Chemo-Mechanical Interactions in the Ettringite Induced Expansion of Sulfate-Bearing Soils
Abstract
:1. Introduction
2. Underlying Mechanisms of Ettringite Formation and Induced Expansion
3. Geochemical Reactions for Ettringite Formation
4. Expansion Resulting from Crystal Growth
4.1. General Concepts and Mathematical Formulations
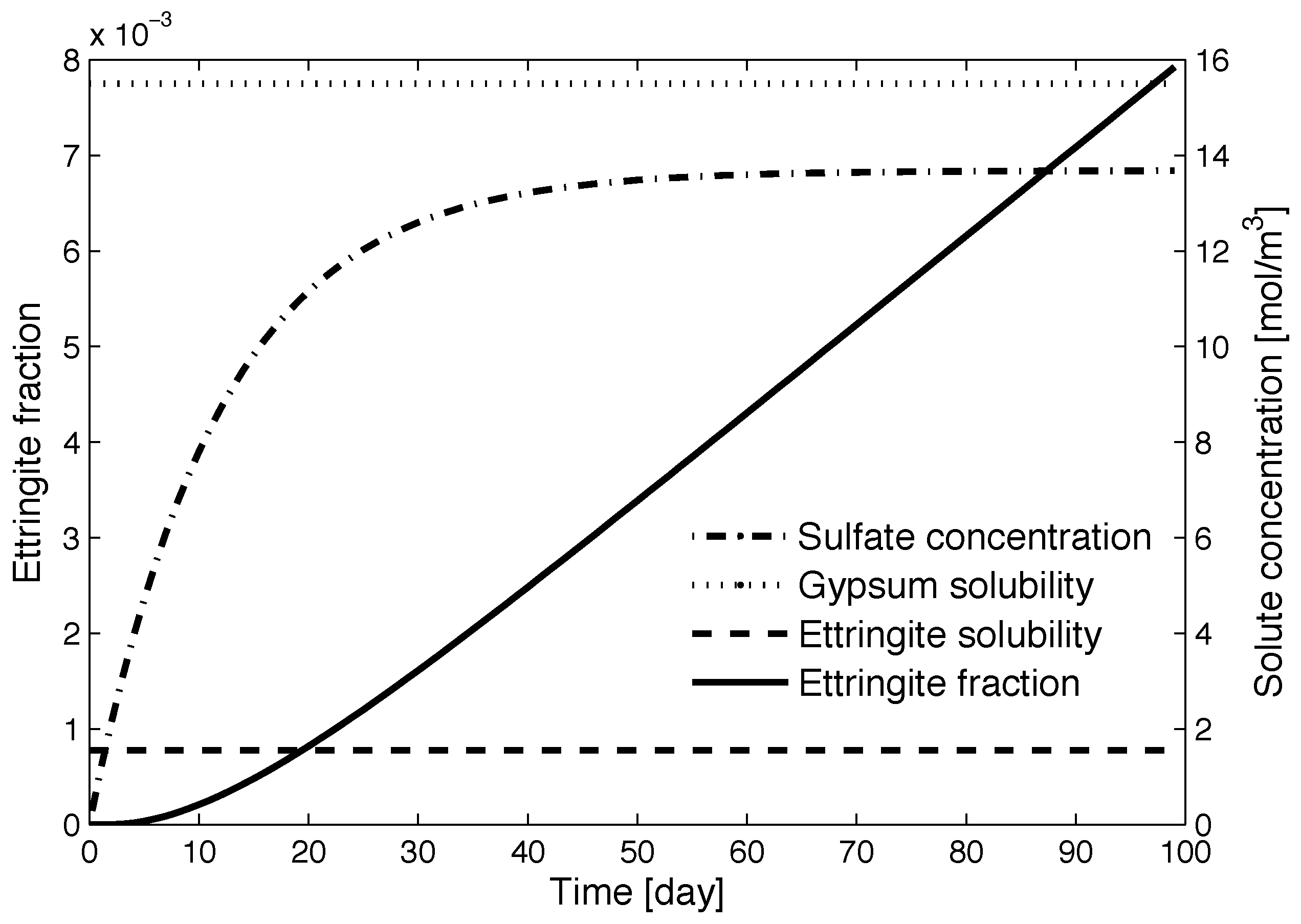
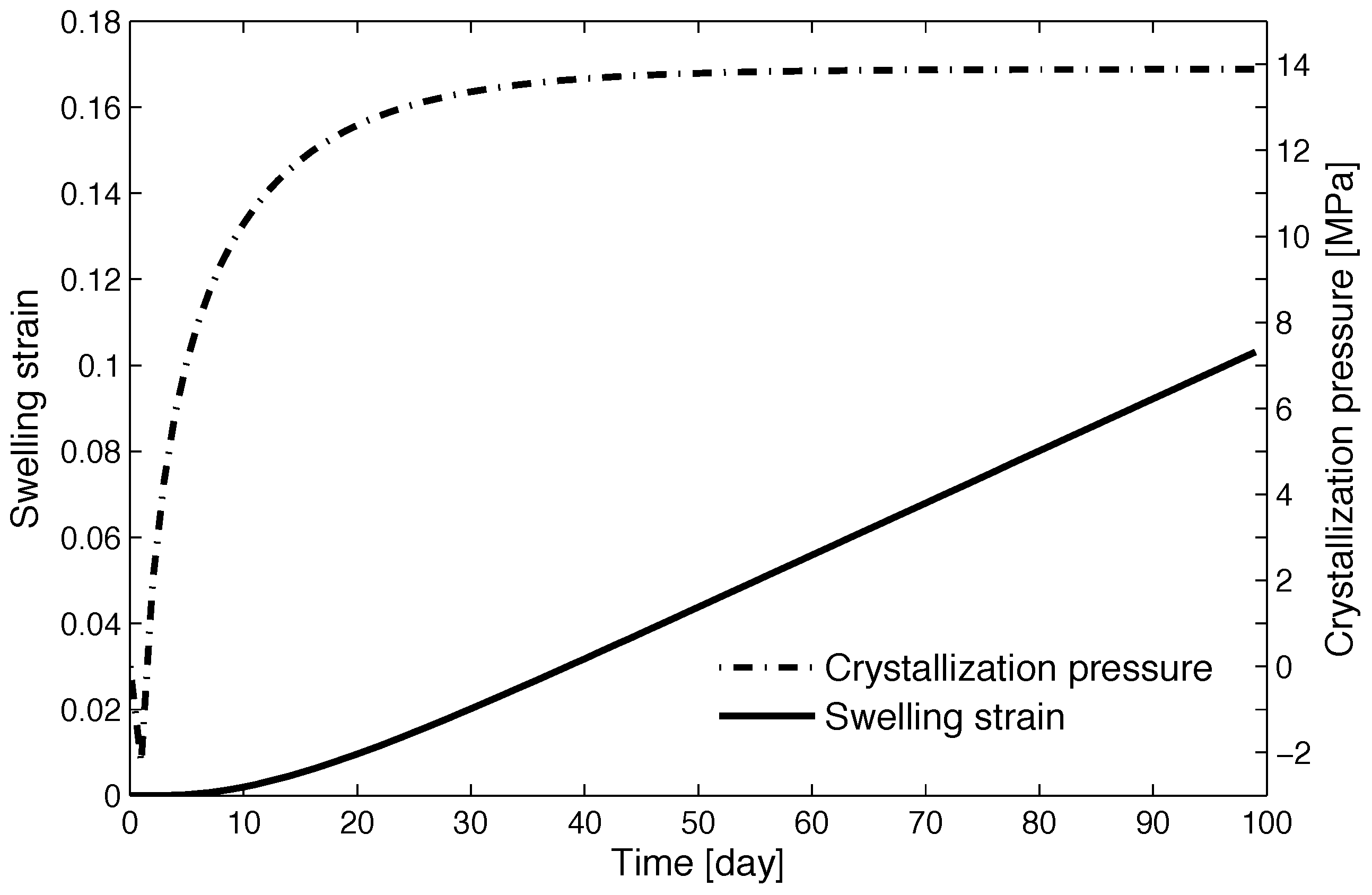
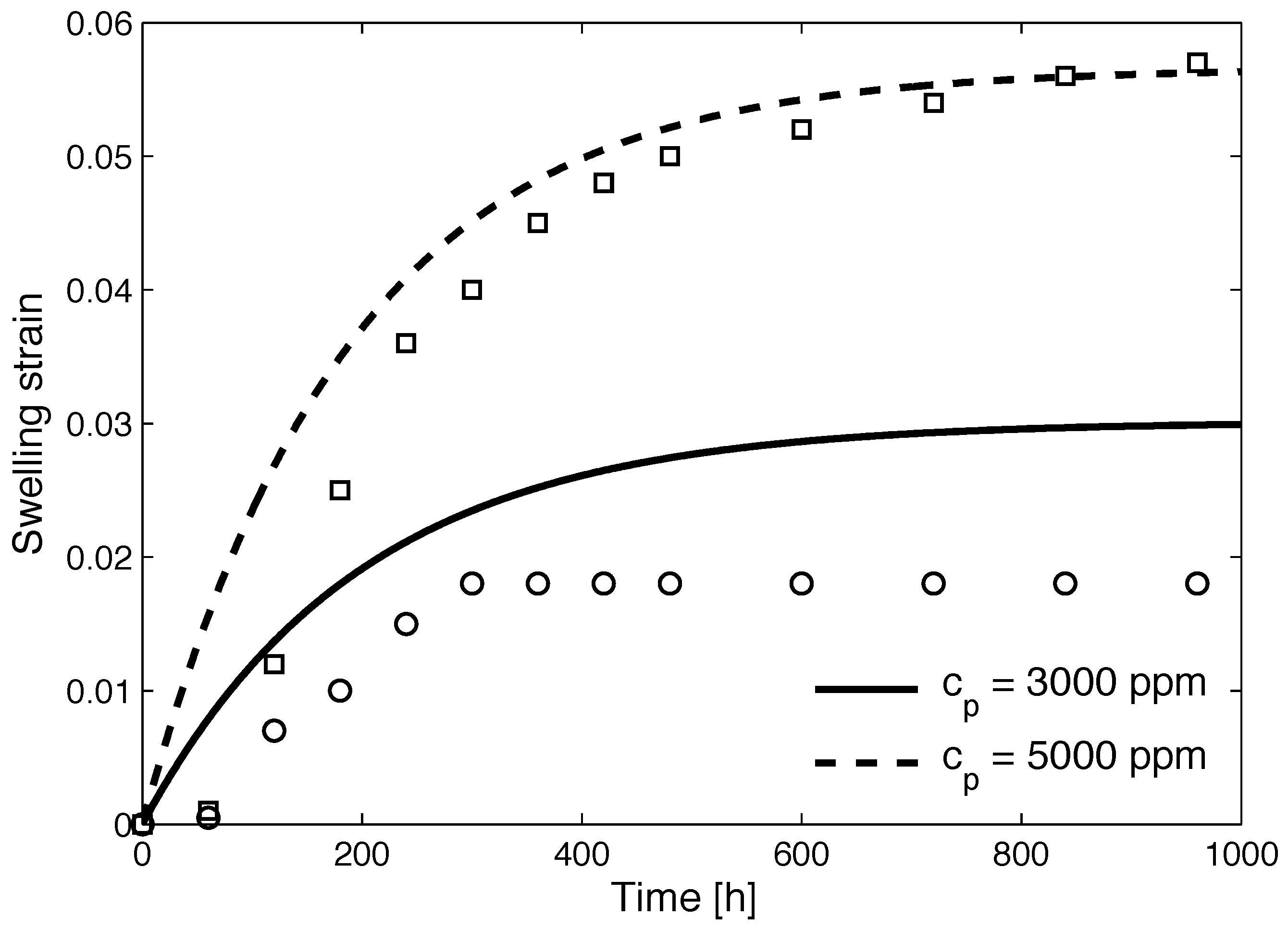
4.2. Numerical Simulations
5. Osmosis-Induced Expansion
5.1. Phenomenological Concepts and Mathematical Formulations
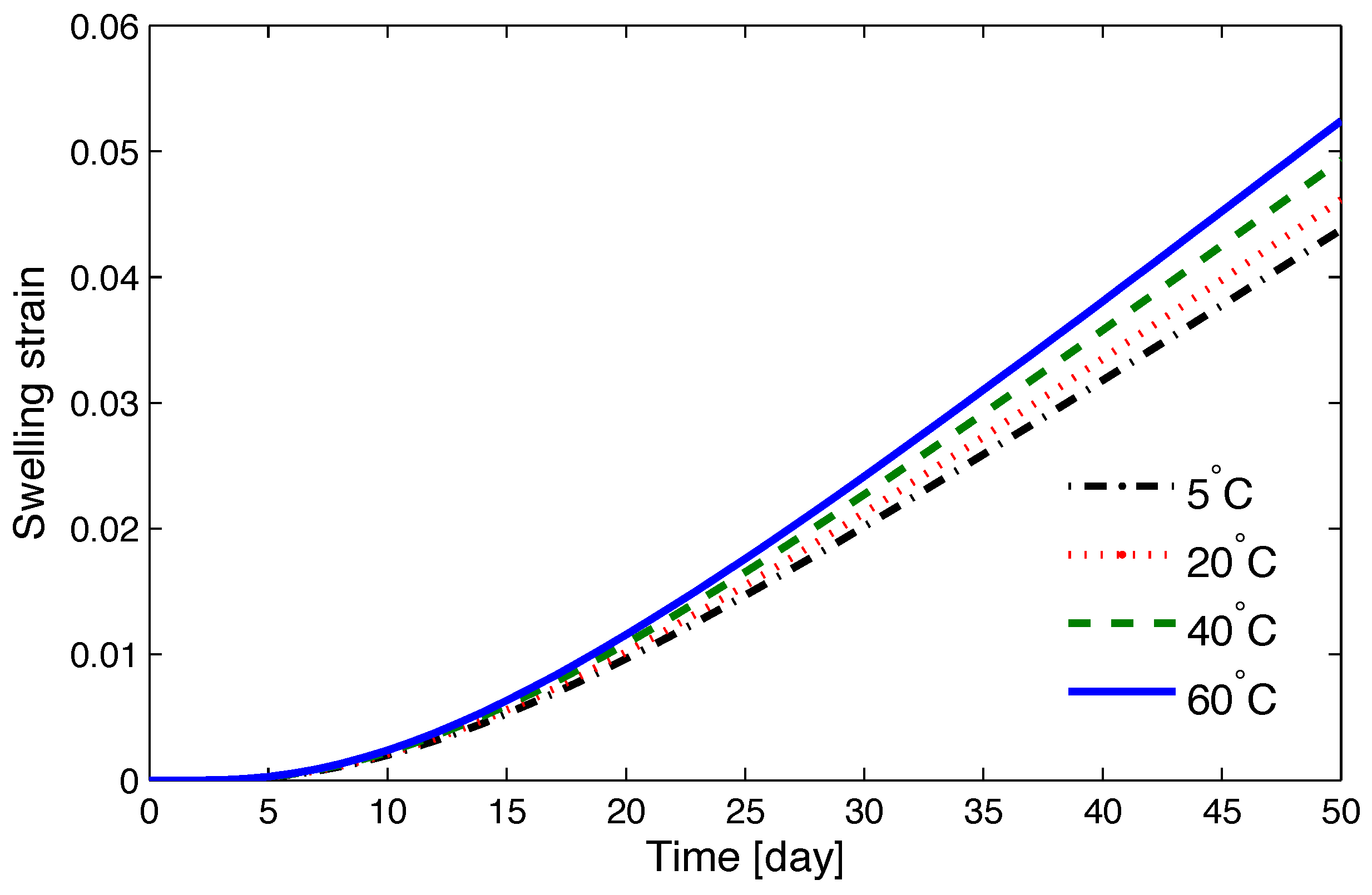
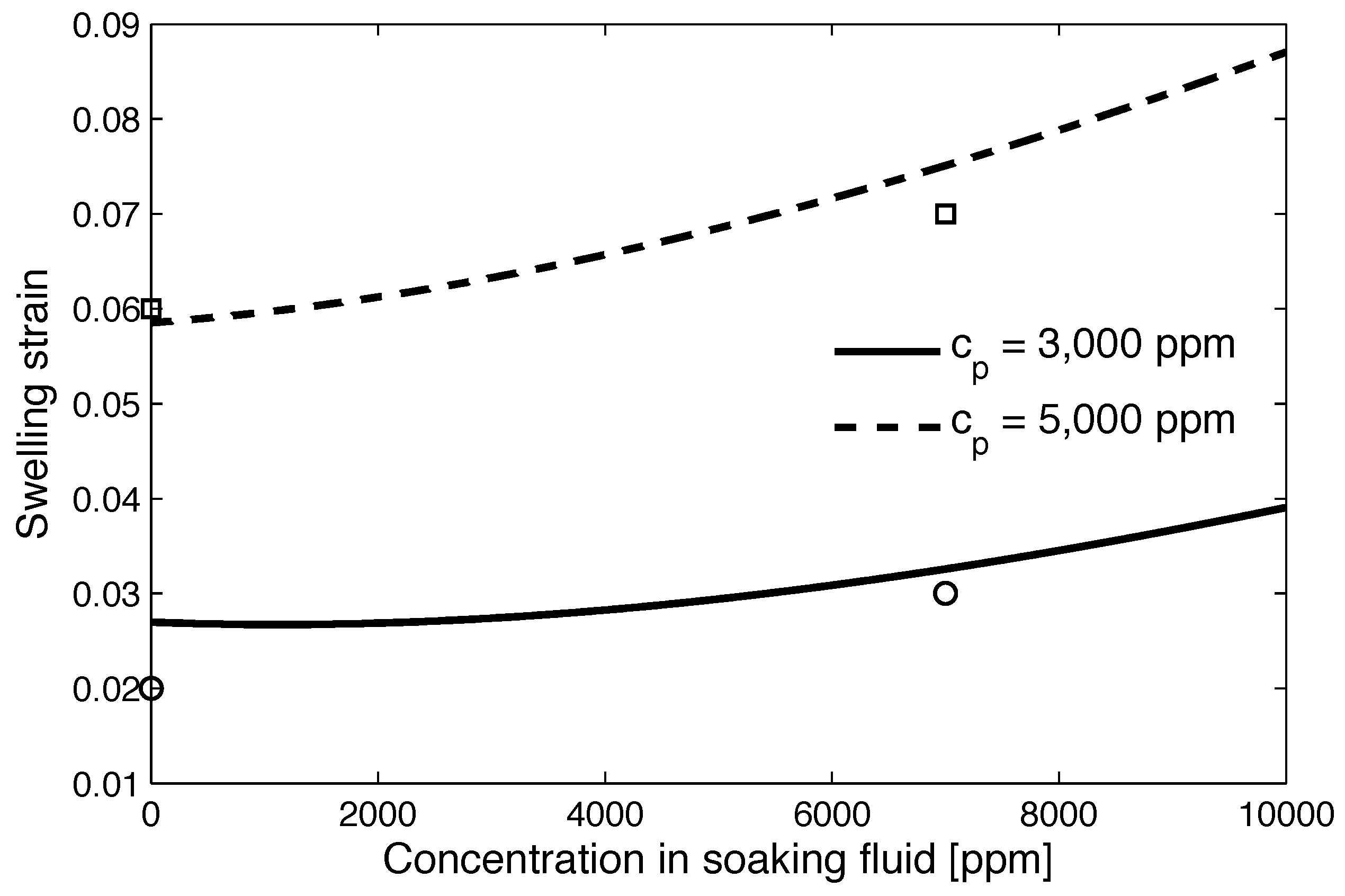
5.2. Numerical Simulations
5.3. Assessing the Kinetics of Osmosis
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Abdi, M.R.; Wild, S. Sulphate expansion of lime-stabilized kaolinite: I. Physical characteristics. Clay Miner. 1993, 28, 569–584. [Google Scholar] [CrossRef]
- Mitchell, J.K. Practical problems from surprising soil behavior. J. Geotech. Eng. 1986, 112, 255–289. [Google Scholar] [CrossRef]
- Hunter, D. Lime-induced heave in sulfate-bearing clay soils. J. Geotech. Eng. 1988, 114, 50–167. [Google Scholar] [CrossRef]
- Puppala, A.J.; Intharasombat, N.; Vempati, R.K. Experimental studies on ettringite-induced heaving in soils. J. Geotech. Eng. 2005, 131, 325–337. [Google Scholar] [CrossRef]
- Mehta, P. Mechanism of expansion associated with ettringite formation. Cem. Concr. Res. 1973, 3, 1–6. [Google Scholar] [CrossRef]
- Xie, P.; Beaudoin, J.J. Mechanism of sulphate expansion. I. Thermodynamic principle of crystallization pressure. Cem. Concr. Res. 1992, 22, 631–640. [Google Scholar]
- Dermatas, D. Ettringite-induced swelling in soils: State-of-the-art. Appl. Mech. Rev. 1995, 48, 659–673. [Google Scholar] [CrossRef]
- Shimada, Y.; Young, J. Structural changes during thermal dehydration of ettringite. Adv. Cem. Res. 2001, 13, 77–81. [Google Scholar] [CrossRef]
- Scherer, G.W. Stress from crystallization of salt. Cem. Concr. Res. 2004, 34, 1613–1624. [Google Scholar] [CrossRef]
- Rajasekaran, G. Sulphate attack and ettringite formation in the lime and cement stabilized marine clays. Ocean Eng. 2005, 32, 1133–1159. [Google Scholar] [CrossRef]
- Moon, D.H.; Dermatas, D.; Wazne, M.; Sanchez, A.M.; Chrysochoou, M.; Grubb, D.G. Swelling related to ettringite crystal formation in chromite ore processing residue. Environ. Geochem. Health 2007, 29, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Wild, S.; Abdi, M.R.; Leng-Ward, G. Sulphate expansion of lime-stabilized kaolinite: II. Reaction products and expansion. Clay Miner. 1993, 28, 569–584. [Google Scholar] [CrossRef]
- Little, D.N.; Nair, S.; Herbert, B. Addressing sulfate-induced heave in lime treated soils. J. Geotech. Geoenviron. Eng. 2011, 136, 110–118. [Google Scholar] [CrossRef]
- Nair, S.; Little, D. Mechanisms of distress associated with sulfate-induced heaving in lime-treated soils. Transp. Res. Rec. J. Transp. Res. Board 2011, 2212, 82–90. [Google Scholar] [CrossRef]
- Narsavage, P.A. Sulfate heaving of cement stabilized soil in Ohio. In Proceedings of the 2011 Ohio Transportation Engineering Conference, Columbus, OH, USA, 25–26 October 2011. [Google Scholar]
- Cutright, T.; Wigdahl, J. Assessment of sulfate bearing soils in Ohio. In Proceedings of the 2013 Ohio Transportation Engineering Conference, Columbus, OH, USA, 22–23 October 2013. [Google Scholar]
- Freese, K. Assessment of Sulfate in Ohio. Master’s Thesis, University of Akron, Akron, OH, USA, 2014. [Google Scholar]
- Weir, M.; Mandell, A.; Farver, J. Role of Sulfates on Highway Heave in Lake County, Ohio; Report # FHWA/OH-2014/4; Ohio Department of Transportation: Columbus, OH, USA, 2014.
- Hueckel, T. Water-mineral interaction in hygro-mechanics of clays exposed to environmental loads: A mixture approach. Can. Geotech. J. 1992, 29, 1071–1086. [Google Scholar] [CrossRef]
- Hueckel, T. Chemo-plasticity of clays subjected to stress and flow of a single contaminant. Int. J. Numer. Anal. Methods Geomech. 1997, 21, 43–72. [Google Scholar] [CrossRef]
- Loret, B.; Hueckel, T.; Gajo, A. Chemo-mechanical coupling in saturated porous media: Elastic-plastic behaviour of homoionic expansive clays. Int. J. Solids Struct. 2002, 39, 2773–2806. [Google Scholar] [CrossRef]
- Gajo, A.; Loret, B.; Hueckel, T. Electro-chemo–mechanical couplings in saturated porous media: Elasto-plastic behaviour of heteroionic expansive clays. Int. J. Solids Struct. 2002, 39, 4327–4362. [Google Scholar] [CrossRef]
- Skoblinskaya, N.; Krasilnikov, K. Changes in crystal structure of ettringite on dehydration. 1. Cem. Concr. Res. 1975, 5, 381–393. [Google Scholar] [CrossRef]
- Scherer, G.W. Crystallization in pores. Cem. Concr. Res. 1999, 29, 1347–1358. [Google Scholar] [CrossRef]
- Oldecop, L.; Alonso, E. Modelling the degradation and swelling of clayey rocks bearing calcium-sulphate. Int. J. Rock Mech. Min. Sci. 2012, 54, 90–102. [Google Scholar] [CrossRef]
- Bary, D. Simplified coupled chemo–mechanical modeling of cement pastes behavior subjected to combined leaching and external sulfate attack. Int. J. Numer. Anal. Methods Geomech. 2008, 32, 1791–1816. [Google Scholar] [CrossRef]
- Correns, C.W. Growth and dissolution of crystals under linear pressure. Discuss. Faraday Soc. 1949, 5, 267–271. [Google Scholar] [CrossRef]
- Sigdel, P.; Hu, L.B. Modelling ettringite induced heaving in cement stabilized soils within a chemo–mechanical constitutive framework. In ASCE Geotechnical Special Publication 256: IFCEE 2015; Iskander, M., Suleiman, M.T., Anderson, J.B., Laefer, D.F., Eds.; ASCE: Reston, VA, USA, 2015; pp. 125–132. [Google Scholar]
- Liu, Z.; Boukpeti, N.; Li, X.; Collin, F.; Radu, J.P.; Hueckel, T.; Charlier, R. Modelling chemo-hydro-mechanical behaviour of unsaturated clays: A feasibility study. Int. J. Numer. Anal. Methods Geomech. 2005, 29, 919–940. [Google Scholar] [CrossRef]
- Wei, C. A theoretical framework for modeling the chemomechanical behavior of unsaturated soils. Vadose Zone J. 2014, 13. [Google Scholar] [CrossRef]
- Guimaraes, L.D.N.; Gens, A.; Sanchez, M.; Olivella, S. A chemo–mechanical constitutive model accounting for cation exchange in expansive clays. Geotechnique 2013, 63, 221–234. [Google Scholar] [CrossRef]
- Witteveen, P.; Ferrari, A.; Laloui, L. An experimental and constitutive investigation on the chemo–mechanical behaviour of a clay. Geotechnique 2013, 63, 244–255. [Google Scholar] [CrossRef]
- Northrop, J.H. The kinetics of osmosis. J. Gen. Physiol. 1927, 10, 883–892. [Google Scholar] [CrossRef]
- Coussy, O. Mechanics of Porous Continua; Wiley: Hoboken, NJ, USA, 1993. [Google Scholar]
| Parameter | Symbol | Value |
|---|---|---|
| Saturated concentration (gypsum) | ||
| Saturated concentration (ettringite) | ||
| Rate constant (gypsum) | ||
| Rate constant (ettringite) | ||
| Molar volume (ettringite) | ||
| Initial volume fraction of gypsum | ||
| Initial volume fraction of ettringite | 0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Sigdel, P.; Hu, L. Chemo-Mechanical Interactions in the Ettringite Induced Expansion of Sulfate-Bearing Soils. Geosciences 2019, 9, 375. https://doi.org/10.3390/geosciences9090375
Wang Z, Sigdel P, Hu L. Chemo-Mechanical Interactions in the Ettringite Induced Expansion of Sulfate-Bearing Soils. Geosciences. 2019; 9(9):375. https://doi.org/10.3390/geosciences9090375
Chicago/Turabian StyleWang, Zhongmei, Pawan Sigdel, and Liangbo Hu. 2019. "Chemo-Mechanical Interactions in the Ettringite Induced Expansion of Sulfate-Bearing Soils" Geosciences 9, no. 9: 375. https://doi.org/10.3390/geosciences9090375




