Long-Term Electroclinical and Employment Follow up in Temporal Lobe Epilepsy Surgery. A Cuban Comprehensive Epilepsy Surgery Program
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population
2.2. Presurgical Evaluation
2.3. Surgical Procedure and Resection Size
2.4. Tissue Characterization and Histopathological Examination
2.5. Post Operative Follow-Up
2.6. Statistics Analysis
2.7. Ethical Considerations
3. Results
3.1. Demographic Profile and Electroclinical Features
3.1.1. Temporal Lobectomy and Complications
3.1.2. Pathology
3.2. Electroclinical Follow Up
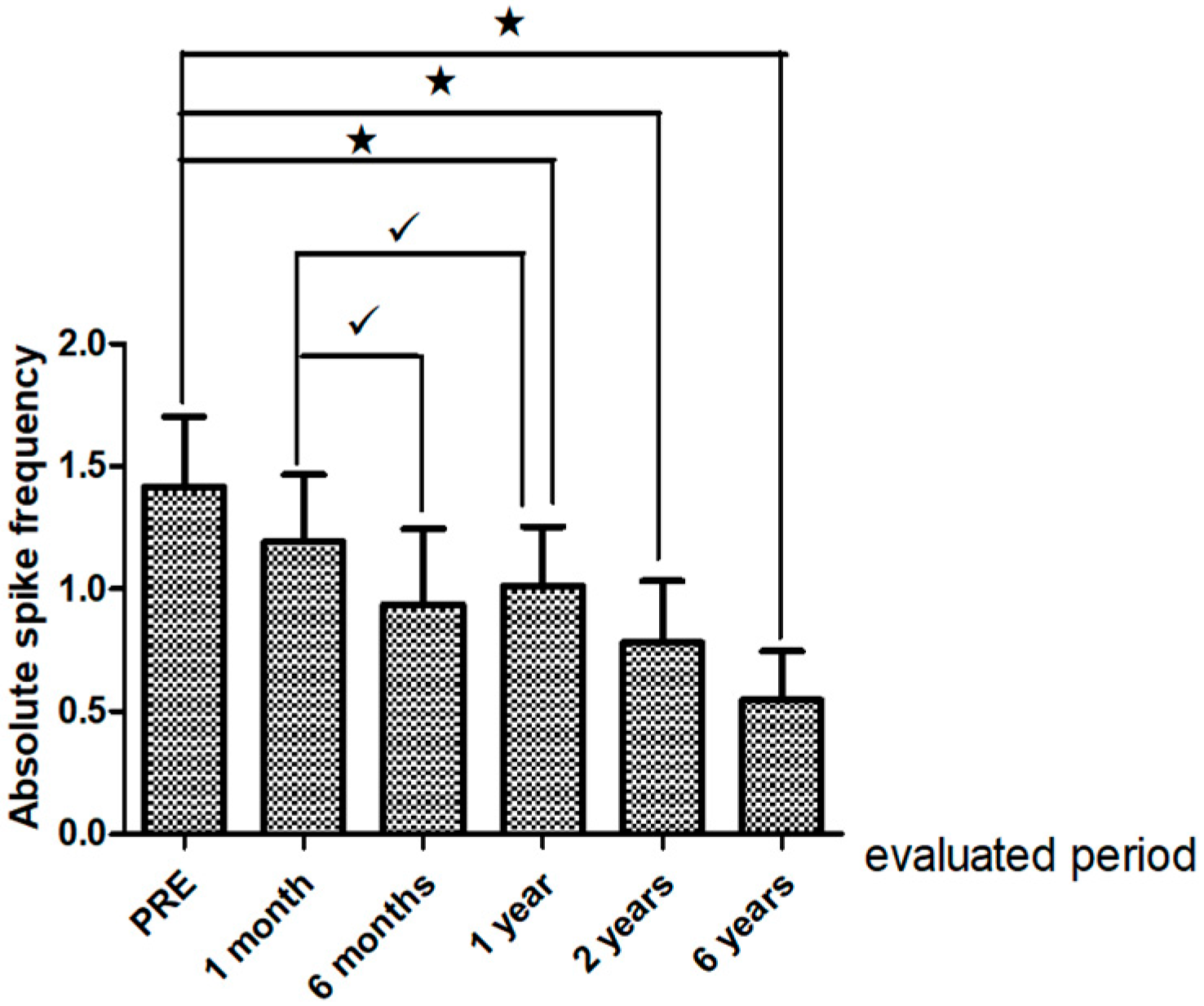
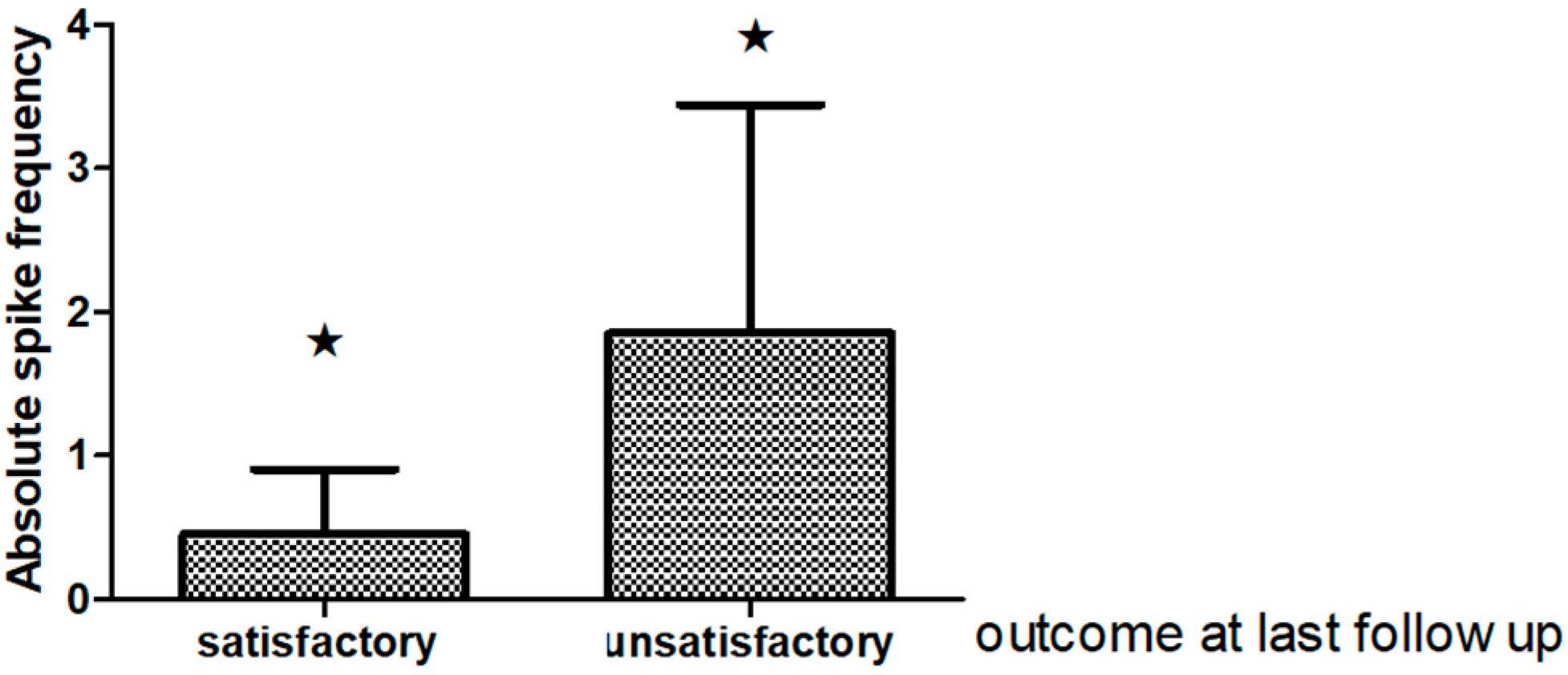
Interictal Epileptiform Discharges on Post-Operative EEG
3.3. Pre and Post-Surgery Education and Employment Status in TLE Patients
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gonzalez-Pal, S.; Quintana, M.J.; Roman Lopez, J.R.; Fernandez-Perez, J.E. The direct cost of epilepsy in Cuba. A study in outpatients. Rev. Neurol. 2005, 41, 379–381. [Google Scholar] [PubMed]
- Asadi-Pooya, A.A.; Sperling, M.R. Strategies for surgical treatment of epilepsies in developing countries. Epilepsia 2008, 49, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Zenteno, J.F.; Ladino, L.D. Temporal epilepsy: Clinical, diagnostic and therapeutic aspects. Rev. Neurol. 2013, 56, 229–242. [Google Scholar] [PubMed]
- Kwon, C.S.; Neal, J.; Tellez-Zenteno, J.; Metcalfe, A.; Fitzgerald, K.; Hernandez-Ronquillo, L.; Hader, W.; Wiebe, S.; Jette, N. Resective focal epilepsy surgery—Has selection of candidates changed? A systematic review. Epilepsy Res. 2016, 122, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr. What can we do for people with drug-resistant epilepsy? The 2016 Wartenberg Lecture. Neurology 2016, 87, 2483–2489. [Google Scholar] [CrossRef] [PubMed]
- Kasradze, S.; Alkhidze, M.; Lomidze, G.; Japaridze, G.; Tsiskaridze, A.; Zangaladze, A. Perspectives of epilepsy surgery in resource-poor countries: A study in Georgia. Acta Neurochir. (Wien) 2015, 157, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, K. Challenges in the management of epilepsy in resource-poor countries. Nat. Rev. Neurol. 2009, 5, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Rathore, C.; Rao, M.B.; Radhakrishnan, K. National epilepsy surgery program: Realistic goals and pragmatic solutions. Neurol. India 2014, 62, 124–129. [Google Scholar] [PubMed]
- Asadi-Pooya, A.A.; Rakei, S.M.; Kamgarpour, A.; Taghipour, M.; Ashjazadeh, N.; Razmkon, A.; Zare, Z.; Bagheri, M.H. Outcome after temporal lobectomy in patients with medically-refractory mesial temporal epilepsy in Iran. J. Neurosurg. Sci. 2017, 61, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Scott, R.A.; Lhatoo, S.D.; Sander, J.W. The treatment of epilepsy in developing countries: Where do we go from here? Bull. World Health Organ. 2001, 79, 344–351. [Google Scholar] [PubMed]
- Mani, K.S.; Subbakrishna, D.K. Perspectives from a developing nation with special reference to rural areas. Epilepsia 2003, 44 (Suppl. 1), 55–57. [Google Scholar] [CrossRef] [PubMed]
- Mbuba, C.K.; Newton, C.R. Packages of care for epilepsy in low- and middle-income countries. PLoS Med. 2009, 6, e1000162. [Google Scholar] [CrossRef] [PubMed]
- Cross, J.H.; Jayakar, P.; Nordli, D.; Delalande, O.; Duchowny, M.; Wieser, H.G.; Guerrini, R.; Mathern, G.W. Proposed criteria for referral and evaluation of children for epilepsy surgery: Recommendations of the Subcommission for Pediatric Epilepsy Surgery. Epilepsia 2006, 47, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Perry, M.S.; Duchowny, M. Surgical versus medical treatment for refractory epilepsy: Outcomes beyond seizure control. Epilepsia 2013, 54, 2060–2070. [Google Scholar] [CrossRef] [PubMed]
- Morales, L.M.; Sanchez, C.; Bender, J.E.; Bosch, J.; Garcia, M.E.; Garcia, I.; Lorigados, L.; Estupinan, B.; Trapaga, O.; Baez, M.; et al. A neurofunctional evaluation strategy for presurgical selection of temporal lobe epilepsy patients. MEDICC Rev. 2009, 11, 29–35. [Google Scholar] [PubMed]
- Morales-Chacón, L.M.; Alfredo Sanchez, C.C.; Minou Baez, M.M.; Rodriguez, R.R.; Lorigados, P.L.; Estupiñan, D.B. Multimodal imaging in nonlesional medically intractable focal epilepsy. Front. Biosci. 2015, 7, 42–57. [Google Scholar] [CrossRef]
- Ebersole, J.S.; Pacia, S.V. Localization of temporal lobe foci by ictal EEG patterns. Epilepsia 1996, 37, 386–399. [Google Scholar] [CrossRef] [PubMed]
- Blumcke, I.; Muhlebner, A. Neuropathological work-up of focal cortical dysplasias using the new ILAE consensus classification system—Practical guideline article invited by the Euro-CNS Research Committee. Clin. Neuropathol. 2011, 30, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr. Update on surgical treatment of the epilepsies. Summary of the Second International Palm Desert Conference on the Surgical Treatment of the Epilepsies (1992). Neurology 1993, 43, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Engel, J., Jr.; McDermott, M.P.; Wiebe, S.; Langfitt, J.T.; Stern, J.M.; Dewar, S.; Sperling, M.R.; Gardiner, I.; Erba, G.; Fried, I.; et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. JAMA 2012, 307, 922–930. [Google Scholar] [CrossRef] [PubMed]
- Tellez-Zenteno, J.F.; Dhar, R.; Wiebe, S. Long-term seizure outcomes following epilepsy surgery: A systematic review and meta-analysis. Brain 2005, 128, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Tonini, C.; Beghi, E.; Berg, A.T.; Bogliun, G.; Giordano, L.; Newton, R.W.; Tetto, A.; Vitelli, E.; Vitezic, D.; Wiebe, S. Predictors of epilepsy surgery outcome: A meta-analysis. Epilepsy Res. 2004, 62, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Goldenholz, D.M.; Jow, A.; Khan, O.I.; Bagic, A.; Sato, S.; Auh, S.; Kufta, C.; Inati, S.; Theodore, W.H. Preoperative prediction of temporal lobe epilepsy surgery outcome. Epilepsy Res. 2016, 127, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Modur, P.N.; Barot, N.; Van Ness, P.C.; Agostini, M.A.; Ding, K.; Gupta, P.; Hays, R.; Mickey, B. Predictors of Postoperative Seizure Recurrence: A Longitudinal Study of Temporal and Extratemporal Resections. Epilepsy Res. Treat. 2016, 2016, 7982494. [Google Scholar] [CrossRef] [PubMed]
- Donadio, M.; D’Giano, C.; Moussalli, M.; Barrios, L.; Ugarnes, G.; Segalovich, M.; Pociecha, J.; Vazquez, C.; Petre, C.; Pomata, H. Epilepsy surgery in Argentina: Long-term results in a comprehensive epilepsy centre. Seizure 2011, 20, 442–445. [Google Scholar] [CrossRef] [PubMed]
- Mikati, M.A.; Ataya, N.; El-Ferezli, J.; Shamseddine, A.; Rahi, A.; Herlopian, A.; Kurdi, R.; Bhar, S.; Hani, A.; Comair, Y.G. Epilepsy surgery in a developing country (Lebanon): Ten years experience and predictors of outcome. Epileptic Disord. 2012, 14, 267–274. [Google Scholar] [PubMed]
- Sylaja, P.N.; Radhakrishnan, K.; Kesavadas, C.; Sarma, P.S. Seizure outcome after anterior temporal lobectomy and its predictors in patients with apparent temporal lobe epilepsy and normal MRI. Epilepsia 2004, 45, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Boling, W.; Palade, A.; Wabulya, A.; Longoni, N.; Warf, B.; Nestor, S.; Alpitsis, R.; Bittar, R.; Howard, C.; Andermann, F. Surgery for pharmacoresistant epilepsy in the developing world: A pilot study. Epilepsia 2009, 50, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, K.H.; Khemiri, E.; Parain, D.; Hattab, N.; Proust, F.; Mrabet, A. Epilepsy surgery program in Tunisia: An example of a Tunisian French collaboration. Seizure 2010, 19, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Campos, M.G. Epilepsy surgery in developing countries. Handb. Clin. Neurol. 2012, 108, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, S.; Panigrahi, M.; Kulkarni, D.K.; Uppin, M.; Somayajula, S.; Challa, S. Outcome of epilepsy surgery in children after evaluation with non-invasive protocol. Neurol. India 2011, 59, 30–36. [Google Scholar] [CrossRef] [PubMed]
- De Tisi, J.; Bell, G.S.; Peacock, J.L.; McEvoy, A.W.; Harkness, W.F.; Sander, J.W.; Duncan, J.S. The long-term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: A cohort study. Lancet 2011, 378, 1388–1395. [Google Scholar] [CrossRef]
- McIntosh, A.M.; Kalnins, R.M.; Mitchell, L.A.; Fabinyi, G.C.; Briellmann, R.S.; Berkovic, S.F. Temporal lobectomy: Long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain 2004, 127, 2018–2030. [Google Scholar] [CrossRef] [PubMed]
- Widdess-Walsh, P.; Kellinghaus, C.; Jeha, L.; Kotagal, P.; Prayson, R.; Bingaman, W.; Najm, I.M. Electro-clinical and imaging characteristics of focal cortical dysplasia: Correlation with pathological subtypes. Epilepsy Res. 2005, 67, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Srikijvilaikul, T.; Najm, I.M.; Hovinga, C.A.; Prayson, R.A.; Gonzalez-Martinez, J.; Bingaman, W.E. Seizure outcome after temporal lobectomy in temporal lobe cortical dysplasia. Epilepsia 2003, 44, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.V.; de Oliveira, R.S.; Machado, H.R. Approach to cortical dysplasia associated with glial and glioneuronal tumors (FCD type IIIb). Child’s Nerv. Syst. 2014, 30, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Schmeiser, B.; Hammen, T.; Steinhoff, B.J.; Zentner, J.; Schulze-Bonhage, A. Long-term outcome characteristics in mesial temporal lobe epilepsy with and without associated cortical dysplasia. Epilepsy Res. 2016, 126, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.S.; Cross, J.H.; Shinnar, S.; Mathern, B.W. Defining the spectrum of international practice in pediatric epilepsy surgery patients. Epilepsia 2008, 49, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Lerner, J.T.; Salamon, N.; Hauptman, J.S.; Velasco, T.R.; Hemb, M.; Wu, J.Y.; Sankar, R.; Donald, S.W.; Engel, J., Jr.; Fried, I.; et al. Assessment and surgical outcomes for mild type I and severe type II cortical dysplasia: A critical review and the UCLA experience. Epilepsia 2009, 50, 1310–1335. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Cai, L.; Dong, S.; Li, Y. Clinical characteristics and post-surgical outcomes of focal cortical dysplasia subtypes. J. Clin. Neurosci. 2016, 23, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Fauser, S.; Bast, T.; Altenmuller, D.M.; Schulte-Monting, J.; Strobl, K.; Steinhoff, B.J.; Zentner, J.; Schulze-Bonhage, A. Factors influencing surgical outcome in patients with focal cortical dysplasia. J. Neurol. Neurosurg. Psychiatry 2008, 79, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Fauser, S.; Zentner, J. Management of cortical dysplasia in epilepsy. Adv. Tech. Stand. Neurosurg. 2012, 38, 137–163. [Google Scholar] [PubMed]
- Fauser, S.; Schulze-Bonhage, A. Epileptogenicity of cortical dysplasia in temporal lobe dual pathology: An electrophysiological study with invasive recordings. Brain 2006, 129, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Holmes, M.D.; Kutsy, R.L.; Ojemann, G.A.; Wilensky, A.J.; Ojemann, L.M. Interictal, unifocal spikes in refractory extratemporal epilepsy predict ictal origin and postsurgical outcome. Clin. Neurophysiol. 2000, 111, 1802–1808. [Google Scholar] [CrossRef]
- Di, G.G.; Quarato, P.P.; Sebastiano, F.; Esposito, V.; Onorati, P.; Mascia, A.; Romanelli, P.; Grammaldo, L.G.; Falco, C.; Scoppetta, C.; et al. Postoperative EEG and seizure outcome in temporal lobe epilepsy surgery. Clin. Neurophysiol. 2004, 115, 1212–1219. [Google Scholar]
- Tuunainen, A.; Nousiainen, U.; Mervaala, E.; Pilke, A.; Vapalahti, M.; Leinonen, E.; Paljarvi, L.; Riekkinen, P. Postoperative EEG and electrocorticography: Relation to clinical outcome in patients with temporal lobe surgery. Epilepsia 1994, 35, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Groppel, G.; Aull-Watschinger, S.; Baumgartner, C. Temporal evolution and prognostic significance of postoperative spikes after selective amygdala-hippocampectomy. J. Clin. Neurophysiol. 2003, 20, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, M.; Schulz, R.; Hoppe, M.; May, T.; Ebner, A. Postoperative routine EEG correlates with long-term seizure outcome after epilepsy surgery. Seizure 2005, 14, 446–451. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.W.; Lee, S.K.; Hong, K.S.; Kim, K.K.; Chung, C.K.; Kim, H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: Longitudinal analysis. Epilepsia 2005, 46, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Jeha, L.E.; Najm, I.M.; Bingaman, W.E.; Khandwala, F.; Widdess-Walsh, P.; Morris, H.H.; Dinner, D.S.; Nair, D.; Foldvary-Schaeffer, N.; Prayson, R.A.; et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology 2006, 66, 1938–1940. [Google Scholar] [CrossRef] [PubMed]
- Velasco, A.L.; Velasco, M.; Velasco, F.; Menes, D.; Gordon, F.; Rocha, L.; Briones, M.; Marquez, I. Subacute and chronic electrical stimulation of the hippocampus on intractable temporal lobe seizures: Preliminary report. Arch. Med. Res. 2000, 31, 316–328. [Google Scholar] [CrossRef]
- Radhakrishnan, A.; Abraham, M.; Vilanilam, G.; Menon, R.; Menon, D.; Kumar, H.; Cherian, A.; Radhakrishnan, N.; Kesavadas, C.; Thomas, B.; et al. Surgery for “Long-term epilepsy associated tumors (LEATs)”: Seizure outcome and its predictors. Clin. Neurol. Neurosurg. 2016, 141, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Godoy, J.; Luders, H.; Dinner, D.S.; Morris, H.H.; Wyllie, E.; Murphy, D. Significance of sharp waves in routine EEGs after epilepsy surgery. Epilepsia 1992, 33, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Mintzer, S.; Nasreddine, W.; Passaro, E.; Beydoun, A. Predictive value of early EEG after epilepsy surgery. J. Clin. Neurophysiol. 2005, 22, 410–414. [Google Scholar] [PubMed]
- Kelemen, A.; Rasonyi, G.; Szucs, A.; Fabo, D.; Halasz, P. Predictive factors for the results of surgical treatment in temporal lobe epilepsy. Ideggyogy Sz 2006, 59, 353–359. [Google Scholar] [PubMed]
- Thorbecke, R.; May, T.W.; Koch-Stoecker, S.; Ebner, A.; Bien, C.G.; Specht, U. Effects of an inpatient rehabilitation program after temporal lobe epilepsy surgery and other factors on employment 2 years after epilepsy surgery. Epilepsia 2014, 55, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Vickrey, B.G.; Hays, R.D.; Rausch, R.; Sutherling, W.W.; Engel, J., Jr.; Brook, R.H. Quality of life of epilepsy surgery patients as compared with outpatients with hypertension, diabetes, heart disease, and/or depressive symptoms. Epilepsia 1994, 35, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Chovaz, C.J.; McLachlan, R.S.; Derry, P.A.; Cummings, A.L. Psychosocial function following temporal lobectomy: Influence of seizure control and learned helplessness. Seizure 1994, 3, 171–176. [Google Scholar] [CrossRef]
- Augustine, E.A.; Novelly, R.A.; Mattson, R.H.; Glaser, G.H.; Williamson, P.D.; Spencer, D.D.; Spencer, S.S. Occupational adjustment following neurosurgical treatment of epilepsy. Ann. Neurol. 1984, 15, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Hamiwka, L.; Macrodimitris, S.; Tellez-Zenteno, J.F.; Metcalfe, A.; Wiebe, S.; Kwon, C.S.; Jette, N. Social outcomes after temporal or extratemporal epilepsy surgery: A systematic review. Epilepsia 2011, 52, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Puka, K.; Smith, M.L. Where are they now? Psychosocial, educational, and vocational outcomes after epilepsy surgery in childhood. Epilepsia 2016, 57, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Sperling, M.R.; O’Connor, M.J.; Saykin, A.J.; Plummer, C. Temporal lobectomy for refractory epilepsy. JAMA 1996, 276, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Guldvog, B. Patient satisfaction and epilepsy surgery. Epilepsia 1994, 35, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Reeves, A.L.; So, E.L.; Evans, R.W.; Cascino, G.D.; Sharbrough, F.W.; O’Brien, P.C.; Trenerry, M.R. Factors associated with work outcome after anterior temporal lobectomy for intractable epilepsy. Epilepsia 1997, 38, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, J.; Ounsted, C.; Richards, P. Long-term outcome in children with temporal lobe seizures. V: Indications and contra-indications for neurosurgery. Dev. Med. Child Neurol. 1984, 26, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Malkki, H. Epilepsy: Beyond seizure control—Vocational outcomes after paediatric epilepsy surgery. Nat. Rev. Neurol. 2016, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Blocher, J.B.; Jackson, D.C. Life outcomes of anterior temporal lobectomy: Serial long-term follow-up evaluations. Neurosurgery 2013, 73, 1018–1025. [Google Scholar] [CrossRef] [PubMed]
- Vickrey, B.G.; Berg, A.T.; Sperling, M.R.; Shinnar, S.; Langfitt, J.T.; Bazil, C.W.; Walczak, T.S.; Pacia, S.; Kim, S.; Spencer, S.S. Relationships between seizure severity and health-related quality of life in refractory localization-related epilepsy. Epilepsia 2000, 41, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Reid, K.; Herbert, A.; Baker, G.A. Epilepsy surgery: Patient-perceived long-term costs and benefits. Epilepsy Behav. 2004, 5, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Asztely, F.; Ekstedt, G.; Rydenhag, B.; Malmgren, K. Long term follow-up of the first 70 operated adults in the Goteborg Epilepsy Surgery Series with respect to seizures, psychosocial outcome and use of antiepileptic drugs. J. Neurol. Neurosurg. Psychiatry 2007, 78, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Vickrey, B.G.; Hays, R.D.; Engel, J., Jr.; Spritzer, K.; Rogers, W.H.; Rausch, R.; Graber, J.; Brook, R.H. Outcome assessment for epilepsy surgery: The impact of measuring health-related quality of life. Ann. Neurol. 1995, 37, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.E.; Berven, N.L.; Ramirez, L.; Woodard, A.; Hermann, B.P. Long-term psychosocial outcomes of anterior temporal lobectomy. Epilepsia 2002, 43, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Alonso, N.B.; Mazetto, L.; de Araujo Filho, G.M.; Vidal-Dourado, M.; Yacubian, E.M.; Centeno, R.S. Psychosocial factors associated with in postsurgical prognosis of temporal lobe epilepsy related to hippocampal sclerosis. Epilepsy Behav. 2015, 53, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Andersson-Roswall, L.; Engman, E.; Samuelsson, H.; Malmgren, K. Psychosocial status 10 years after temporal lobe resection for epilepsy, a longitudinal controlled study. Epilepsy Behav. 2013, 28, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.C.; McMackin, D.; Staunton, H.; Delanty, N.; Phillips, J. Patients’ aims for epilepsy surgery: Desires beyond seizure freedom. Epilepsia 2001, 42, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Baca, C.B.; Cheng, E.M.; Spencer, S.S.; Vassar, S.; Vickrey, B.G. Racial differences in patient expectations prior to resective epilepsy surgery. Epilepsy Behav. 2009, 15, 452–455. [Google Scholar] [CrossRef] [PubMed]
- Ozanne, A.; Graneheim, U.H.; Ekstedt, G.; Malmgren, K. Patients’ expectations and experiences of epilepsy surgery—A population-based long-term qualitative study. Epilepsia 2016, 57, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Saling, M.M.; Kincade, P.; Bladin, P.F. Patient expectations of temporal lobe surgery. Epilepsia 1998, 39, 167–174. [Google Scholar] [CrossRef] [PubMed]
| Mean age at surgery (years ± SD range) | 33.5 ± 9.7, (range 16–58) |
| Mean age at seizure onset (years ± SD range) | 13.7 ± 11.3, (range 9–52) |
| Gender | Male: 22 (55%) Female: 18 (45%) |
| Mean epilepsy duration (year ± SD range) | 19.6 ± 10.18 (range 2–42) |
| Precipitant event n (%) | 31 (77.5%), febrile seizures 47.6% |
| Mean number of antiepileptic drugs tried ± SD (range) | 5.95 ± 2.02, (range 3–10) |
| Resection lateralization n (%) | Right 19 (47.5%) Left 21 (52.5%) |
| Mean follow-up (month range) (year ± SD range) | 8.6 ± 3.9 years (range 1–14) |
| Seizure types | Complex partial seizures: 100% |
| Simple partial seizures: 76% | |
| History of secondary generalized tonic-clonic seizures: 84% | |
| Ictal EEG pattern n (%) | Type I (5–9 Hz) 24 (60%) |
| Type II (2–5 Hz) 16 (40%) | |
| Ictal EEG topography, n (%) | Concordant 31 (77.4%) |
| Discordant 8 (22.5%) | |
| Preoperative interictal EEG, n (%) | Unilateral/concordant 15 (37.5%); Bilateral with ipsilateral predominance 5:1, 25 (62.5%) |
| Left hemisphere resection longitude (mean ± SD), Right hemisphere resection longitude (mean ± SD) | mesial 18.23 ± 6.76 mm, neocortical 41.6 ± 9.8 mm |
| mesial 15.06 ± 5.19 mm, neocortical 40.19 ± 12.07 |
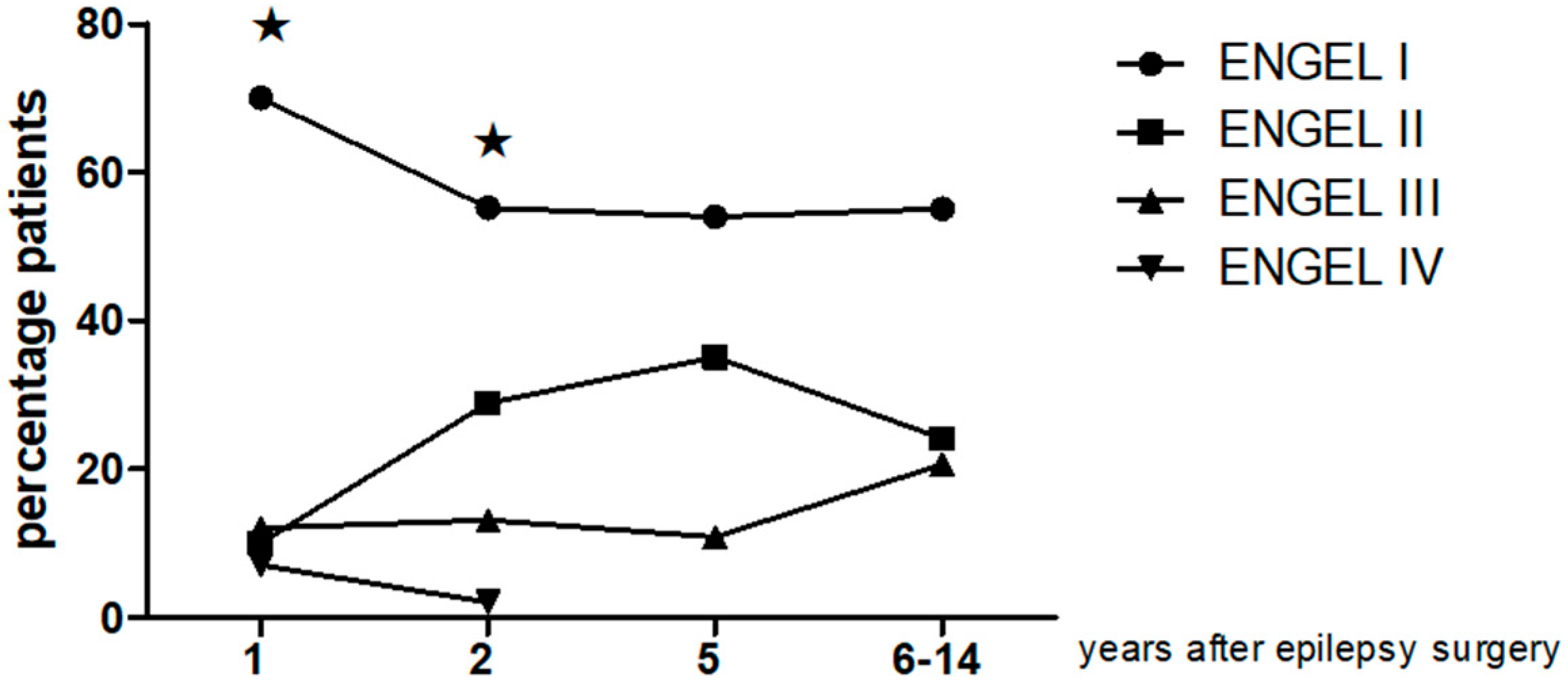
| Follow-Up | Class I Patients n, (%) | Class II Patients n, (%) | Class III Patients n, (%) | Class IV Patients n, (%) |
|---|---|---|---|---|
| 1 year, n = 40 | 28, (70%) | 4, (10%) | 5, (12%) | 3 (7%) |
| 2 year, n = 38 | 21, (55.2%) | 11, (28.9) | 5, (13.1%) | 1 (2%) |
| 5 year, n = 37 | 20, (54.05) | 13, (35.1%) | 4, (10.8%) | |
| 6–14 year mean 9.7y n = 29 | 16, (55.1) | 7, (24.1%) | 6, (20.6%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales Chacón, L.M.; Garcia Maeso, I.; Baez Martin, M.M.; Bender del Busto, J.E.; García Navarro, M.E.; Quintanal Cordero, N.; Estupiñan Díaz, B.; Lorigados Pedre, L.; Valdés Yerena, R.; Gonzalez, J.; et al. Long-Term Electroclinical and Employment Follow up in Temporal Lobe Epilepsy Surgery. A Cuban Comprehensive Epilepsy Surgery Program. Behav. Sci. 2018, 8, 19. https://doi.org/10.3390/bs8020019
Morales Chacón LM, Garcia Maeso I, Baez Martin MM, Bender del Busto JE, García Navarro ME, Quintanal Cordero N, Estupiñan Díaz B, Lorigados Pedre L, Valdés Yerena R, Gonzalez J, et al. Long-Term Electroclinical and Employment Follow up in Temporal Lobe Epilepsy Surgery. A Cuban Comprehensive Epilepsy Surgery Program. Behavioral Sciences. 2018; 8(2):19. https://doi.org/10.3390/bs8020019
Chicago/Turabian StyleMorales Chacón, Lilia Maria, Ivan Garcia Maeso, Margarita M. Baez Martin, Juan E. Bender del Busto, María Eugenia García Navarro, Nelson Quintanal Cordero, Bárbara Estupiñan Díaz, Lourdes Lorigados Pedre, Ricardo Valdés Yerena, Judith Gonzalez, and et al. 2018. "Long-Term Electroclinical and Employment Follow up in Temporal Lobe Epilepsy Surgery. A Cuban Comprehensive Epilepsy Surgery Program" Behavioral Sciences 8, no. 2: 19. https://doi.org/10.3390/bs8020019





