar] [CrossRef]
- Marshall, W.L.; Jones, E.V. Second dissociation constant of sulfuric acid from 25 to 350 °C evaluated from solubilities of calcium sulfate in sulfuric acid solutions. J. Phys. Chem. 1966, 70, 4028–4040. [Google Scholar] [CrossRef]
- Ling, Y.; Demopoulos, G.P. Solubility of calcium sulfate hydrates in (0 to 3.5) mol·kg−1 sulfuric acid solutions at 100 °C. J. Chem. Eng. Data 2004, 49, 1263–1268. [Google Scholar] [CrossRef]
- Braitsch, O. The temperature of evaporite formation. In Problems in Paleoclimatology; Nairn, A.E.M., Ed.; Wiley: New York, NY, USA, 1964; pp. 479–490. [Google Scholar]
- Dutrizac, J.E. Calcium sulfate solubilities in simulated zinc processing solutions. Hydrometallurgy 2002, 65, 109–135. [Google Scholar] [CrossRef]
- Lindberg, R.D.; Runnels, D.D. Ground water redox reactions: An analysis of equilibrium state applied to Eh measurements and geochemical modeling. Science 1984, 225, 925–927. [Google Scholar] [CrossRef] [PubMed]
- Huggenberger, P.; Zidane, A.; Zechner, E.; Gechter, D. The role of tectonic structures and density-driven groundwater flow for salt karst formation. In Engeneering Geology for Society and Territory; Lollino, G., Manconi, A., Guzzetti, F., Culshaw, M., Bobrowsky, P., Luino, F., Eds.; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Jenks, W.F. Origins of some massive pyritic ore deposits of Western Europe. Econ. Geol. 1994, 70, 488–498. [Google Scholar] [CrossRef]
- Petrini, R.; Slejko, F.; Lutman, A.; Pison, S.; Franceschini, G.; Zini, L.; Italiano, F.; Galic, A. Natural arsenic contamination in waters from the Pesariis village, NE Italy. Environ. Earth Sci. 2011, 62, 481–491. [Google Scholar] [CrossRef]
- Appelo, C.A.J.; Postma, D. Geochemistry, Groundwater and Pollution; A.A. Balkema Publishers: Avereest, The Netherlands, 2005. [Google Scholar]
- Piper, A.M. A graphic procedure in the geochemical interpretation of water-analyses. Am. Geophys. Union 1944, 25, 914–928. [Google Scholar] [CrossRef]
- Bock, E. On the solubility of anhydrous calcium sulphate and gypsum in concentrated solutions of sodium chloride at 25 °C, 30 °C, 40 °C and 50 °C. Can. J. Chem. 1961, 39, 1746–1751. [Google Scholar] [CrossRef]
- Gobran, G.R.; Miyamoto, S. Dissolution rate of gypsum in aqueous salt solutions. Soil Sci. 1985, 40, 89–93. [Google Scholar] [CrossRef]
- Blount, C.W.; Dickson, F.W. The solubility of anhydrite (CaSO4) in NaCl-H2O from 100 to 450 °C and 1 to 1000 bars. Geochim. Cosmochim. Acta 1969, 33, 227–245. [Google Scholar] [CrossRef]
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium brines: A global perspective. Rev. Econ. Geol. 2016, 18, 339–365. [Google Scholar]
- Carlson, E.H. Celestite replacements of evaporates in Salina Group. Sediment. Geol. 1987, 54, 93–112. [Google Scholar] [CrossRef]
- West, I.M. Vanished evaporates; significance of strontium minerals. J. Sediment. Petrol. 1973, 43, 278–279. [Google Scholar]
- Taberner, C.; Marshall, J.D.; Hendry, J.P.; Pierre, C.; Thirlwall, M.F. Celestite formation, Bactyerial sulphate reduction and carbonate cementation in Eocene reefs and basinal sediments (Igualada, NE Spain). Sedimentology 2002, 49, 171–190. [Google Scholar] [CrossRef]
- Hanor, J.S. A model for the origin of large carbonate and evaporite hosted Celestine (SrSO4) deposits. J. Sediment. Res. 2004, 74, 168–175. [Google Scholar] [CrossRef]










| ID | Sample Depth [b.g.l.] | Weight A [g] | Weight B [g] | Weight Loss [g] | % Weight Loss | Density [g/cm3] | Surface [cm2] | Days of Immersion | % Time of Immersion | R = Dissolution Rate [mm/yr] |
|---|---|---|---|---|---|---|---|---|---|---|
| PZ3/1 | 2.0 | 209.00 | 185.50 | 23.50 | 11.2 | 2.32 | 136.2 | 0 | 0 | 0.74 |
| PZ3/2 | 10.0 | 158.60 | 50.20 | 108.40 | 68.3 | 2.64 | 97.2 | 43 | 10 | 4.22 |
| PZ3/3 | 14.3 | 234.30 | 0.00 | 234.30 | 100.0 | 2.39 | 146.6 | 122 | 30 | 6.68 |
| PZ3/4 | 19.7 | 254.20 | 38.50 | 215.70 | 84.9 | 2.54 | 149.2 | 410 | 100 | 5.68 |
| PZ3/5 | 19.7 | 251.20 | 5.50 | 245.70 | 97.8 | 2.28 | 162.2 | 410 | 100 | 6.63 |
| PZ7/1 | 2.0 | 736.50 | 727.90 | 8.60 | 1.2 | 2.73 | 284.83 | 0 | 0 | 0.11 |
| PZ7/2 | 9.8 | 609.60 | 523.60 | 86.00 | 14.1 | 2.39 | 271.19 | 34 | 8 | 1.33 |
| PZ7/3 | 19.8 | 621.50 | 293.90 | 327.60 | 52.7 | 2.39 | 294.55 | 85 | 21 | 4.64 |
| PZ7/4 | 37.3 | 1521.80 | 426.50 | 1095.30 | 72.0 | 2.32 | 693.75 | 410 | 100 | 6.79 |
| PZ7/5 | 37.3 | 1486.90 | 432.60 | 1054.30 | 70.9 | 2.29 | 688.68 | 410 | 100 | 6.68 |
| PZ8/1 | 2.0 | 670.00 | 669.10 | 0.9 | 0.1 | 2.68 | 263.76 | 0 | 0 | 0.01 |
| PZ8/2 | 10.0 | 743.90 | 680.30 | 63.6 | 0.0 | 2.66 | 343.45 | 17 | 4 | 0.69 |
| PZ8/3 | 20.0 | 617.00 | 30.40 | 586.6 | 95.1 | 2.47 | 309.35 | 60 | 15 | 7.67 |
| PZ8/4 | 30.0 | 654.30 | 0.00 | 654.3 | 100.0 | 2.42 | 332.08 | 250 | 61 | 8.12 |
| PZ8/5 | 35.5 | 1621.20 | 952.30 | 668.9 | 41.3 | 2.32 | 820.97 | 410 | 100 | 3.52 |
| PZ8/6 | 35.5 | 1219.70 | 772.80 | 446.9 | 36.6 | 2.28 | 633.37 | 410 | 100 | 3.08 |
| PZ16/1 | 2.0 | 294.20 | 294.10 | 0.10 | 0.0 | 2.45 | 147.83 | 0 | 0 | 0.00 |
| PZ16/2 | 9.4 | 394.10 | 392.40 | 1.70 | 0.4 | 2.46 | 183.50 | 205 | 50 | 0.03 |
| PZ16/3 | 14.8 | 388.00 | 387.10 | 0.90 | 0.2 | 2.28 | 192.43 | 410 | 100 | 0.02 |
| PZ16/4 | 17.9 | 740.90 | 740.90 | 0.00 | 0.0 | 2.39 | 317.27 | 410 | 100 | 0.00 |
| PZ16/5 | 17.9 | 927.90 | 926.60 | 1.30 | 0.1 | 2.32 | 397.52 | 410 | 100 | 0.01 |
| PZ22/1 | 2 (A) | 225.40 | 225.00 | 0 | 0.0 | 2.50 | 127.44 | 0 | 0 | 0.01 |
| 2 (B) | 138.30 | 138.30 | 0 | 0.0 | 2.38 | 94.59 | 0 | 0 | 0.00 | |
| PZ22/2 | 9.8 | 348.40 | 319.90 | 28.5 | 8.2 | 2.21 | 224.52 | 17 | 4 | 0.57 |
| PZ22/3 | 14.8 (A) | 239.90 | 212.40 | 27.50 | 11.5 | 2.40 | 149.17 | 60 | 15 | 0.77 |
| 14.8 (B) | 145.70 | 111.80 | 33.90 | 23.3 | 2.43 | 97.18 | 60 | 15 | 1.43 | |
| PZ22/4 | 19.7 | 432.50 | 308.90 | 123.6 | 28.6 | 2.28 | 266.11 | 118 | 29 | 2.04 |
| PZ22/5 | 29.2 | 325.90 | 253.40 | 72.5 | 22.2 | 3.10 | 155.66 | 410 | 100 | 1.50 |
| PZ22/6 | 29.2 | 378.40 | 288.00 | 90.4 | 23.9 | 2.91 | 188.15 | 410 | 100 | 1.65 |
| PZ22/7 | 49.2 | 374.80 | 350.90 | 23.9 | 6.4 | 2.88 | 188.15 | 410 | 100 | 0.44 |
| PZ22/8 | 59.1 | 355.00 | 353.30 | 1.7 | 0.5 | 2.84 | 181.64 | 410 | 100 | 0.03 |
| PZ23/1 | 2.0 | 358.70 | 178.50 | 180.2 | 50.2 | 2.24 | 227.13 | 0 | 0 | 3.54 |
| PZ23/2 | 5.0 | 377.30 | 246.20 | 131.1 | 34.7 | 2.43 | 220.63 | 14 | 3 | 2.43 |
| PZ23/3 | 5.0 | 381.50 | 276.80 | 104.7 | 27.4 | 2.38 | 227.13 | 14 | 3 | 1.93 |
| PZ23/4 | 10.0 | 546.10 | 384.60 | 161.5 | 29.6 | 2.76 | 276.51 | 112 | 27 | 2.12 |
| PZ23/5 | 15.0 | 588.00 | 240.00 | 348 | 59.2 | 2.73 | 298.59 | 410 | 100 | 4.25 |
| PZ23/6 | 24.6 | 489.70 | 360.40 | 129.3 | 26.4 | 2.80 | 246.61 | 410 | 100 | 1.86 |
| PZ23/7 | 34.5 | 603.50 | 531.80 | 71.7 | 11.9 | 2.74 | 305.08 | 410 | 100 | 0.85 |
| PZ25/1 | 3.1 | 247.10 | 246.90 | 0.20 | 0.1 | 2.29 | 137.14 | 0 | 0 | 0.00 |
| PZ25/2 | 10.2 | 356.60 | 354.60 | 2.00 | 0.6 | 2.38 | 174.58 | 0 | 0 | 0.05 |
| PZ25/3 | 13.1 | 337.10 | 336.40 | 0.70 | 0.2 | 2.41 | 165.67 | 18 | 4 | 0.02 |
| PZ25/4 | 17.1 | 852.30 | 851.80 | 0.50 | 0.1 | 2.40 | 357.39 | 410 | 100 | 0.00 |
| PZ25/5 | 17.1 | 886.10 | 885.50 | 0.60 | 0.1 | 2.36 | 357.22 | 410 | 100 | 0.00 |
| Sample | Water-Type * | T | EC | TDS | pH | Eh | SIgy ** | SIcal ** | SIcel ** | Na+ | K+ | Mg2+ | Ca2+ | Cl− | NO3− | SO42− | HCO3− | Ion Balance |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (C°) | (mS/cm) | (mg/L) | (V) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | (mg/L) | |||||||
| PZ3/5 | Ca-SO4 | 11.0 | 0.95 | 629 | 7.82 | 0.22 | −1.18 | 0.67 | −1.16 | 4.4 | 4.1 | 21 | 145 | 5.6 | 5.7 | 236 | 226 | 2.3 |
| PZ7/5 | Ca-SO4 | 12.2 | 2.38 | 2061 | 7.55 | −0.07 | −0.25 | 0.81 | −0.41 | 7.7 | 5.8 | 51 | 582 | 9.7 | 9.2 | 1208 | 236 | 6.6 |
| PZ8/6 | Ca-SO4 | 12.1 | 2.36 | 2261 | 7.63 | 0.26 | −0.17 | 0.78 | −0.38 | 9 | 4.5 | 39 | 619 | 8.9 | 1440 | 185 | 2.0 | |
| PZ10/1 | Ca-SO4 | 12.1 | 1.83 | 1467 | 7.85 | 0.18 | −0.43 | 0.94 | −0.54 | 8.4 | 3.6 | 26 | 432 | 8.4 | 6.9 | 829 | 186 | 7.9 |
| PZ10/2 | Ca-SO4 | 11.7 | 3.35 | 2504 | 7.88 | −0.08 | −0.31 | 1.07 | −0.40 | 274 | 11 | 43 | 519 | 258 | 1211 | 238 | 6.7 | |
| PZ16/3 | Ca-SO4 | 13.1 | 2.69 | 2586 | 7.79 | 0.18 | −0.12 | 0.87 | −0.36 | 88 | 8.7 | 25 | 644 | 66 | 1650 | 154 | −0.7 | |
| PZ16/4 | Na-Cl | 13.0 | 9.37 | 6173 | 7.71 | −0.06 | −0.22 | 0.94 | −0.43 | 1407 | 10.1 | 34 | 808 | 2150 | 1668 | 232 | 2.7 | |
| PZ22/6 | Ca-SO4 | 11.9 | 2.20 | 1878 | 7.93 | −0.11 | −0.26 | 1.08 | −0.72 | 11 | 5.2 | 18 | 567 | 12 | 2.3 | 1123 | 181 | 6.4 |
| PZ22/7 | Ca-SO4 | 11.7 | 2.33 | 2081 | 7.96 | −0.12 | −0.22 | 1.16 | −0.65 | 26 | 5.7 | 19 | 580 | 23 | 1260 | 211 | 2.3 | |
| PZ22/8 | Na-Cl | 11.5 | 18.41 | 11935 | 8.01 | −0.08 | −0.26 | 1.00 | −0.18 | 3577 | 54 | 75 | 766 | 4997 | 2559 | 217 | 0.9 | |
| PZ23/5 | Ca-HCO3 | 11.8 | 0.22 | 166 | 8.01 | 0.17 | −2.73 | 0.02 | −3.49 | 8.4 | 4.4 | 1 | 31 | 12 | 15 | 98 | −2.8 | |
| PZ23/7 | Ca-SO4 | 11.8 | 1.73 | 1446 | 7.81 | 0.18 | −0.4 | 0.89 | −0.75 | 12 | 5.2 | 14 | 413 | 12 | 5.5 | 828 | 191 | 3.8 |
| PZ24/1 | Ca-HCO3 | 12.4 | 1.42 | 951 | 8.12 | 0.16 | −1.27 | 1.16 | −1.17 | 115 | 6.7 | 24 | 175 | 162 | 9.8 | 186 | 299 | 8.0 |
| PZ24/2 | Na-Cl | 12.0 | 33.70 | 21485 | 8 | −0.09 | −0.39 | 0.91 | −0.35 | 7273 | 99 | 226 | 661 | 9847 | 3740 | 301 | 1.4 | |
| PZ24/3 | Na-Cl | 12.4 | 52.30 | 36660 | 8.05 | −0.15 | −0.16 | 1.12 | −0.29 | 12919 | 188 | 388 | 1080 | 16237 | 6840 | 390 | 3.6 | |
| PZ25/3 | Na-SO4 | 12.6 | 5.20 | 4306 | 7.94 | −0.05 | −0.13 | 1.17 | −0.12 | 635 | 34 | 83 | 563 | 273 | 2490 | 320 | −1.0 | |
| PZ25/4 | Na-SO4 | 12.8 | 7.77 | 5988 | 8.18 | −0.09 | −0.16 | 1.25 | −0.27 | 1455 | 44 | 67 | 494 | 575 | 3191 | 297 | 3.9 | |
| PZ25/6 | Na-SO4 | 13.1 | 6.79 | 5213 | 8.24 | −0.07 | −0.19 | 1.39 | −0.26 | 1117 | 41 | 82 | 499 | 562 | 2702 | 323 | 2.4 |
| Sample | Li | Mn | Co | Ni | Sr | Mo | Sb | Ba | Tl | Pb | U | V | Cr | Fe | As |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PZ3/5 | 11.7 | 0.91 | 0.36 | 7.8 | 3270 | 2.29 | 0.26 | 43 | <0.2 | 2.31 | 0.43 | <0.1 | <0.3 | 86 | <0.2 |
| PZ7/5 | 15.8 | 719 | 1.94 | 32.2 | 8771 | <1 | 0.31 | 59 | <0.6 | <2 | 0.97 | 1.68 | <1 | 983 | 15.3 |
| PZ8/6 | 17 | 19.7 | 1.78 | 32.5 | 8263 | 1.96 | 0.72 | 15.8 | <0.6 | 6.4 | 1.12 | 0.9 | <1 | 644 | <0.6 |
| PZ10/1 | 13.6 | 3.4 | 0.88 | 24.5 | 7430 | 3.8 | 0.32 | 57 | <0.3 | 2.68 | 0.73 | <0.2 | 2.96 | 218 | <0.3 |
| PZ10/2 | 148 | 81 | 1.6 | 26.8 | 9066 | <1 | <0.6 | 26.9 | <0.6 | <2 | 0.33 | <0.4 | <1 | 331 | <0.6 |
| PZ16/3 | 40 | 4.8 | 1.57 | 35 | 8130 | 17.1 | 1.49 | 25 | <0.6 | 2.47 | 2.61 | <0.4 | <1 | 327 | 0.67 |
| PZ16/4 | 27 | 1977 | 4 | 44 | 10156 | 10.1 | <1 | 47 | <1 | <4 | 5.4 | 1.7 | <2 | 822 | 2.9 |
| PZ22/6 | 15.5 | 25.3 | 1.37 | 34.3 | 4210 | <1 | 2.14 | 72 | <0.6 | <2 | 0.41 | <0.4 | <1 | 359 | <0.6 |
| PZ22/7 | 21 | 26.3 | 1.29 | 31.6 | 4693 | <1 | 1.17 | 60 | <0.6 | <2 | 0.5 | <0.4 | <1 | 594 | <0.6 |
| PZ22/8 | 564 | 1007 | 1.78 | 42 | 15397 | <4 | <2 | 41 | <2 | <8 | <0.2 | 3.6 | <4 | 1870 | <2 |
| PZ23/5 | 3.7 | 76 | 0.55 | 2 | 119 | 0.95 | 5.9 | 19.5 | <0.05 | 7.19 | 0.07 | 1.16 | 0.55 | 109 | 0.49 |
| PZ23/7 | 10.9 | 35 | 1.45 | 25.7 | 4369 | 4.6 | 2.78 | 28.1 | <0.3 | 6.3 | 0.62 | 0.17 | <0.6 | 248 | <0.3 |
| PZ24/1 | 55 | 34 | 1.06 | 39 | 4755 | 3.6 | 1.23 | 123 | <0.3 | 4.2 | 0.53 | 0.73 | 25.6 | 407 | 0.57 |
| PZ24/2 | 2686 | 542 | 1.56 | <40 | 12390 | <9 | <5 | <70 | <5 | <20 | <0.4 | 7.5 | <10 | 734 | <5 |
| PZ24/3 | 5322 | 461 | 5.8 | 420 | 12651 | <18 | <10 | <140 | <10 | <40 | <0.8 | 14.1 | <20 | 1491 | 32.4 |
| PZ25/3 | 453 | 38 | 1.24 | 30.9 | 9855 | <2 | <1 | <15 | <1 | 7.3 | 2.84 | <0.8 | <2 | 462 | <1 |
| PZ25/4 | 846 | 28.3 | 1.04 | 24.1 | 8356 | <2 | <1 | <15 | <1 | <4 | 0.99 | <0.8 | <2 | 327 | <1 |
| PZ25/6 | 714 | 72 | 1.93 | 29.8 | 9000 | 8.1 | <1 | <15 | <1 | <4 | 12.4 | 2.3 | <2 | 469 | 3.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calligaris, C.; Ghezzi, L.; Petrini, R.; Lenaz, D.; Zini, L. Evaporite Dissolution Rate through an on-site Experiment into Piezometric Tubes Applied to the Real Case-Study of Quinis (NE Italy). Geosciences 2019, 9, 298. https://doi.org/10.3390/geosciences9070298
Calligaris C, Ghezzi L, Petrini R, Lenaz D, Zini L. Evaporite Dissolution Rate through an on-site Experiment into Piezometric Tubes Applied to the Real Case-Study of Quinis (NE Italy). Geosciences. 2019; 9(7):298. https://doi.org/10.3390/geosciences9070298
Chicago/Turabian StyleCalligaris, Chiara, Lisa Ghezzi, Riccardo Petrini, Davide Lenaz, and Luca Zini. 2019. "Evaporite Dissolution Rate through an on-site Experiment into Piezometric Tubes Applied to the Real Case-Study of Quinis (NE Italy)" Geosciences 9, no. 7: 298. https://doi.org/10.3390/geosciences9070298







