The Association between Sleep and Theory of Mind in School Aged Children with ADHD
Abstract
:1. Introduction
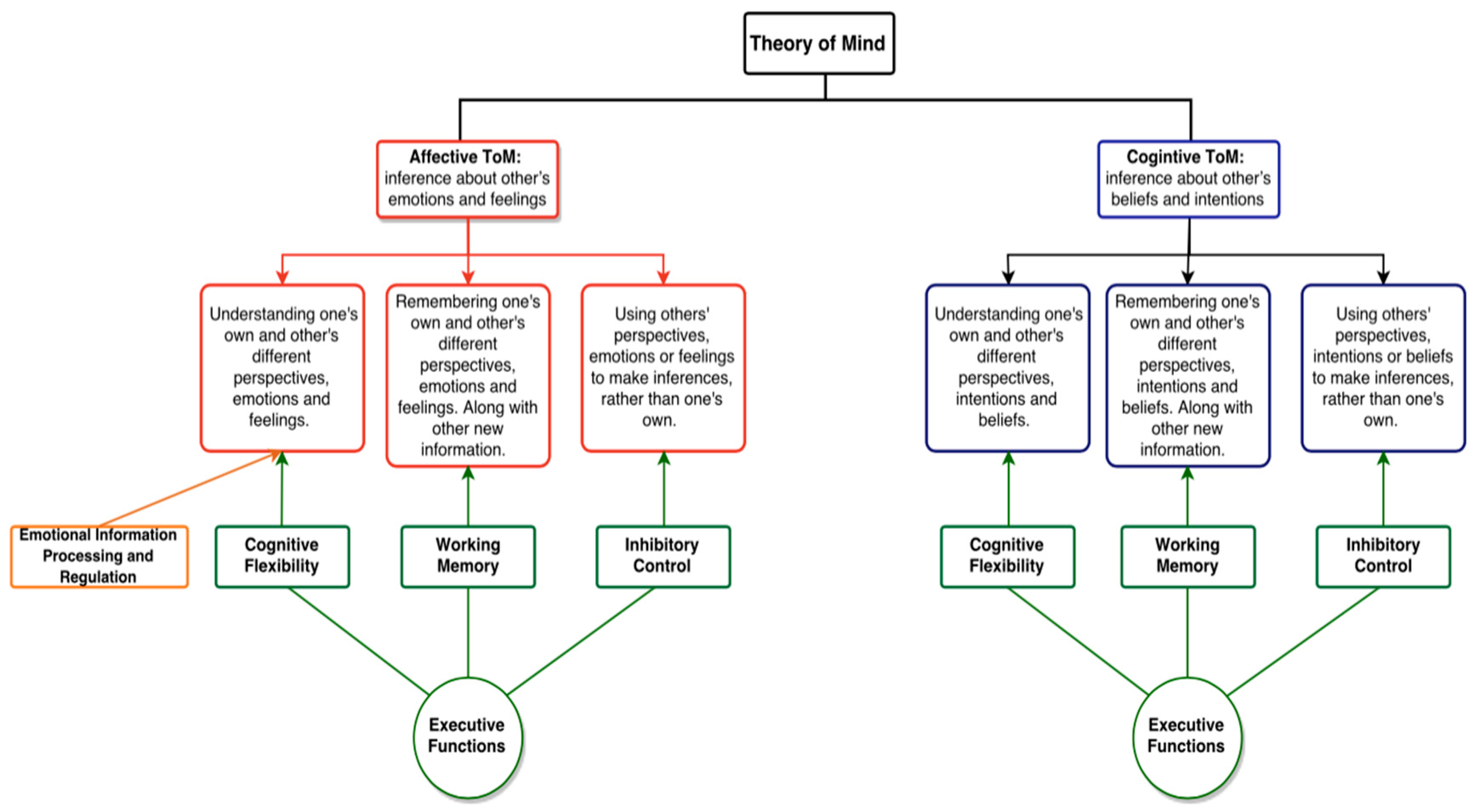
2. Theory of Mind
Executive Functioning and Theory of Mind
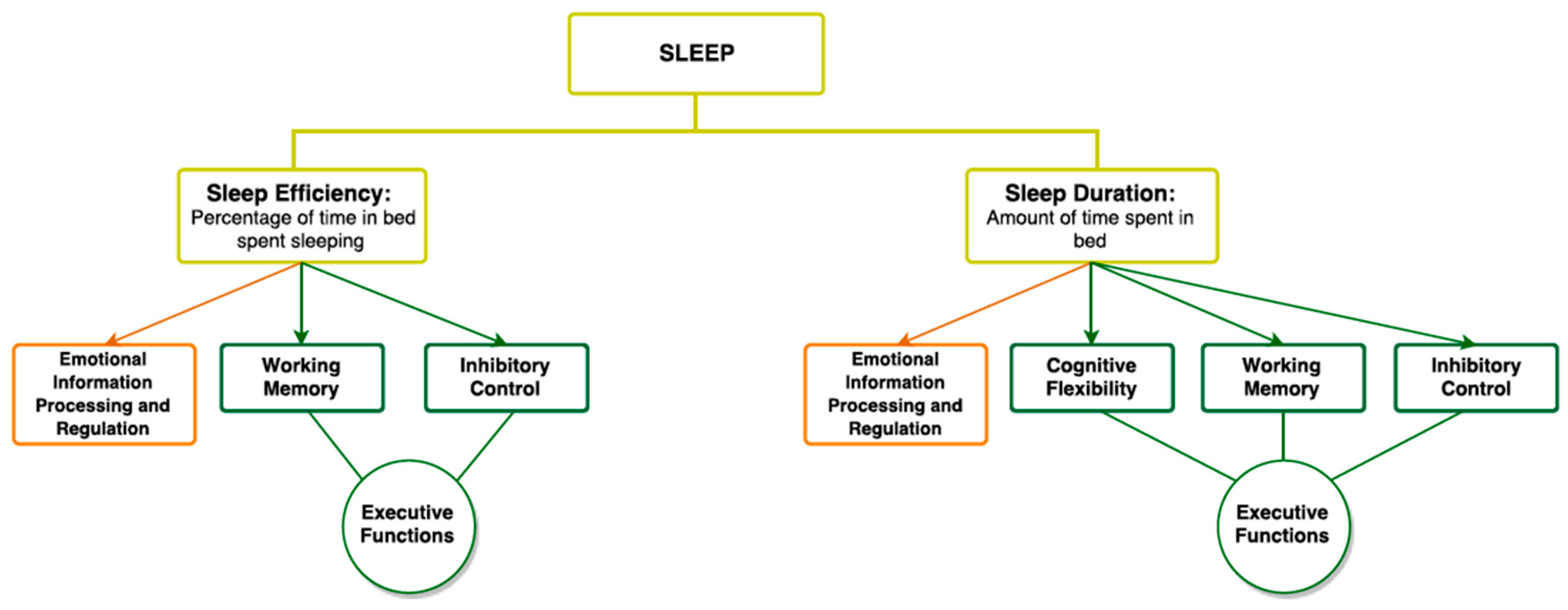
3. Sleep
3.1. Sleep and Executive Functioning
3.2. Sleep and Emotional Information Processing
4. Sleep and ToM: Are They Associated?
5. Attention Deficit/Hyperactivity Disorder
5.1. ADHD, EF, and Social Functioning
5.2. ADHD and ToM
5.3. ADHD and Sleep
6. Conclusions
Author Contributions
Conflicts of Interest
References
- Premack, D.; Woodruff, G. Does the chimpanzee have a theory of mind? Behav. Brain Sci. 1978, 1, 515–526. [Google Scholar] [CrossRef]
- Wellman, H.M. The child’s Theory of Mind; The MIT Press: Cambridge, MA, USA, 1990. [Google Scholar]
- Peterson, C.; Slaughter, V.; Moore, C.; Wellman, H.M. Peer social skills and theory of mind in children with autism, deafness, or typical development. Dev. Psychol. 2016, 52, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Watson, A.C.; Nixon, C.L.; Wilson, A.; Capage, L. Social interaction skills and theory of mind in young children. Dev. Psychol. 1999, 35, 386–391. [Google Scholar] [CrossRef] [PubMed]
- Imuta, K.; Henry, J.D.; Slaughter, V.; Selcuk, B.; Ruffman, T. Theory of mind and prosocial behavior in childhood: A meta-analytic review. Dev. Psychol. 2016, 52, 1192–1205. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Watling, D.; Caputi, M. Peer relations and the understanding of faux pas: Longitudinal evidence for bidirectional associations. Child Dev. 2011, 82, 1887–1905. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, V.; Imuta, K.; Peterson, C.C.; Henry, J.D. Meta-analysis of theory of mind and peer popularity in the preschool and early school years. Child Dev. 2015, 86, 1159–1174. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Berk, M. Theory of mind in major depressive disorder: A meta-analysis. J. Affect. Disord. 2016, 191, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Harkness, K.L.; Sabbagh, M.A.; Jacobson, J.A. Mental state decoding abilities in clinical depression. J. Affect. Disord. 2005, 86, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hezel, D.M.; McNally, R.J. Theory of mind impairments in social anxiety disorder. Behav. Ther. 2014, 45, 530–540. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S. Theory of mind and autism: A review. Int. Rev. Res. Ment. Retard. 2000, 23, 169–184. [Google Scholar] [CrossRef]
- Corkum, P.; Tannock, R.; Moldofsky, H.; Hogg-Johnson, S.; Humphries, T. Actigraphy and parental ratings of sleep in children with attention-deficit/hyperactivity disorder. Sleep 2001, 24, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Owens, J.A. The ADHD and sleep conundrum: A review. J. Dev. Behav. Pediatr. 2005, 26, 312–322. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, (DSM-5®); American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Visser, S.N.; Danielson, M.L.; Bitsko, R.H.; Holbrook, J.R.; Kogan, M.D.; Ghandour, R.M.; Blumberg, S.J. Trends in the parent-report of health care provider-diagnosed and medicated attention-deficit/hyperactivity disorder: United States, 2003–2011. J. Am. Acad. Child Adolesc. Psychiatry 2014, 53, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Hoza, B.; Mrug, S.; Gerdes, A.C.; Hinshaw, S.P.; Bukowski, W.M.; Gold, J.A.; Arnold, L.E. What aspects of peer relationships are impaired in children with attention-deficit/hyperactivity disorder? J. Consult. Clin. Psychol. 2005, 73, 411. [Google Scholar] [CrossRef] [PubMed]
- Mikami, A.Y. The importance of friendship for youth with attention-deficit/hyperactivity disorder. Clin. Child Fam. Psychol. Rev. 2010, 13, 181–198. [Google Scholar] [CrossRef] [PubMed]
- Braaten, E.B.; Rosén, L.A. Self-regulation of affect in attention deficit-hyperactivity disorder (ADHD) and non-ADHD boys: Differences in empathic responding. J. Consult. Clin. Psychol. 2000, 68, 313. [Google Scholar] [CrossRef] [PubMed]
- Ronk, M.J.; Hund, A.M.; Landau, S. Assessment of social competence of boys with attention-deficit/hyperactivity disorder: Problematic peer entry, host responses, and evaluations. J. Abnorm. Child Psychol. 2011, 39, 829–840. [Google Scholar] [CrossRef] [PubMed]
- Greene, R.W.; Biederman, J.; Faraone, S.V.; Sienna, M.; Garcia-Jetton, J. Adolescent outcome of boys with attention-deficit/hyperactivity disorder and social disability: Results from a 4-year longitudinal follow-up study. J. Consult. Clin. Psychol. 1997, 65, 758. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.G.; Mannuzza, S. Long-term outcome of hyperactive children: A review. J. Am. Acad. Child Adolesc. Psychiatry 1991, 30, 383–387. [Google Scholar] [CrossRef] [PubMed]
- Derefinko, K.J.; Bailey, U.L.; Milich, R.; Lorch, E.P.; Riley, E. The effects of stimulant medication on the online story narrations of children with ADHD. School Ment. Health 2009, 1, 171–182. [Google Scholar] [CrossRef]
- King, S.; Waschbusch, D.A.; Pelham, W.E., Jr.; Frankland, B.W.; Andrade, B.F.; Jacques, S.; Corkum, P.V. Social information processing in elementary-school aged children with ADHD: Medication effects and comparisons with typical children. J. Abnorm. Child Psychol. 2009, 37, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Sodian, B.; Schuwerk, T.; Kristen, S. Implicit and spontaneous theory of mind reasoning in autism spectrum disorders. In Autism Spectrum Disorder-Recent Advances; InTech: Rijeka, Croatia, 2015. [Google Scholar] [CrossRef]
- Schuwerk, T.; Vuori, M.; Sodian, B. Implicit and explicit theory of mind reasoning in autism spectrum disorders: the impact of experience. Autism 2015, 19, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Kovács, Á.M.; Téglás, E.; Endress, A.D. The social sense: Susceptibility to others’ beliefs in human infants and adults. Science 2010, 330, 1830–1834. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, H.; Perner, J. Beliefs about beliefs: Representation and constraining function of wrong beliefs in young children's understanding of deception. Cognition 1983, 13, 103–128. [Google Scholar] [CrossRef]
- Perner, J. Understanding the Representational Mind; The MIT Press: Cambridge, MA, US, 1991; p. 348. [Google Scholar]
- Wellman, H.M.; Cross, D.; Watson, J. Meta-analysis of theory-of-mind development: The truth about false belief. Child Dev. 2001, 72, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Frith, U. Interacting minds—A biological basis. Science (New York, N.Y.) 1999, 286, 1692–1695. [Google Scholar] [CrossRef]
- Hughes, C.; Devine, R.T.; Ensor, R.; Masuo, K.; Ai, M.; Lecce, S. Lost in translation? Comparing british, japanese, and italian children’s theory-of-mind performance. Child Dev. Res. 2014, 1–10. [Google Scholar] [CrossRef]
- Sabbagh, M.A.; Xu, F.; Carlson, S.M.; Moses, L.J.; Lee, K. The development of executive functioning and theory of mind. A comparison of Chinese and U.S. Preschoolers. Psychol. Sci. 2006, 17, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.A. Children’s understanding of second-order mental states. Psychol. Bull. 2009, 135, 749–773. [Google Scholar] [CrossRef] [PubMed]
- Perner, J.; Wimmer, H. “John thinks that mary thinks that…” attribution of second-order beliefs by 5- to 10-year-old children. J. Exp. Child Psychol. 1985, 39, 437–471. [Google Scholar] [CrossRef]
- Happe, F. Theory of mind and the self. Ann. N. Y. Acad. Sci. 2003, 1001, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Segrin, C. Social skills deficits associated with depression. Clin. Psychol. Rev. 2000, 20, 379–403. [Google Scholar] [CrossRef]
- Spitzberg, B.H.; Cupach, W.R. Issues in interpersonal competence research. In Handbook of Interpersonal Competence Research; Springer: New York, NY, USA, 1989; pp. 52–75. [Google Scholar]
- Caputi, M.; Lecce, S.; Pagnin, A.; Banerjee, R. Longitudinal effects of theory of mind on later peer relations: The role of prosocial behavior. Dev. Psychol. 2012, 48, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Baurain, C.; Nader-Grosbois, N. Compétences Sociales et émotionnelles: Enfant Typique et Déficient Intellectuel; Presses Académiques Francophones: Saarbrücken, Germany, 2013. [Google Scholar]
- Riggs, N.R.; Greenberg, M.T.; Kusche, C.A.; Pentz, M.A. The mediational role of neurocognition in the behavioral outcomes of a social-emotional prevention program in elementary school students: Effects of the paths curriculum. Prev. Sci. Off. J. Soc. Prev. Res. 2006, 7, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.J.; Fletcher, J.; French, D.J. Social reasoning: A source of influence on aggression. Clin. Psychol. Rev. 2001, 21, 447–469. [Google Scholar] [CrossRef]
- Renouf, A.; Brendgen, M.; Parent, S.; Vitaro, F.; David Zelazo, P.; Boivin, M.; Dionne, G.; Tremblay, R.E.; Pérusse, D.; Séguin, J.R. Relations between theory of mind and indirect and physical aggression in kindergarten: Evidence of the moderating role of prosocial behaviors. Soc. Dev. 2010, 19, 535–555. [Google Scholar] [CrossRef]
- Kalbe, E.; Schlegel, M.; Sack, A.T.; Nowak, D.A.; Dafotakis, M.; Bangard, C.; Brand, M.; Shamay-Tsoory, S.; Onur, O.A.; Kessler, J. Dissociating cognitive from affective theory of mind: A tms study. Cortex J. Devot. Study Nerv. Syst. Behav. 2010, 46, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Shur, S.; Barcai-Goodman, L.; Medlovich, S.; Harari, H.; Levkovitz, Y. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007, 149, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Abu-Akel, A.; Shamay-Tsoory, S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia 2011, 49, 2971–2984. [Google Scholar] [CrossRef] [PubMed]
- Bottiroli, S.; Cavallini, E.; Ceccato, I.; Vecchi, T.; Lecce, S. Theory of mind in aging: Comparing cognitive and affective components in the faux pas test. Arch. Gerontol. Geriatr. 2016, 62, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Roca, M.; Parr, A.; Thompson, R.; Woolgar, A.; Torralva, T.; Antoun, N.; Manes, F.; Duncan, J. Executive function and fluid intelligence after frontal lobe lesions. Brain J. Neurol. 2010, 133, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, C.L.; Fontaine, N.M.; Bird, G.; Blakemore, S.J.; Brito, S.A.; McCrory, E.J.; Viding, E. Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Soc. Cognit. Affect. Neurosci. 2012, 7, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J. Dissociable prefrontal networks for cognitive and affective theory of mind: A lesion study. Neuropsychologia 2007, 45, 3054–3067. [Google Scholar] [CrossRef] [PubMed]
- Shamay-Tsoory, S.G.; Tibi-Elhanany, Y.; Aharon-Peretz, J. The ventromedial prefrontal cortex is involved in understanding affective but not cognitive theory of mind stories. Soc. Neurosci. 2006, 1, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Van Overwalle, F.; Baetens, K. Understanding others’ actions and goals by mirror and mentalizing systems: A meta-analysis. NeuroImage 2009, 48, 564–584. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.D.; Frith, U. The neural basis of mentalizing. Neuron 2006, 50, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Schurz, M.; Radua, J.; Aichhorn, M.; Richlan, F.; Perner, J. Fractionating theory of mind: A meta-analysis of functional brain imaging studies. Neurosci. Biobehav. Rev. 2014, 42, 9–34. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Jolliffe, T.; Mortimore, C.; Robertson, M. Another advanced test of theory of mind: Evidence from very high functioning adults with autism or asperger syndrome. J. Child Psychol. Psychiatry Allied Discip. 1997, 38, 813–822. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “reading the mind in the eyes” test revised version: A study with normal adults, and adults with asperger syndrome or high-functioning autism. J. Child Psychol. Psychiatry Allied Discip. 2001, 42, 241–251. [Google Scholar] [CrossRef]
- Adolphs, R.; Sears, L.; Piven, J. Abnormal processing of social information from faces in autism. J. Cognit. Neurosci. 2001, 13, 232–240. [Google Scholar] [CrossRef]
- Yoo, S.S.; Gujar, N.; Hu, P.; Jolesz, F.A.; Walker, M.P. The human emotional brain without sleep—A prefrontal amygdala disconnect. Curr. Biol. 2007, 17, R877–R878. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.A. Emotion regulation: A theme in search of definition. Monogr.Soc. Res. Child Dev. 1994, 59, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.E.; Baron-Cohen, S.; Knight, R.T. Frontal lobe contributions to theory of mind. J. Cognit. Neurosci. 1998, 10, 640–656. [Google Scholar] [CrossRef]
- Carlson, S.M.; Zelazo, P.D.; Faja, S. Executive function. In The Oxford Handbook of Developmental Psychology; Zelazo, P.D., Ed.; Oxford University Press: New York, NY, USA, 2013; Volume 1, pp. 706–743. [Google Scholar]
- Diamond, A. Executive functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Miyake, A. The relations among inhibition and interference control functions: A latent-variable analysis. J. Exp. Psychol. Gen. 2004, 133, 101–135. [Google Scholar] [CrossRef] [PubMed]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognit. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef] [PubMed]
- Devine, R.T.; Hughes, C. Relations between false belief understanding and executive function in early childhood: A meta-analysis. Child Dev. 2014, 85, 1777–1794. [Google Scholar] [CrossRef] [PubMed]
- Fuster, J.M. The Prefrontal Cortex, 4th ed.; Academic Press: Boston, MA, USA, 2008. [Google Scholar]
- Davidson, M.C.; Amso, D.; Anderson, L.C.; Diamond, A. Development of cognitive control and executive functions from 4 to 13 years: Evidence from manipulations of memory, inhibition, and task switching. Neuropsychologia 2006, 44, 2037–2078. [Google Scholar] [CrossRef] [PubMed]
- Apperly, I.A.; Warren, F.; Andrews, B.J.; Grant, J.; Todd, S. Developmental continuity in theory of mind: Speed and accuracy of belief-desire reasoning in children and adults. Child Dev. 2011, 82, 1691–1703. [Google Scholar] [CrossRef] [PubMed]
- Devine, R.T.; Hughes, C. Silent films and strange stories: Theory of mind, gender, and social experiences in middle childhood. Child Dev. 2013, 84, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Carlson, S.M.; Moses, L.J. Individual differences in inhibitory control and children's theory of mind. Child Dev. 2001, 72, 1032–1053. [Google Scholar] [CrossRef] [PubMed]
- Austin, G.; Groppe, K.; Elsner, B. The reciprocal relationship between executive function and theory of mind in middle childhood: A 1-year longitudinal perspective. Front. Psychol. 2014, 5, 655. [Google Scholar] [CrossRef] [PubMed]
- Bock, A.M.; Gallaway, K.C.; Hund, A.M. Specifying links between executive functioning and theory of mind during middle childhood: Cognitive flexibility predicts social understanding. J. Cognit. Dev. 2015, 16, 509–521. [Google Scholar] [CrossRef]
- Perner, J.; Lang, B. Theory of mind and executive function: Is there a developmental relationship? In Understanding Other Minds: Perspectives from Developmental Cognitive Neuroscience, 2nd ed.; Oxford University Press: New York, NY, USA, 2000; pp. 150–181. [Google Scholar]
- Borbely, A.A. A two process model of sleep regulation. Hum. Neurobiol. 1982, 1, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Hirshkowitz, M.; Whiton, K.; Albert, S.M.; Alessi, C.; Bruni, O.; DonCarlos, L.; Neubauer, D.N. National Sleep Foundation’s sleep time duration recommendations: Methodology and results summary. Sleep Health 2015, 1, 40–43. [Google Scholar] [CrossRef]
- Paruthi, S.; Brooks, L.J.; D’Ambrosio, C.; Hall, W.A.; Kotagal, S.; Lloyd, R.M.; Rosen, C.L. Recommended amount of sleep for pediatric populations: A consensus statement of the American Academy of Sleep Medicine. J. Clin. Sleep Med. JCSM Off. Publ. Am. Acad. Sleep Med. 2016, 12, 785. [Google Scholar] [CrossRef] [PubMed]
- Tremblay, M.S.; Carson, V.; Chaput, J.P.; Connor Gorber, S.; Dinh, T.; Duggan, M.; Janssen, I. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep 1. Appl. Physiol. Nutr. Metab. 2016, 41, S311–S327. [Google Scholar] [CrossRef] [PubMed]
- Beebe, D.W.; Wells, C.T.; Jeffries, J.; Chini, B.; Kalra, M.; Amin, R. Neuropsychological effects of pediatric obstructive sleep apnea. J. Int. Neuropsychol. Soc. JINS 2004, 10, 962–975. [Google Scholar] [CrossRef] [PubMed]
- Bourke, R.; Anderson, V.; Yang, J.S.; Jackman, A.R.; Killedar, A.; Nixon, G.M.; Davey, M.J.; Walker, A.M.; Trinder, J.; Horne, R.S. Cognitive and academic functions are impaired in children with all severities of sleep-disordered breathing. Sleep Med. 2011, 12, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Dahl, R.E. The impact of inadequate sleep on children’s daytime cognitive function. Semin. Pediatr. Neurol. 1996, 3, 44–50. [Google Scholar] [CrossRef]
- Friedman, N.P.; Corley, R.P.; Hewitt, J.K.; Wright, K.P., Jr. Individual differences in childhood sleep problems predict later cognitive executive control. Sleep 2009, 32, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.M.; Caspi, A.; Moffitt, T.E.; Poulton, R. Sleep problems in childhood predict neuropsychological functioning in adolescence. Pediatrics 2009, 123, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T.D.; Nelson, J.M.; Kidwell, K.M.; James, T.D.; Espy, K.A. Preschool sleep problems and differential associations with specific aspects of executive control in early elementary school. Dev. Neuropsychol. 2015, 40, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, J.P.; Soderstrom, M.; Karlsson, A.U.; Lekander, M.; Akerstedt, T.; Lindroth, N.E.; Axelsson, J. Less effective executive functioning after one night’s sleep deprivation. J. Sleep Res. 2005, 14, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Taveras, E.M.; Rifas-Shiman, S.L.; Bub, K.L.; Gillman, M.W.; Oken, E. Prospective study of insufficient sleep and neurobehavioral functioning among school-age children. Acad. Pediatr. 2017, 17, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Owens, J. Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics 2014, 134, e921–e932. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, M.; Wickwire, E.M.; Hirshkowitz, M.; Albert, S.M.; Avidan, A.; Daly, F.J.; Dauvilliers, Y.; Ferri, R.; Fung, C.; Gozal, D.; et al. National sleep foundation's sleep quality recommendations: First report. Sleep Health 2017, 3, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Durmer, J.S.; Dinges, D.F. Neurocognitive consequences of sleep deprivation. Semin. Neurol. 2005, 25, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Edinger, J.D.; Bonnet, M.H.; Bootzin, R.R.; Doghramji, K.; Dorsey, C.M.; Espie, C.A.; Stepanski, E.J. Derivation of research diagnostic criteria for insomnia: Report of an American Academy of Sleep Medicine Work Group. Sleep 2004, 27, 1567–1596. [Google Scholar] [CrossRef] [PubMed]
- Morin, C.M.; Benca, R. Chronic insomnia. Lancet 2012, 379, 1129–1141. [Google Scholar] [CrossRef]
- Lu, F.M.; Liu, C.H.; Lu, S.L.; Tang, L.R.; Tie, C.L.; Zhang, J.; Yuan, Z. Disrupted topology of frontostriatal circuits is linked to the severity of insomnia. Front. Neurosci. 2017, 11, 214. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.D.; Moore, S.; Cramer, S.C.; Lin, J.J. Caudate atrophy and impaired frontostriatal connections are linked to executive dysfunction in temporal lobe epilepsy. Epilepsy Behav. 2011, 21, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Chuah, Y.M.; Venkatraman, V.; Dinges, D.F.; Chee, M.W. The neural basis of interindividual variability in inhibitory efficiency after sleep deprivation. J. Neuroscience Off. J. Soc. Neurosci. 2006, 26, 7156–7162. [Google Scholar] [CrossRef] [PubMed]
- Drummond, S.P.; Paulus, M.P.; Tapert, S.F. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J. Sleep Res. 2006, 15, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Wiebe, S.; Montecalvo, L.; Brunetti, B.; Amsel, R.; Carrier, J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep 2011, 34, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Maski, K.P.; Kothare, S.V. Sleep deprivation and neurobehavioral functioning in children. Int. J. Psychophysiol. Off. J. Int. Organ. Psychophysiol. 2013, 89, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Molfese, D.L.; Ivanenko, A.; Key, A.F.; Roman, A.; Molfese, V.J.; O’Brien, L.M.; Gozal, D.; Kota, S.; Hudac, C.M. A one-hour sleep restriction impacts brain processing in young children across tasks: Evidence from event-related potentials. Dev. Neuropsychol. 2013, 38, 317–336. [Google Scholar] [CrossRef] [PubMed]
- Sadeh, A.; Gruber, R.; Raviv, A. Sleep, neurobehavioral functioning, and behavior problems in school-age children. Child Dev. 2002, 73, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Halperin, J.M.; Sharma, V.; Greenblatt, E.; Schwartz, S.T. Assessment of the continuous performance test: Reliability and validity in a nonreferred sample. Psychol. Assess. A J. Consult. Clin. Psychol. 1991, 3, 603–608. [Google Scholar] [CrossRef]
- Sadeh, A.; Gruber, R.; Raviv, A. The effects of sleep restriction and extension on school-age children: What a difference an hour makes. Child Dev. 2003, 74, 444–455. [Google Scholar] [CrossRef] [PubMed]
- Gruber, R.; Michaelsen, S.; Bergmame, L.; Frenette, S.; Bruni, O.; Fontil, L.; Carrier, J. Short sleep duration is associated with teacher-reported inattention and cognitive problems in healthy school-aged children. Nat. Sci. Sleep 2012, 4, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Kopasz, M.; Loessl, B.; Hornyak, M.; Riemann, D.; Nissen, C.; Piosczyk, H.; Voderholzer, U. Sleep and memory in healthy children and adolescents—A critical review. Sleep Med. Rev. 2010, 14, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Steenari, M.R.; Vuontela, V.; Paavonen, E.J.; Carlson, S.; Fjallberg, M.; Aronen, E. Working memory and sleep in 6- to 13-year-old schoolchildren. J. Am. Acad. Child Adolesc. Psychiatry 2003, 42, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Vriend, J.L.; Davidson, F.D.; Corkum, P.V.; Rusak, B.; Chambers, C.T.; McLaughlin, E.N. Manipulating sleep duration alters emotional functioning and cognitive performance in children. J. Pediatr. Psychol. 2013, 38, 1058–1069. [Google Scholar] [CrossRef] [PubMed]
- Dewald-Kaufmann, J.F.; Oort, F.J.; Meijer, A.M. The effects of sleep extension on sleep and cognitive performance in adolescents with chronic sleep reduction: An experimental study. Sleep Med. 2013, 14, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.C.; Buchsbaum, M.; Bunney, W.E., Jr. Clinical neurochemical implications of sleep deprivation’s effects on the anterior cingulate of depressed responders. Neuropsychopharmacol. Off. Publ. Am. Coll. Neuropsychopharmacol. 2001, 25, S74–S78. [Google Scholar] [CrossRef]
- Perlman, S.B.; Pelphrey, K.A. Developing connections for affective regulation: Age-related changes in emotional brain connectivity. J. Exp. Child Psychol. 2011, 108, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.H.; Miller, A.L.; Seifer, R.; Cares, S.R.; LeBourgeois, M.K. Acute sleep restriction effects on emotion responses in 30- to 36-month-old children. J. Sleep Res. 2012, 21, 235–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deliens, G.; Gilson, M.; Peigneux, P. Sleep and the processing of emotions. Exp. Brain Res. 2014, 232, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Soffer-Dudek, N.; Shahar, G. Daily stress interacts with trait dissociation to predict sleep-related experiences in young adults. J. Abnorm. Psychol. 2011, 120, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Killgore, W.D. Self-reported sleep correlates with prefrontal-amygdala functional connectivity and emotional functioning. Sleep 2013, 36, 1597–1608. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Webb, C.A.; Deldonno, S.R.; Kipman, M.; Schwab, Z.J.; Weiner, M.R.; Killgore, W.D. Habitual ‘sleep credit’ is associated with greater grey matter volume of the medial prefrontal cortex, higher emotional intelligence and better mental health. J. Sleep Res. 2013, 22, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Doyle, A.E. Executive functions in attention-deficit/hyperactivity disorder. J. Clin. Psychiatry 2005, 67, 21–26. [Google Scholar]
- Barkley, R.A. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol. Bull. 1997, 121, 65. [Google Scholar] [CrossRef] [PubMed]
- Willcutt, E.G.; Doyle, A.E.; Nigg, J.T.; Faraone, S.V.; Pennington, B.F. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol. Psychiatry 2005, 57, 1336–1346. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, K.; Bunte, T.; Wiebe, S.A.; Espy, K.A.; Deković, M.; Matthys, W. Executive function deficits in preschool children with ADHD and DBD. J. Child Psychol. Psychiatry 2012, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Durston, S.; Mulder, M.; Casey, B.J.; Ziermans, T.; van Engeland, H. Activation in ventral prefrontal cortex is sensitive to genetic vulnerability for attention-deficit hyperactivity disorder. Biol. Psychiatry 2006, 60, 1062–1070. [Google Scholar] [CrossRef] [PubMed]
- Shaw, P.; Rabin, C. New insights into attention-deficit/hyperactivity disorder using structural neuroimaging. Curr. Psychiatry Rep. 2009, 11, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.P.; Newcorn, J.H.; Fan, J.I.N.; Tang, C.Y.; Halperin, J.M. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Loe, I.M.; Feldman, H.M. Academic and educational outcomes of children with ADHD. J. Pediatr. Psychol. 2007, 32, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Biederman, J.; Monuteaux, M.C.; Doyle, A.E.; Seidman, L.J.; Wilens, T.E.; Ferrero, F.; Faraone, S.V. Impact of executive function deficits and attention-deficit/hyperactivity disorder (ADHD) on academic outcomes in children. J. Consult. Clin. Psychol. 2004, 72, 757. [Google Scholar] [CrossRef] [PubMed]
- Savitz, J.B.; Jansen, P. The Stroop Color-Word Interference Test as an indicator of ADHD in poor readers. J. Genet. Psychol. 2003, 164, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Langberg, J.M.; Epstein, J.N.; Urbanowicz, C.; Simon, J.; Graham, A. Efficacy of an organization skills intervention to improve the academic functioning of students with ADHD. School Psychol. Q. 2008, 23, 407–417. [Google Scholar] [CrossRef]
- Gureasko-Moore, S.; Dupaul, G.J.; White, G.P. The effects of self-management in general education classrooms on the organizational skills of adolescents with ADHD. Behav. Modif. 2008, 30, 159–183. [Google Scholar] [CrossRef] [PubMed]
- DuPaul, G.J.; McGoey, K.E.; Eckert, T.L.; VanBrakle, J. Preschool children with attention-deficit/hyperactivity disorder: impairments in behavioral, social, and school functioning. J. Am. Acad. Child Adolesc. Psychiatry 2001, 40, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Frankel, F.; Feinberg, D. Social problems associated with ADHD vs. ODD in children referred for friendship problems. Child Psychiatry Hum. Dev. 2002, 33, 125–146. [Google Scholar] [CrossRef] [PubMed]
- Grygiel, P.; Humenny, G.; Rębisz, S.; Bajcar, E.; Świtaj, P. Peer Rejection and Perceived Quality of Relations With Schoolmates Among Children With ADHD. J. Atten. Disord. 2014. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, D.; Hinshaw, S.P. Initial sociometric impressions of attention-deficit hyperactivity disorder and comparison boys: Predictions from social behaviors and from nonbehavioral variables. J. Consult. Clin. Psychol. 1994, 62, 833. [Google Scholar] [CrossRef] [PubMed]
- Pelham, W.E.; Bender, M.E. Peer relationships in hyperactive children: Description and treatment. Adv. Learn. Behav. Disabil. 1982, 1, 365–436. [Google Scholar]
- Gresham, F.M.; MacMillan, D.L.; Bocian, K.M.; Ward, S.L.; Forness, S.R. Comorbidity of hyperactivity-impulsivity-inattention and conduct problems: Risk factors in social, affective, and academic domains. J. Abnorm. Child Psychol. 1998, 26, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Hoza, B.; Gerdes, A.C.; Hinshaw, S.P.; Arnold, L.E.; Pelham, W.E., Jr.; Molina, B.S.; Odbert, C. Self-perceptions of competence in children with ADHD and comparison children. J. Consult. Clin. Psychol. 2004, 72, 382. [Google Scholar] [CrossRef] [PubMed]
- Mary, A.; Slama, H.; Mousty, P.; Massat, I.; Capiau, T.; Drabs, V.; Peigneux, P. Executive and attentional contributions to Theory of Mind deficit in attention deficit/hyperactivity disorder (ADHD). Child Neuropsychol. 2016, 22, 345–365. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, T.C.; Panayiotou, G.; Spanoudis, G.; Natsopoulos, D. Evidence of poor planning in children with attention deficits. J. Abnorm. Child Psychol. 2005, 33, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Wheeler Maedgen, J.; Carlson, C.L. Social functioning and emotional regulation in the attention deficit hyperactivity disorder subtypes. J. Clin. Child Psychol. 2000, 29, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.M.; Hermens, D.F.; Palmer, D.; Kohn, M.; Clarke, S.; Keage, H.; Gordon, E. Misinterpreting emotional expressions in attention-deficit/hyperactivity disorder: Evidence for a neural marker and stimulant effects. Biol. Psychiatry 2008, 63, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.A.; Blair, R.J.R. Deficits in facial affect recognition among antisocial populations: A meta-analysis. Neurosci. Biobehav. Rev. 2008, 32, 454–465. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.L.; Galán, C.A.; Tottenham, N.; Lee, S.S. Impaired social decision-making mediates the association between ADHD and social problems. J. Abnorm. Child Psychol. 2016, 44, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.F.; Waschbusch, D.A.; Doucet, A.; King, S.; MacKinnon, M.; McGrath, P.J.; Corkum, P. Social information processing of positive and negative hypothetical events in children with ADHD and conduct problems and controls. J. Atten. Disord. 2012, 16, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Chervin, R.D.; Archbold, K.H.; Dillon, J.E.; Pituch, K.J.; Panahi, P.; Dahl, R.E.; Guilleminault, C. Associations between symptoms of inattention, hyperactivity, restless legs, and periodic leg movements. Sleep 2002, 25, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Picchietti, D.L.; England, S.J.; Walters, A.S.; Willis, K.; Verrico, T. Periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. J. Child Neurol. 1998, 13, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Golan, N.; Shahar, E.; Ravid, S.; Pillar, G. Sleep disorders and daytime sleepiness in children with attention-deficit/hyperactive disorder. Sleep 2004, 27, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Konofal, E.; Lecendreux, M.; Cortese, S. Sleep and ADHD. Sleep Med. 2010, 11, 652–658. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tesfaye, R.; Gruber, R. The Association between Sleep and Theory of Mind in School Aged Children with ADHD. Med. Sci. 2017, 5, 18. https://doi.org/10.3390/medsci5030018
Tesfaye R, Gruber R. The Association between Sleep and Theory of Mind in School Aged Children with ADHD. Medical Sciences. 2017; 5(3):18. https://doi.org/10.3390/medsci5030018
Chicago/Turabian StyleTesfaye, Rackeb, and Reut Gruber. 2017. "The Association between Sleep and Theory of Mind in School Aged Children with ADHD" Medical Sciences 5, no. 3: 18. https://doi.org/10.3390/medsci5030018




