Major Adverse Cardiac and Cerebrovascular Events in Geriatric Patients with Obstructive Sleep Apnea: An Inpatient Sample Analysis
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Study Population and Data Collection
2.3. Statistical Analysis
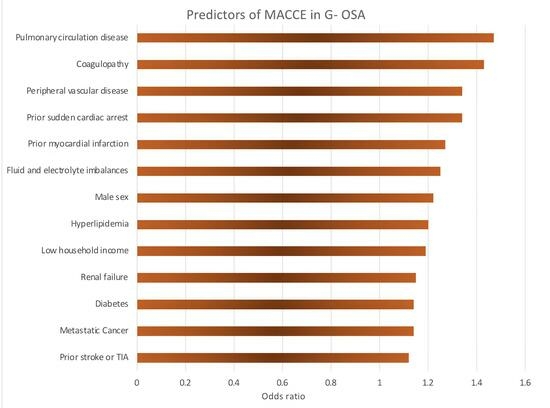
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghavami, T.; Kazeminia, M.; Ahmadi, N.; Rajati, F. Global Prevalence of Obstructive Sleep Apnea in the Elderly and Related Factors: A Systematic Review and Meta-Analysis Study. J. PeriAnesthesia Nurs. 2023. [Google Scholar] [CrossRef]
- Alexander, K.P.; Newby, L.K.; Armstrong, P.W.; Cannon, C.P.; Gibler, W.B.; Rich, M.W.; Van de Werf, F.; White, H.D.; Weaver, W.D.; Naylor, M.D.; et al. Acute Coronary Care in the Elderly, Part II. Circulation 2007, 115, 2570–2589. [Google Scholar] [CrossRef] [PubMed]
- Fietze, I.; Laharnar, N.; Obst, A.; Ewert, R.; Felix, S.B.; Garcia, C.; Gläser, S.; Glos, M.; Schmidt, C.O.; Stubbe, B.; et al. Prevalence and association analysis of obstructive sleep apnea with gender and age differences—Results of SHIP-Trend. J. Sleep Res. 2019, 28, e12770. [Google Scholar] [CrossRef] [PubMed]
- Lv, R.; Liu, X.; Zhang, Y.; Dong, N.; Wang, X.; He, Y.; Yue, H.; Yin, Q. Pathophysiological mechanisms and therapeutic approaches in obstructive sleep apnea syndrome. Signal Transduct. Target. Ther. 2023, 8, 218. [Google Scholar] [CrossRef] [PubMed]
- Serra, P.C.; Soler, X. Obstructive sleep apnea and cardiovascular events in elderly patients. Expert Rev. Respir. Med. 2022, 16, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- HCUP. Healthcare Cost & Utilization Project. (n.d.). Available online: https://hcup-us.ahrq.gov/nisoverview.jsp (accessed on 26 October 2023).
- Neumann, J.T.; Thao, L.T.P.; Callander, E.; Chowdhury, E.; Williamson, J.D.; Nelson, M.R.; Donnan, G.; Woods, R.L.; Reid, C.M.; Poppe, K.K.; et al. Cardiovascular risk prediction in healthy older people. GeroScience 2022, 44, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Browne, H.A.K.; Adams, L.; Simonds, A.K.; Morrell, M.J. Impact of age on breathing and resistive pressure in people with and without sleep apnea. J. Appl. Physiol. 2001, 90, 1074–1082. [Google Scholar] [CrossRef]
- Malhotra, A.; Huang, Y.; Fogel, R.; Lazic, S.; Pillar, G.; Jakab, M.; Kikinis, R.; White, D.P. Aging Influences on Pharyngeal Anatomy and Physiology: The Predisposition to Pharyngeal Collapse. Am. J. Med. 2006, 119, 72.e9–72.e14. [Google Scholar] [CrossRef]
- Browne, H.; Adams, L.; Simonds, A.; Morrell, M. Sleep apnoea and daytime function in the elderly—What is the impact of arousal frequency? Respir. Med. 2003, 97, 1102–1108. [Google Scholar] [CrossRef]
- Jordan, A.S.; McSharry, D.G.; Malhotra, A. Adult obstructive sleep apnoea. Lancet 2014, 383, 736–747. [Google Scholar] [CrossRef]
- Shamsuzzaman, A.S.M.; Gersh, B.J.; Somers, V.K. Obstructive Sleep Apnea: Implications for Cardiac and Vascular Disease. JAMA 2003, 290, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Eichhorn, L.; Dolscheid-Pommerich, R.; Erdfelder, F.; Ayub, M.A.; Schmitz, T.; Werner, N.; Jansen, F. Sustained apnea induces endothelial activation. Clin. Cardiol. 2017, 40, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Maeder, M.T.; Schoch, O.D.; Rickli, H. A clinical approach to obstructive sleep apnea as a risk factor for cardiovascular disease. Vasc. Health Risk Manag. 2016, 12, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Yumino, D.; Tsurumi, Y.; Takagi, A.; Suzuki, K.; Kasanuki, H. Impact of Obstructive Sleep Apnea on Clinical and Angiographic Outcomes Following Percutaneous Coronary Intervention in Patients with Acute Coronary Syndrome. Am. J. Cardiol. 2007, 99, 26–30. [Google Scholar] [CrossRef]
- Lee, C.-H.; Khoo, S.-M.; Chan, M.Y.; Wong, H.-B.; Low, A.F.; Phua, Q.-H.; Richards, A.M.; Tan, H.-C.; Yeo, T.-C. Severe Obstructive Sleep Apnea and Outcomes Following Myocardial Infarction. Sleep Med. 2011, 7, 616–621. [Google Scholar] [CrossRef]
- Loo, G.; Tan, A.Y.; Koo, C.-Y.; Tai, B.-C.; Richards, M.; Lee, C.-H. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014, 15, 631–636. [Google Scholar] [CrossRef]
- Nakashima, H.; Kurobe, M.; Minami, K.; Furudono, S.; Uchida, Y.; Amenomori, K.; Nunohiro, T.; Takeshita, S.; Maemura, K. Effects of moderate-to-severe obstructive sleep apnea on the clinical manifestations of plaque vulnerability and the progression of coronary atherosclerosis in patients with acute coronary syndrome. Eur. Heart J. Acute Cardiovasc. Care 2015, 4, 75–84. [Google Scholar] [CrossRef]
- Lee, C.-H.; Sethi, R.; Li, R.; Ho, H.-H.; Hein, T.; Jim, M.-H.; Loo, G.; Koo, C.-Y.; Gao, X.-F.; Chandra, S.; et al. Obstructive Sleep Apnea and Cardiovascular Events after Percutaneous Coronary Intervention. Circulation 2016, 133, 2008–2017. [Google Scholar] [CrossRef]
- Yang, D.; Li, L.; Dong, J.; Yang, W.; Liu, Z. Effects of continuous positive airway pressure on cardiac events and metabolic components in patients with moderate to severe obstructive sleep apnea and coronary artery disease: A meta-analysis. J. Clin. Sleep Med. 2023, jcsm–10740. [Google Scholar] [CrossRef]
- Munoz, R.; Duran-Cantolla, J.; Martínez-Vila, E.; Gallego, J.; Rubio, R.; Aizpuru, F.; De La Torre, G. Severe Sleep Apnea and Risk of Ischemic Stroke in the Elderly. Stroke 2006, 37, 2317–2321. [Google Scholar] [CrossRef]
- Martínez-García, M.Á.; Galiano-Blancart, R.; Román-Sánchez, P.; Soler-Cataluña, J.J.; Cabero-Salt, L.; Salcedo-Maiques, E. Continuous Positive Airway Pressure Treatment in Sleep Apnea Prevents New Vascular Events after Ischemic Stroke. Chest 2005, 128, 2123–2129. [Google Scholar] [CrossRef]
- Wolk, R.; Somers, V.K. Cardiovascular consequences of obstructive sleep apnea. Clin. Chest Med. 2003, 24, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.A.; Lindberg, E. Obstructive sleep apnea is a common disorder in the population—A review on the epidemiology of sleep apnea. J. Thorac. Dis. 2015, 7, 1311–1322. [Google Scholar] [CrossRef]
- Punjabi, N.M.; Caffo, B.S.; Goodwin, J.L.; Gottlieb, D.J.; Newman, A.B.; O’Connor, G.T.; Rapoport, D.M.; Redline, S.; Resnick, H.E.; Robbins, J.A.; et al. Sleep-Disordered Breathing and Mortality: A Prospective Cohort Study. PLOS Med. 2009, 6, e1000132. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Simon-Tuval, T.; Reuveni, H.; Greenberg-Dotan, S.; Oksenberg, A.; Tal, A.; Tarasiuk, A. Php16 low socioeconomic status is a risk factor for cpap acceptance among adult osas patients requiring treatment. Value Health 2010, 13, A84. [Google Scholar] [CrossRef]
- Yeghiazarians, Y.; Jneid, H.; Tietjens, J.R.; Redline, S.; Brown, D.L.; El-Sherif, N.; Mehra, R.; Bozkurt, B.; Ndumele, C.E.; Somers, V.K. Obstructive Sleep Apnea and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 144, E56–E67. [Google Scholar] [CrossRef] [PubMed]
- Testelmans, D.; Spruit, M.A.; Vrijsen, B.; Sastry, M.; Belge, C.; Kalkanis, A.; Gaffron, S.; Wouters, E.F.M.; Buyse, B. Comorbidity clusters in patients with moderate-to-severe OSA. Sleep Breath. 2021, 26, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Grigg-Damberger, M. Why a Polysomnogram Should Become Part of the Diagnostic Evaluation of Stroke and Transient Ischemic Attack. J. Clin. Neurophysiol. 2006, 23, 21–38. [Google Scholar] [CrossRef]
- Wang, G.; Miao, H.; Hao, W.; Zhao, G.; Yan, Y.; Gong, W.; Fan, J.; Ai, H.; Que, B.; Wang, X.; et al. Association of obstructive sleep apnoea with long-term cardiovascular events in patients with acute coronary syndrome with or without hypertension: Insight from the OSA-ACS project. BMJ Open Respir. Res. 2023, 10, e001662. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; Kannel, W.B. Obesity, Diabetes, and Risk of Cardiovascular Disease in the Elderly. Am. J. Geriatr. Cardiol. 2002, 11, 119–124. [Google Scholar] [CrossRef]
- Sharafkhaneh, A.; Agrawal, R.; Nambi, V.; BaHammam, A.; Razjouyan, J. Obesity paradox or hypoxia preconditioning: How obstructive sleep apnea modifies the Obesity-MI relationship. Sleep Med. 2023, 110, 132–136. [Google Scholar] [CrossRef]
- Gupta, P.P.; Fonarow, G.C.; Horwich, T.B. Obesity and the Obesity Paradox in Heart Failure. Can. J. Cardiol. 2015, 31, 195–202. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chen, P.-Y.; Chuang, L.-P.; Chen, N.-H.; Tu, Y.-K.; Hsieh, Y.-J.; Wang, Y.-C.; Guilleminault, C. Diagnostic accuracy of the Berlin questionnaire, STOP-BANG, STOP, and Epworth sleepiness scale in detecting obstructive sleep apnea: A bivariate meta-analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef]
- Xie, J.; Sert Kuniyoshi, F.H.; Covassin, N.; Singh, P.; Gami, A.S.; Wang, S.; Chahal, C.A.A.; Wei, Y.; Somers, V.K. Nocturnal Hypoxemia Due to Obstructive Sleep Apnea Is an Independent Predictor of Poor Prognosis after Myocardial Infarction. J. Am. Heart Assoc. 2016, 5, e003162. [Google Scholar] [CrossRef]
- Force, U.P.S.T.; Mangione, C.M.; Barry, M.J.; Nicholson, W.K.; Cabana, M.; Chelmow, D.; Coker, T.R.; Davidson, K.W.; Davis, E.M.; Donahue, K.E.; et al. Screening for Obstructive Sleep Apnea in Adults. JAMA 2022, 328, 1945–1950. [Google Scholar] [CrossRef]
- Boulos, M.I.; Kamra, M.; Colelli, D.R.; Kirolos, N.; Gladstone, D.J.; Boyle, K.; Sundaram, A.; Hopyan, J.J.; Swartz, R.H.; Mamdani, M.; et al. SLEAP SMART (Sleep Apnea Screening Using Mobile Ambulatory Recorders after TIA/Stroke): A Randomized Controlled Trial. Stroke 2022, 53, 710–718. [Google Scholar] [CrossRef] [PubMed]
| Variable | MACCE All-Cause Mortality, AMI, Cardiac Arrest and Stroke | Overall Geriatric OSA Admissions | ||
|---|---|---|---|---|
| YES | NO | |||
| N = 113,295 | N = 102,785 | N = 1,141,120 | ||
| Age (years) | 73 (69–79) | 74 (69–80) | 73 (69–79) | |
| Yes | No | |||
| Sex | Male | 63.6% | 57.0% | 57.7% |
| Female | 36.4% | 43.0% | 42.3% | |
| Race (Ref: White) | White | 82.1% | 82.5% | 82.5% |
| Black | 9.6% | 9.7% | 9.7% | |
| Hispanic | 4.9% | 4.9% | 4.9% | |
| Asian Pacific | 1.5% | 1.1% | 1.1% | |
| Native Americans | 0.4% | 0.4% | 0.4% | |
| Other Ethnicities | 1.5% | 1.4% | 1.4% | |
| Bed Size (Ref: Small) | Small | 17.6% | 21.3% | 21.0% |
| Medium | 28.9% | 28.5% | 28.6% | |
| Large | 53.5% | 50.1% | 50.5% | |
| Location/Teaching status of hospital (Ref: Rural) | Rural | 7.3% | 8.6% | 8.5% |
| Urban non-teaching | 18.6% | 20.3% | 20.2% | |
| Urban teaching | 74.0% | 71.1% | 71.4% | |
| Region of hospital (Ref: Northeast) | Northeast | 14.6% | 15.9% | 15.8% |
| Midwest | 31.2% | 30.6% | 30.7% | |
| South | 35.3% | 35.6% | 35.6% | |
| West | 18.9% | 17.9% | 18.0% | |
| Primary Expected Payer (Ref: Medicare) | Medicare | 88.3% | 89.5% | 89.4% |
| Medicaid | 0.6% | 0.6% | 0.6% | |
| Private including HMO | 7.9% | 8.4% | 8.4% | |
| Self-pay | 0.3% | 0.3% | 0.3% | |
| No charges | 0.0% | 0.0% | 0.0% | |
| Others | 1.6% | 2.4% | 1.7% | |
| Hypertension | 63.7% | 68.8% | 68.3% | |
| Diabetes Mellitus (with and without chronic complications) | 55.7% | 49.7% | 50.3% | |
| Hyperlipidemia | 68.0% | 62.9% | 63.4% | |
| Smoking | 40.3% | 42.4% | 42.2% | |
| Peripheral Vascular Disease | 12.9% | 9.3% | 9.6% | |
| Obesity | 39.6% | 41.8% | 41.5% | |
| Renal Failure | 39.4% | 32.1% | 32.8% | |
| Prior MI | 15.6% | 11.1% | 11.6% | |
| Prior PCI | 1.4% | 1.0% | 1.0% | |
| Prior CABG | 13.7% | 10.3% | 10.7% | |
| Prior TIA or stroke | 12.3% | 10.6% | 10.7% | |
| Prior SCA | 0.6% | 0.4% | 0.4% | |
| Prior history of VTE | 6.5% | 8.8% | 8.5% | |
| Personal history of cancer | 14.1% | 16.7% | 16.4% | |
| Congestive heart failure | 27.0% | 26.1% | 26.2% | |
| Valvular heart disease | 9.1% | 8.0% | 8.1% | |
| Pulmonary circulation disease | 1.7% | 1.1% | 1.2% | |
| Chronic pulmonary disease | 39.2% | 39.8% | 39.8% | |
| Liver disease | 3.9% | 4.3% | 4.2% | |
| Metastatic cancer | 2.4% | 2.1% | 2.1% | |
| Solid tumor without metastasis | 2.8% | 2.9% | 2.9% | |
| Rheumatoid arthritis | 3.9% | 5.2% | 5.1% | |
| Coagulopathy | 10.7% | 7.0% | 7.4% | |
| Fluid and electrolyte disorders | 30.8% | 39.4% | 31.7% | |
| Deficiency Anemias | 25.2% | 23.4% | 23.5% | |
| Alcohol Abuse | 2.1% | 1.8% | 1.9% | |
| Drug abuse | 1.1% | 1.3% | 1.3% | |
| Other neurological disorders | 10.5% | 12.2% | 12.0% | |
| Depression | 15.9% | 19.0% | 18.7% | |
| Age (years) | ||||
| Variable | Odds Ratio | 95 Confidence | p Value | ||
|---|---|---|---|---|---|
| Lower | Upper | ||||
| Sex | Male vs. Female | 1.22 | 1.18 | 1.26 | <0.001 |
| Race (Ref: White) | Black | 0.89 | 0.84 | 0.95 | <0.001 |
| Hispanic | 0.90 | 0.83 | 0.96 | ||
| Asian Pacific | 1.08 | 0.94 | 1.24 | ||
| Native Americans | 1.04 | 0.78 | 1.37 | ||
| Others | 1.05 | 0.93 | 1.19 | ||
| Bed Size (Ref: Small) | Medium | 1.17 | 1.09 | 1.25 | <0.001 |
| Large | 1.25 | 1.18 | 1.33 | ||
| Location/Teaching status of hospital (Ref: Rural) | Urban non-teaching | 1.15 | 1.05 | 1.25 | <0.001 |
| Urban teaching | 1.34 | 1.23 | 1.46 | ||
| Region of hospital (Ref: Northeast) | Midwest | 1.11 | 1.02 | 1.20 | <0.035 |
| South | 1.06 | 0.98 | 1.15 | ||
| West | 1.12 | 1.02 | 1.21 | ||
| Median household income national quartile (Ref: 76–100th) | 0–25th percentile | 1.19 | 1.13 | 1.26 | <0.001 |
| 26–50th percentile | 1.15 | 1.09 | 1.21 | ||
| 51–75th percentile | 1.05 | 1.00 | 1.10 | ||
| Elective vs. non-elective admission | Non-elective vs. Elective admission | 4.12 | 3.82 | 4.42 | <0.001 |
| Primary Expected Payer (Ref: Medicare) | Medicaid | 0.88 | 0.72 | 1.08 | <0.001 |
| Private including HMO | 1.17 | 1.11 | 1.24 | ||
| Hypertension | 0.89 | 0.86 | 0.92 | <0.001 | |
| Diabetes Mellitus (with and without chronic complications) | 1.14 | 1.10 | 1.17 | <0.001 | |
| Hyperlipidemia | 1.20 | 1.16 | 1.24 | <0.001 | |
| Smoking | 0.86 | 0.84 | 0.89 | <0.001 | |
| Peripheral Vascular Disease | 1.34 | 1.28 | 1.40 | <0.001 | |
| Obesity | 0.92 | 0.90 | 0.95 | <0.001 | |
| Renal Failure | 1.15 | 1.12 | 1.19 | <0.001 | |
| Prior MI | 1.27 | 1.22 | 1.33 | <0.001 | |
| Prior PCI | 1.16 | 1.02 | 1.32 | 0.020 | |
| Prior CABG | 1.09 | 1.04 | 1.14 | <0.001 | |
| Prior TIA or stroke | 1.12 | 1.07 | 1.17 | <0.001 | |
| Prior SCA | 1.34 | 1.11 | 1.62 | 0.003 | |
| Prior history of VTE | 0.73 | 0.69 | 0.77 | <0.001 | |
| Personal history of cancer | 0.82 | 0.78 | 0.85 | <0.001 | |
| Congestive heart failure | 0.87 | 0.84 | 0.90 | <0.001 | |
| Valvular heart disease | 1.11 | 1.05 | 1.17 | <0.001 | |
| Pulmonary circulation disease | 1.47 | 1.31 | 1.66 | <0.001 | |
| Chronic pulmonary disease | 0.92 | 0.89 | 0.94 | <0.001 | |
| Liver disease | 0.80 | 0.74 | 0.86 | <0.001 | |
| Acquired immune deficiency syndrome | 0.18 | 0.04 | 0.74 | 0.018 | |
| Metastatic cancer | 1.14 | 1.03 | 1.25 | 0.013 | |
| Solid tumor without metastasis | 0.88 | 0.81 | 0.96 | 0.004 | |
| Rheumatoid arthritis | 0.81 | 0.75 | 0.87 | <0.001 | |
| Coagulopathy | 1.43 | 1.35 | 1.50 | <0.001 | |
| Fluid and electrolyte disorders | 1.25 | 1.20 | 1.29 | <0.001 | |
| Deficiency Anemias | 0.89 | 0.86 | 0.92 | <0.001 | |
| Alcohol Abuse | 1.10 | 0.99 | 1.22 | 0.070 | |
| Drug abuse | 0.87 | 0.76 | 1.00 | 0.048 | |
| Other neurological disorders | 0.82 | 0.78 | 0.86 | <0.001 | |
| Depression | 0.86 | 0.82 | 0.89 | <0.001 | |
| Age (years) | 1.01 | 1.00 | 1.01 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desai, R.; Mellacheruvu, S.P.; Akella, S.A.; Mohammed, A.S.; Saketha, P.; Mohammed, A.A.; Hussain, M.; Bavanasi, A.; Gummadi, J.; Sunkara, P. Major Adverse Cardiac and Cerebrovascular Events in Geriatric Patients with Obstructive Sleep Apnea: An Inpatient Sample Analysis. Med. Sci. 2023, 11, 69. https://doi.org/10.3390/medsci11040069
Desai R, Mellacheruvu SP, Akella SA, Mohammed AS, Saketha P, Mohammed AA, Hussain M, Bavanasi A, Gummadi J, Sunkara P. Major Adverse Cardiac and Cerebrovascular Events in Geriatric Patients with Obstructive Sleep Apnea: An Inpatient Sample Analysis. Medical Sciences. 2023; 11(4):69. https://doi.org/10.3390/medsci11040069
Chicago/Turabian StyleDesai, Rupak, Sai Priyanka Mellacheruvu, Sai Anusha Akella, Adil Sarvar Mohammed, Pakhal Saketha, Abdul Aziz Mohammed, Mushfequa Hussain, Aamani Bavanasi, Jyotsna Gummadi, and Praveena Sunkara. 2023. "Major Adverse Cardiac and Cerebrovascular Events in Geriatric Patients with Obstructive Sleep Apnea: An Inpatient Sample Analysis" Medical Sciences 11, no. 4: 69. https://doi.org/10.3390/medsci11040069