Tyrosine Hydroxylase, Vesicular Monoamine Transporter and Dopamine Transporter mRNA Expression in Nigrostriatal Tissue of Rats with Pedunculopontine Neurotoxic Lesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Subjects
2.2. Surgical Procedure
2.3. Molecular Biology Studies
2.4. Morphological Studies
2.5. Immunohistochemistry
2.6. Data Processing and Statistical Analysis
3. Results
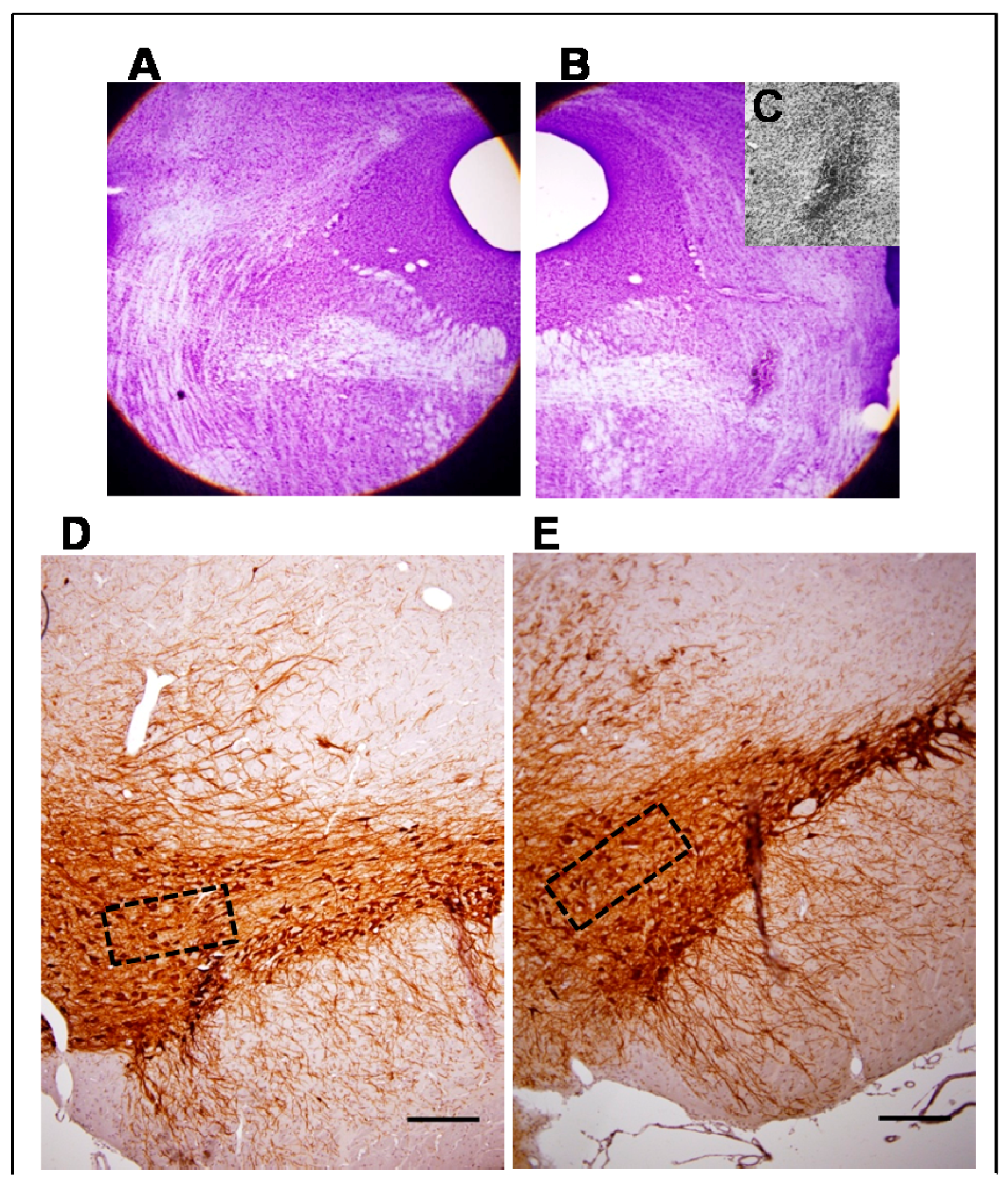
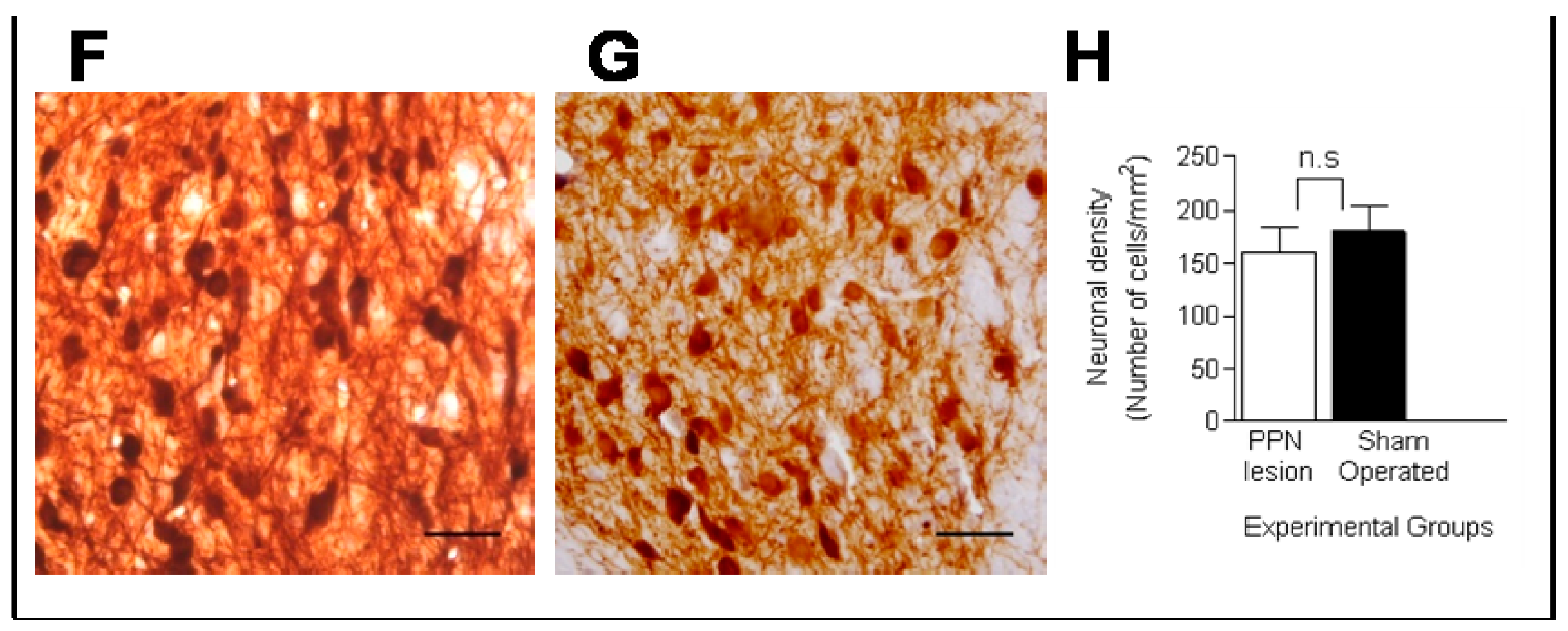
3.1. Morphological Studies
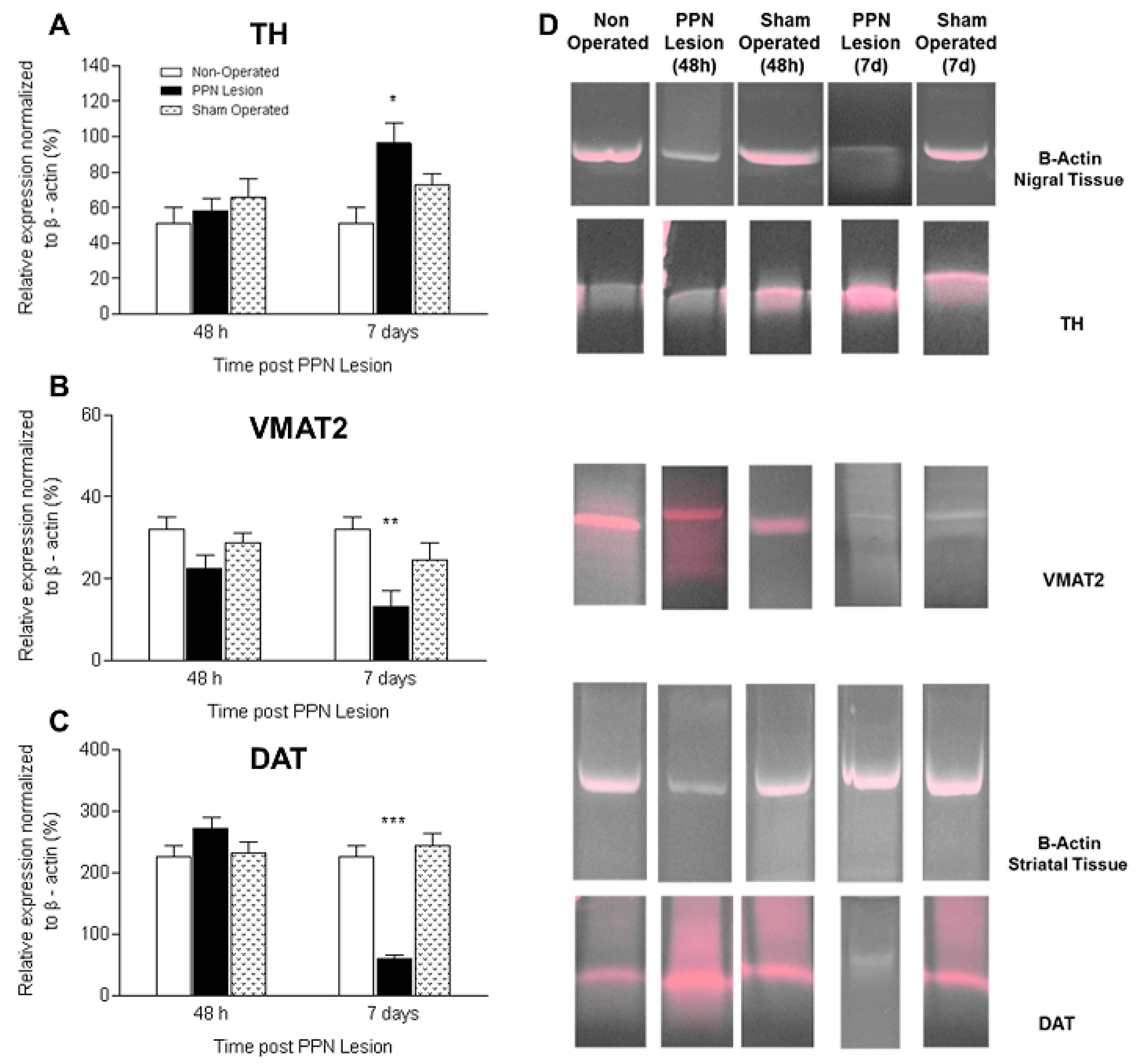
3.2. Molecular Biology Studies
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wolters, E.C.; Braak, H. Parkinson’s disease: Premotor clinic-pathological correlations. J. Neural Transm. Suppl. 2006, 70, 309–319. [Google Scholar]
- Jellinger, K. The pedunculopontine nucleus in Parkinson’s disease, progressive supranuclear palsy and Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 540–543. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Schwabe, K.; Krauss, J.K. The pedunculopontine nucleus area: Critical evaluation of interspecies differences relevant for its use as a target for deep brain stimulation. Brain 2011, 134, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, C.; Andel, J.; Bolam, J.P.; Mena-Segovia, J. Divergent motor projections from the pedunculopontine nucleus are differentially regulated in Parkinsonism. Brain Struct. Funct. 2014, 219, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Mori, F.; Okada, K.; Nomura, T.; Kobayashi, Y. The Pedunculopontine Tegmental Nucleus as a Motor and Cognitive Interface between the Cerebellum and Basal Ganglia. Front. Neuroanat. 2016, 10, 109. [Google Scholar] [CrossRef] [PubMed]
- Saper, C.B.; Loewy, A.D. Projections of the pedunculopontine tegmental nucleus in the rat: Evidence for additional extrapyramidal circuitry. Brain Res. 1982, 252, 367–372. [Google Scholar] [CrossRef]
- Futami, T.; Takakusaki, K.; Kitai, S.T. Glutamatergic and tegmental nucleus to cholinergic inputs from the pedunculopontine dopamine neurons in the substantia nigra pars compacta. Neurosci. Res. 1995, 21, 331–342. [Google Scholar] [CrossRef]
- Bohnen, N.; Albin, R.L. The Cholinergic System and Parkinson Disease. Behav. Brain Res. 2011, 221, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Graybiel, A.M.; Duyckaerts, C.; Javoy-Agid, F. Neuronal loss in the pedunculopontine tegmental nucleus in Parkinson disease and in progressive supranuclear palsy. Proc. Natl. Acad. Sci. USA 1987, 84, 5976–5980. [Google Scholar] [CrossRef] [PubMed]
- Zweig, R.M.; Jankel, W.R.; Hedreen, J.C.; Mayeux, R.; Price, D.L. The pedunculopontine nucleus in Parkinson’s disease. Ann. Neurol. 1989, 26, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Pienaar, I.S.; Elson, J.L.; Racca, C.; Nelson, G.; Turnbull, D.M.; Morris, C.M. Mitochondrial Abnormality Associates with Type-Specific Neuronal Loss and Cell Morphology Changes in the Pedunculopontine Nucleus in Parkinson Disease. Am. J. Pathol. 2013, 183, 1826–1840. [Google Scholar] [CrossRef] [PubMed]
- Kojima, J.; Yamaji, Y.; Matsumura, M.; Nambu, A.; Inase, M.; Tokuno, H.; Takada, M.; Imai, H. Excitotoxic lesions of the pedunculopontine tegmental nucleus produce contralateral hemiparkinsonism in the monkey. Neurosci. Lett. 1997, 226, 111–114. [Google Scholar] [CrossRef]
- Aziz, T.Z.; Davies, L.; Stein, J.; France, S. The role of descending basal ganglia connections to the brain stem in parkinsonian akinesia. Br. J. Neurosurg. 1998, 12, 245–249. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, M.; Kojima, J. The role of the pedunculopontine tegmental nucleus in experimental Parkinsonism in primates. Stereotact. Funct. Neurosurg. 2001, 77, 108–115. [Google Scholar] [CrossRef] [PubMed]
- McGeer, E.G.; McGeer, P.L. Substantia nigra cell death from kainic acid or folic acid injections into the pontine tegmentum. Brain Res. 1984, 298, 339–342. [Google Scholar] [CrossRef]
- Gonzalez-Hernandez, T.; Abdala, P.; Rodríguez, M. NOS Expression in Nigral Cells after Excitotoxic and Non-excitotoxic Lesion of the Pedunculopontine Tegmental Nucleus. Eur. J. Neurosci. 1997, 9, 2658–2667. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Martin, J.; Blanco-Lezcano, L.; González-Fraguela, M.E.; Díaz-Hung, M.L.; Serrano-Sánchez, T.; Almenares, J.L.; Francis-Turner, L. Effect of neurotoxic lesion of Pedunculopontine nucleus in nigral and striatal redox balance and motor performance in rats. Neuroscience 2015, 289, 300–314. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Lezcano, L.; Jiménez-Martín, J.; Diaz-Hung, M.; Alberti-Amador, E.; Wong-Guerra, M.; González-Fraguela, M.E.; Estupinán-Díaz, B.; Serrano-Sánchez, T.; Francis-Turner, L.; Delgado-Ocaña, S.; et al. Motor Dysfunction and alterations in gluthathione concentration, cholinesterase activity and BDNF expression in substantia nigra pars compacta in rats with pedunculopontine lesion. Neuroscience 2017, 348, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Michel, P.P.; Toulorge, D.; Guerreiro, S.; Hirsch, E.C. Specific needs of dopamine neurons for stimulation in order to survive: Implication for Parkinson disease. FASEB J. 2013, 27, 3414–3423. [Google Scholar] [CrossRef] [PubMed]
- Bensaid, M.; Michel, P.P.; Clark, S.; Hirsch, E.C.; François, C. Role of the pedunculopontine cholinergic neurons in the vulnerability of nigral dopaminergic neurons in Parkinson’s disease. Exp. Neurol. 2016, 275, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Bezard, E.; Gross, Ch. Compensatory mechanisms in experimental and human Parkinsonism: Towards a dynamic approach. Prog. Neurobiol. 1998, 55, 93–116. [Google Scholar] [CrossRef]
- Meiser, J.; Weindl, D.; Hiller, K. Complexity of dopamine metabolism. Cell Commun. Signal. 2013, 11, 34–51. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Trigo-Damas, I.; Quiroga-Varela, A.; Jackson-Lewis, V. Oxidative stress and Parkinson’s disease. Front. Neuroanat. 2015, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Berry, M.D.; Juorio, A.V.; Paterson, I.A. The functional role of monoamine oxidases A and B in the mammalian central nervous system. Prog. Neurobiol. 1994, 42, 375–391. [Google Scholar] [CrossRef]
- Caudle, W.M.; Colebrooke, R.E.; Emson, P.C.; Miller, G.W. Altered vesicular dopamine storage in Parkinson’s disease: A premature demise. TINS 2008, 31, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and toxic roles of dopamine in Parkinson’s disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Kumer, S.C.; Vrana, K.E. Intricate Regulation of Tyrosine Hydroxylase Activity and Gene Expression. J. Neurochem. 1996, 67, 443–462. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.G. Oxidative pathways for catecholamines in the genesis of neuromelanin and cytotoxic quinones. Mol. Pharmacol. 1978, 14, 633–643. [Google Scholar] [PubMed]
- Wimalasena, K. Vesicular Monoamine Transporters: Structure-Function, Pharmacology, and Medicinal Chemistry. Med. Res. Rev. 2011, 31, 483–519. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Caudle, W.M.; Miller, G.W. VMAT2-DeficientMice Display Nigral and Extranigral Pathology and Motor and Nonmotor Symptoms of Parkinson’s Disease. Parkinson Dis. 2011, 2011, 124165. [Google Scholar] [CrossRef]
- Masoud, S.T.; Vecchio, L.M.; Bergeron, Y.; Hossain, M.M.; Nguyen, L.T.; Bermejo, M.K.; Kile, B.; Sotnikova, T.D.; Siesser, W.B.; Gainetdinov, R.R.; et al. Increased expression of the dopamine transporter leads to loss of dopamine neurons, oxidative stress and L-DOPA reversible motor deficits. Neurobiol. Dis. 2015, 74, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, W.; Zhou, Y.; Park, S.; Wang, L.; Mitchell, N.; Stone, M.D.; Becker, K.G.; Martin, B.; Maudsley, S. Minimal Peroxide Exposure of Neuronal Cells Induces Multifaceted Adaptive Responses. PLoS ONE 2010, 5, e14352. [Google Scholar] [CrossRef] [PubMed]
- Spiers, J.G.; Chen, H.J.; Sernia, C.; Lavidis, N.A. Activation of the hypothalamic-pituitary-adrenal stress axis induces cellular oxidative stress. Front. Neurosci. 2015, 8, 456. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2007. [Google Scholar]
- Dautan, D.; Souza, A.; Huerta-Ocampo, I.; Valencia, M.; Assous, M.; Witten, I.; Deisseroth, K.; Tepper, J.M.; Bolam, J.P.; Gerdjikov, T.V.; et al. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. J. Neurosci. 1995, 15, 5859–5869. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Melián, W.; Mercerón, D.; Pavón, N.; Alberti, E.; Frey, J.U. Novelty exposure overcomes foot shock-induced spatial-memory impairment by processes of synaptic-tagging in rats. Proc. Natl. Acad. Sci. USA 2012, 109, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Almaguer-Melián, W.; Mercerón, D.; Pavón, N.; Alberti, E.; León, R.; Ledón, N.; Delgado, S.; Bergado, J. Erythropoietin Promotes Neural Plasticity and Spatial Memory Recovery in Fimbria-Fornix-Lesioned Rats. Neurorehabilit. Neural Repair 2015, 29, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Toulorge, D.; Guerreiro, S.; Hild, A.; Maskos, U.; Hirsch, E.C.; Michel, P.P. Neuroprotection of midbrain dopamine neurons by nicotine is gated by cytoplasmic Ca2+. FASEB J. 2011, 25, 2563–2573. [Google Scholar] [CrossRef] [PubMed]
- Bordia, T.; McGregor, M.; Papke, R.L.; Decker, M.W.; McIntosh, M.; Quik, M. The α7 nicotinic receptor agonist ABT-107 protects against nigrostriatal damage in rats with unilateral 6-hydroxydopamine lesions. Exp. Neurol. 2015, 263, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, M.J.; Hastings, T.; Abercrombie, E. Neurochemical responses to 6-hydroxydopamine and L-dopa therapy: Implications for Parkinson’s disease. Ann. N. Y. Acad. Sci. 1992, 648, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Hastings, T.G.; Lewis, D.A.; Zigmond, M.J. Reactive dopamine metabolites and neurotoxicity: Implications for Parkinson’s disease. Adv. Exp. Med. Biol. 1996, 387, 97–106. [Google Scholar] [PubMed]
- Caudle, W.M.; Richardson, J.R.; Wang, M.Z.; Taylor, T.N.; Guillot, T.S.; McCormack, A.L.; Colebrooke, R.E.; Di Monte, D.A.; Emson, P.C.; Miller, G.W. Reduced Vesicular Storage of Dopamine Causes Progressive Nigrostriatal Neurodegeneration. J. Neurosci. 2007, 27, 8138–8148. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Kopin, I.J.; Sharabi, Y. Catecholamine autotoxicity. Implications for pharmacology and therapeutics of Parkinson disease and related disorders. Pharmacol. Ther. 2014, 44, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, D.S.; Holmes, C.; Sullivan, P.; Mash, D.C.; Sidransky, E.; Stefani, A.; Kopin, I.J.; Sharabi, Y. Deficient Vesicular Storage: A Common Theme in Catecholaminergic Neurodegeneration. Parkinsonism Relat. Disord. 2015, 21, 1013–1022. [Google Scholar] [CrossRef] [PubMed]
- Aquilano, K.; Baldelli, S.; Ciriolo, M. Glutathione: New roles in redox signaling for an old antioxidant. Front. Pharmacol. 2014, 5, 196. [Google Scholar] [CrossRef] [PubMed]
- Do, T.; Kerr, B.; Kuzhikandathil, E.V. Brain-derived neurotrophic factor regulates the expression of D1 dopamine receptors. J. Neurochem. 2007, 100, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Nordberg, J.; Arner, E.S.J. Reactive oxygen species, antioxidants, and the mammalian thioredoxin system. Free Radic. Biol. Med. 2001, 31, 1287–1312. [Google Scholar] [CrossRef]
- Dalton, T.P.; Shertzer, H.G.; Puga, A. Regulation of gene expression by reactive oxygen. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 67–101. [Google Scholar] [CrossRef] [PubMed]
- Daubner, S.C.; Le, T.; Wang, S. Tyrosine Hydroxylase and Regulation of Dopamine Synthesis. Arch. Biochem. Biophys. 2011, 508, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bademci, B.; Vance, J.M.; Wang, L. Tyrosine Hydroxylase Gene: Another Piece of the Genetic Puzzle of Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2012, 11, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Amstad, P.A.; Krupitza, G.; Cerutti, P.A. Mechanism of c-fos induction by active oxygen. Cancer Res. 1992, 52, 3952–3960. [Google Scholar] [PubMed]
- Pahl, H.L.; Baeuerle, P.A. Oxygen and the control of gene expression. BioEssays 1994, 16, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Bauer, M.K.; Vogt, M.; Wesselborg, S. Oxidative stress and signal transduction. Int. J. Vitam. Nutr. Res. 1997, 67, 336–342. [Google Scholar] [PubMed]
- Suzuki, Y.J.; Forman, H.J.; Sevanian, A. Oxidants as stimulators of signal transduction. Free Radic. Biol. Med. 1997, 22, 269–285. [Google Scholar] [CrossRef]
- Eyerman, D.J.; Yamamoto, B.K. A rapid oxidation and persistent decrease in the vesicular monoamine transporter 2 after methamphetamine. J. Neurochem. 2007, 103, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Taylor, T.N.; Caudle, M.W.; Shepherd, K.R.; Noorian, A.; Jackson, R.; Luvone, P.N.; Weinshenker, D.; Greene, J.G.; Miller, G.W. Non-motor symptoms of Parkinson’s disease revealed in an animal model with reduced monoamine storage capacity. J. Neurosci. 2009, 29, 8103–8113. [Google Scholar] [CrossRef] [PubMed]
- Berman, S.B.; Zigmond, M.J.; Hasting, T.G. Modification of Dopamine Transporter Function: Effect of Reactive Oxygen Species and Dopamine. J. Neurochem. 1996, 67, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.B.; Moriwaki, A.; Uhl, G.R. Dopamine Transporter Cysteine Mutants: Second Extracellular Loop Cysteines Are Required for Transporter Expression. J. Neurochem. 1995, 64, 1416–1419. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, R.E.; Ferrer, J.V.; Javitch, J.A.; Justice, J.B. Reaction of oxidized dopamine with endogenous cysteine residues in the human dopamine transporter. J. Neurochem. 2001, 76, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Samii, A.; Sossi, V.; Ruth, T.J.; Schulzer, M.; Holden, J.E.; Wudel, J.; Pal, P.K.; de la Fuente-Fernandez, R.; Calne, D.B.; et al. In Vivo Positron Emission Tomographic Evidence for Compensatory Changes in Presynaptic Dopaminergic Nerve Terminals in Parkinson’s disease. Ann. Neurol. 2000, 47, 493–503. [Google Scholar] [CrossRef]
- Hartmann, A. Postmortem studies in Parkinson’s disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar] [PubMed]
- Sossi, V.; De la Fuente-Fernández, R.; Schulzer, M.; Troiano, A.R.; Ruth, T.J.; Stoessl, A.J. Dopamine Transporter Relation to Dopamine Turnover in Parkinson’s disease: A Positron Emission Tomography Study. Ann. Neurol. 2007, 62, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An evaluation of 6-OHDA lesions of the nigrostriatal pathways. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Rolland, A.S.; Tandé, D.; Herrero, M.T.; Luquin, M.R.; Vazquez-Claverie, M.; Karachi, C.; Hirsch, E.C.; François, C. Evidence for a dopaminergic innervation of the pedunculopontine nucleus in monkeys, and its drastic reduction after MPTP intoxication. J. Neurochem. 2009, 110, 1321–1329. [Google Scholar] [CrossRef] [PubMed]
- Sgádo, P.; Viaggi, C.; Pinna, A.; Marrone, C.; Vaglini, F.; Pontis, S.; Mercuri, N.B.; Morelli, M.; Corsini, G.U. Behavioral, Neurochemical, and Electrophysiological Changes in an Early Spontaneous Mouse Model of Nigrostriatal Degeneration. Neurotox. Res. 2011, 20, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Porras, G.; Li, Q.; Bezard, E. Modeling Parkinson’s disease in Primates: The MPTP Model. Cold Spring Harb. Perspect. Med. 2012, 2, a009308. [Google Scholar] [CrossRef] [PubMed]
- Blesa, J.; Przedborski, S. Parkinson’s disease: Animal models and dopaminergic cell vulnerability. Front. Neuroanat. 2014, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Chan, N.G.; Góngora-Alfaro, J.L.; Álvarez-Cervera, F.J.; Solís-Rodríguez, F.A.; Heredia-López, F.J.; Arankowsky-Sandoval, G.A. Quinolinic acid lesions of the pedunculopontine nucleus impair sleep architecture, but not locomotion, exploration, emotionality or working memory in the rat. Behav. Brain Res. 2011, 225, 482–490. [Google Scholar] [CrossRef] [PubMed]
- Deneris, S.; Hobert, O. Maintenance of postmitotic neuronal cell identity. Nat. Neurosci. 2014, 17, 899–907. [Google Scholar] [CrossRef] [PubMed]
| Neurotoxin employed | N-methyl-D-aspartate (NMDA) (Sigma, St. Louis, MO, USA) Concentration: 0.1 M, Volume: 0.5 µL Rate: 0.1 μL/min by means of 1-μL Hamilton syringe |
| Stereotactic coordinates (mm) | AP: −7.80, ML: 1.60, DV: 7.60 |
| Experimental design | In order to study the early effect of the PPN neurotoxic lesion on the gene expression of TH, VMAT2 and DAT, the mRNA expression of the above-mentioned proteins was evaluated 48 h and 7 days after injury. The following experimental groups were organized: Healthy rats (non-operated) (n = 12), rats-PPN injury (48 h post lesion) (n = 13), rats-PPN injury (7 days post lesion) (n = 12), rats Sham operated (48 h after surgery) (n = 13), rats Sham operated (7 days after surgery) (n = 12). Rats were lesioned, assigned randomly to the experimental groups and sacrificed in the temporal window already described |
| Gene Product | Sequence of Primers | Product Length (bp) | Annealing Temperature (°C) |
|---|---|---|---|
| TH | 5′ TTC CCC ATG TTC AAC GGA CC-3′ 5′ GCG AGC ACA GTA ATC ACC TTC-3′ | 449 | 56 |
| DAT | 5′ GGA AGC TGG TCA GCC CCT GCT T-3′ 5′ GAA TTG GCG CAC CTC CCC TCT G-3′ | 266 | 60 |
| VMAT2 | 5′ CGC AAA CTG ATC CTG TTC AT-3′ 5′ AGA AGA TGC TTT CGC AGG TG-3′ | 175 | 60 |
| B ACTIN (endogenous control) | 5′ ATT TGG CAC CAC ACT TTC TAC A-3′ 5′ TCA CGC ACG ATT TCC CTC TCA G-3′ | 379 | 55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blanco-Lezcano, L.; Alberti-Amador, E.; Díaz-Hung, M.-L.; González-Fraguela, M.E.; Estupiñán-Díaz, B.; Serrano-Sánchez, T.; Francis-Turner, L.; Jiménez-Martín, J.; Vega-Hurtado, Y.; Fernández-Jiménez, I. Tyrosine Hydroxylase, Vesicular Monoamine Transporter and Dopamine Transporter mRNA Expression in Nigrostriatal Tissue of Rats with Pedunculopontine Neurotoxic Lesion. Behav. Sci. 2018, 8, 20. https://doi.org/10.3390/bs8020020
Blanco-Lezcano L, Alberti-Amador E, Díaz-Hung M-L, González-Fraguela ME, Estupiñán-Díaz B, Serrano-Sánchez T, Francis-Turner L, Jiménez-Martín J, Vega-Hurtado Y, Fernández-Jiménez I. Tyrosine Hydroxylase, Vesicular Monoamine Transporter and Dopamine Transporter mRNA Expression in Nigrostriatal Tissue of Rats with Pedunculopontine Neurotoxic Lesion. Behavioral Sciences. 2018; 8(2):20. https://doi.org/10.3390/bs8020020
Chicago/Turabian StyleBlanco-Lezcano, Lisette, Esteban Alberti-Amador, Mei-Li Díaz-Hung, María Elena González-Fraguela, Bárbara Estupiñán-Díaz, Teresa Serrano-Sánchez, Liliana Francis-Turner, Javier Jiménez-Martín, Yamilé Vega-Hurtado, and Isabel Fernández-Jiménez. 2018. "Tyrosine Hydroxylase, Vesicular Monoamine Transporter and Dopamine Transporter mRNA Expression in Nigrostriatal Tissue of Rats with Pedunculopontine Neurotoxic Lesion" Behavioral Sciences 8, no. 2: 20. https://doi.org/10.3390/bs8020020




