Magnesium/Silica/Lanthanum@Activated Carbon for the Remediation of As(III) from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
Synthesis of Hybrid Mg-Si-La@AC
2.2. Adsorption Experiments
2.2.1. Adsorption Isotherms
- 1/n = 0 means heterogeneity;
- 1/n < 1 means a normality; and
- 1/n > 1 designates a cooperativeness.
2.2.2. Kinetics
2.3. Arsenic Determination
2.4. Thermodynamics
2.5. Characterization Techniques
3. Results and Discussion
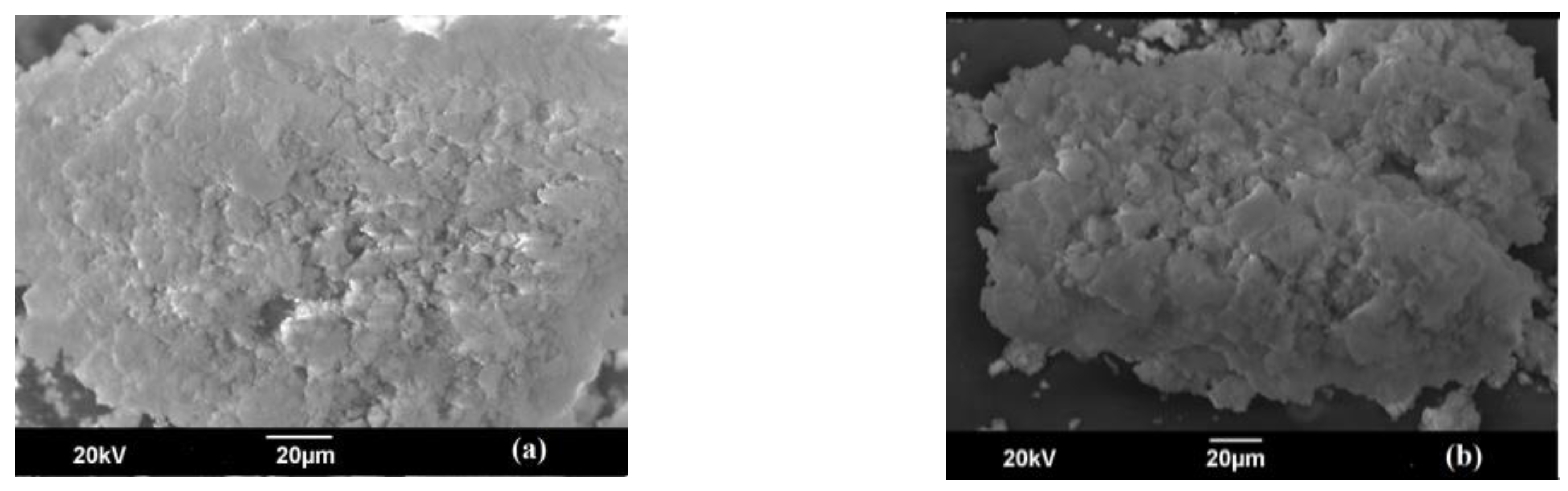
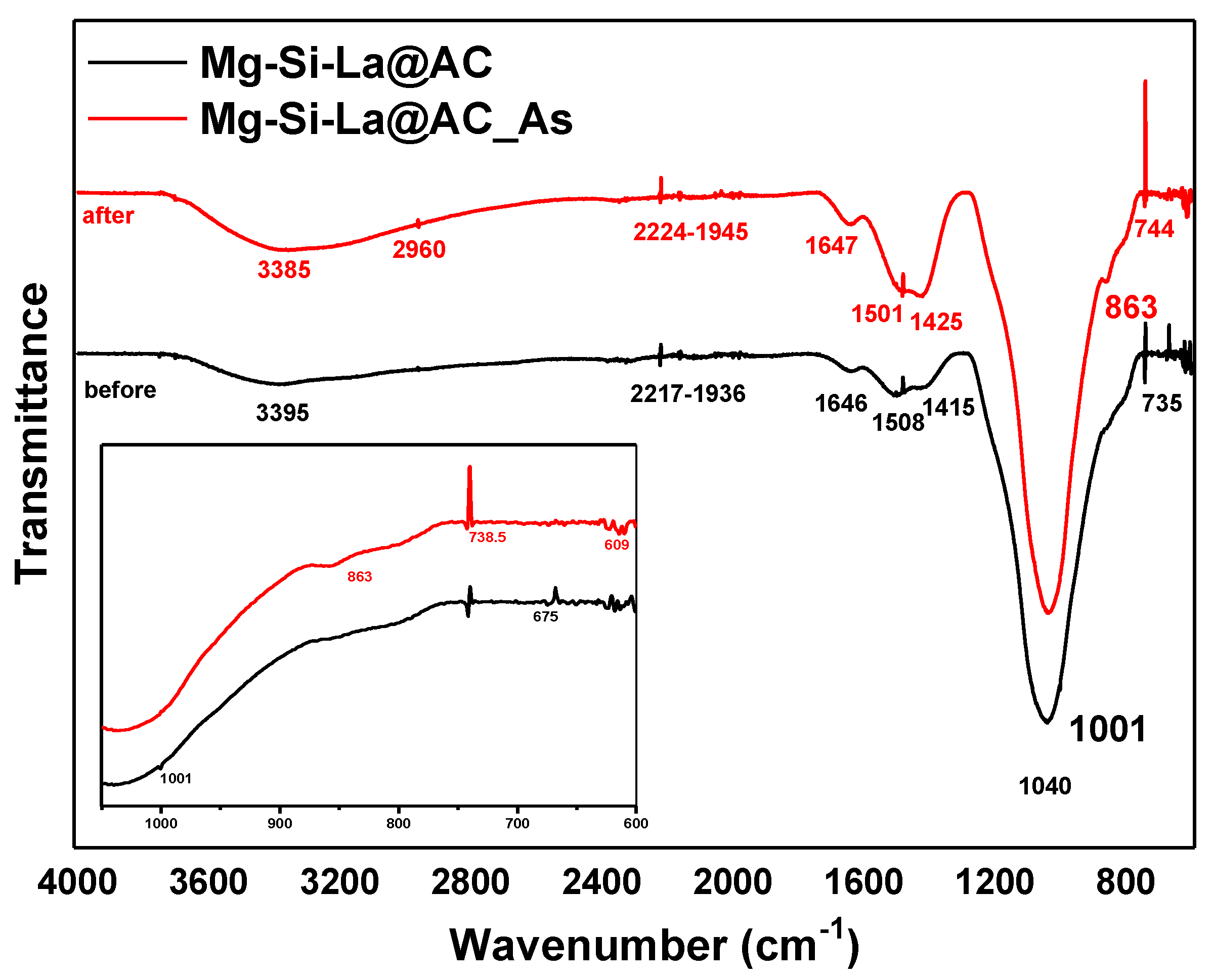
3.1. Characterization of Mg-Si-La@AC
3.2. Batch Adsorption Experiments
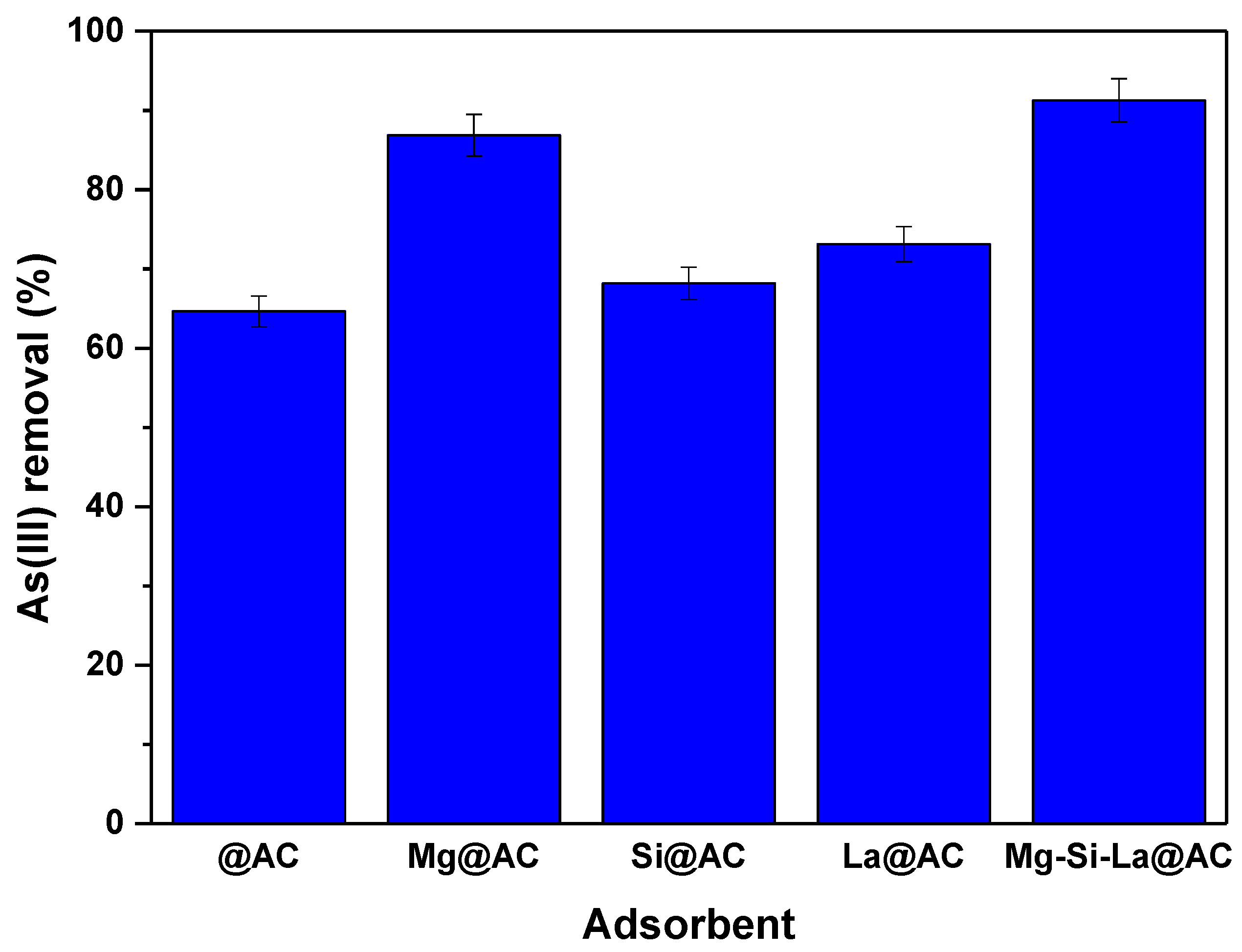
Effect of Modification of Activated Carbon
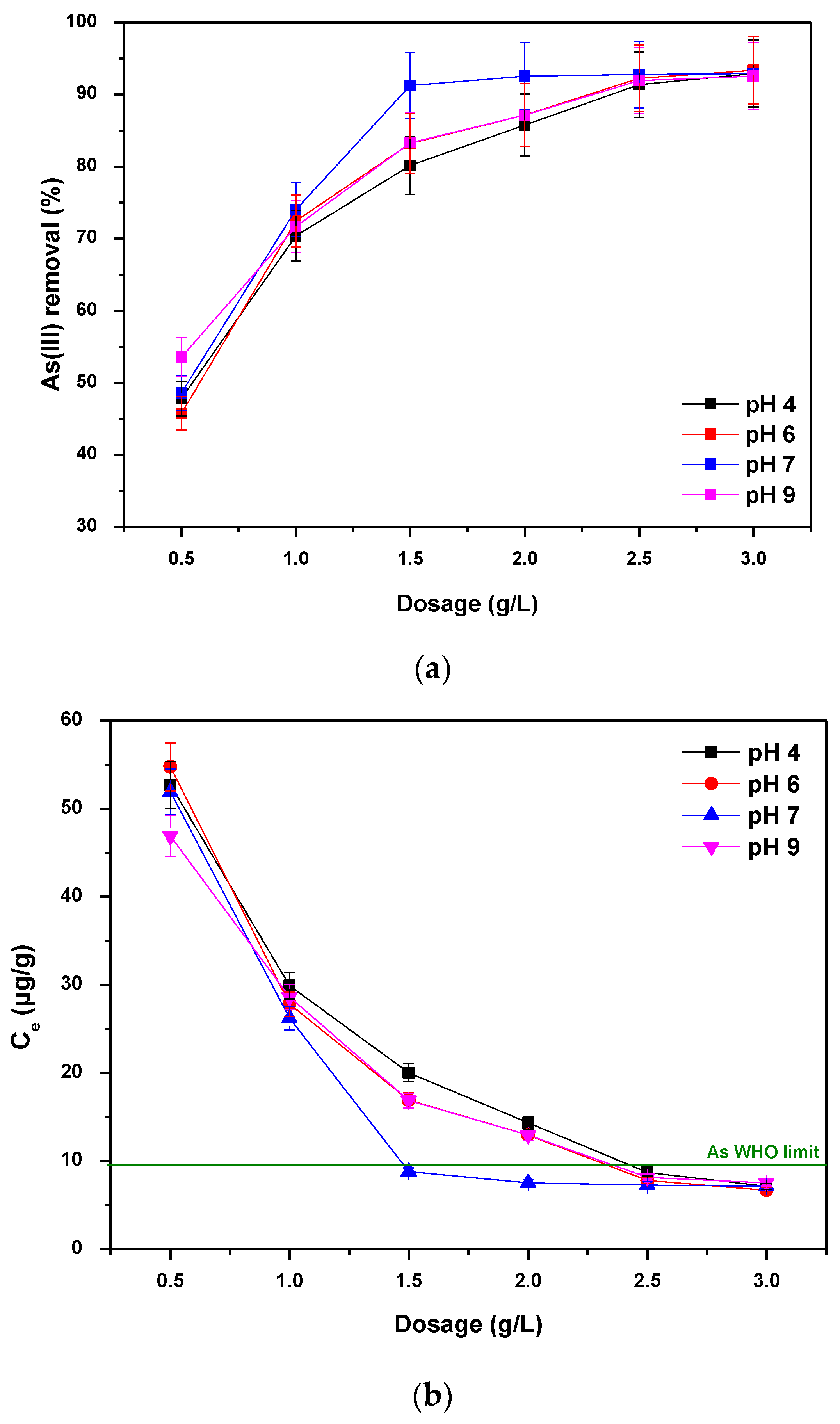
3.3. Effect of Dosage and Initial Solution pH
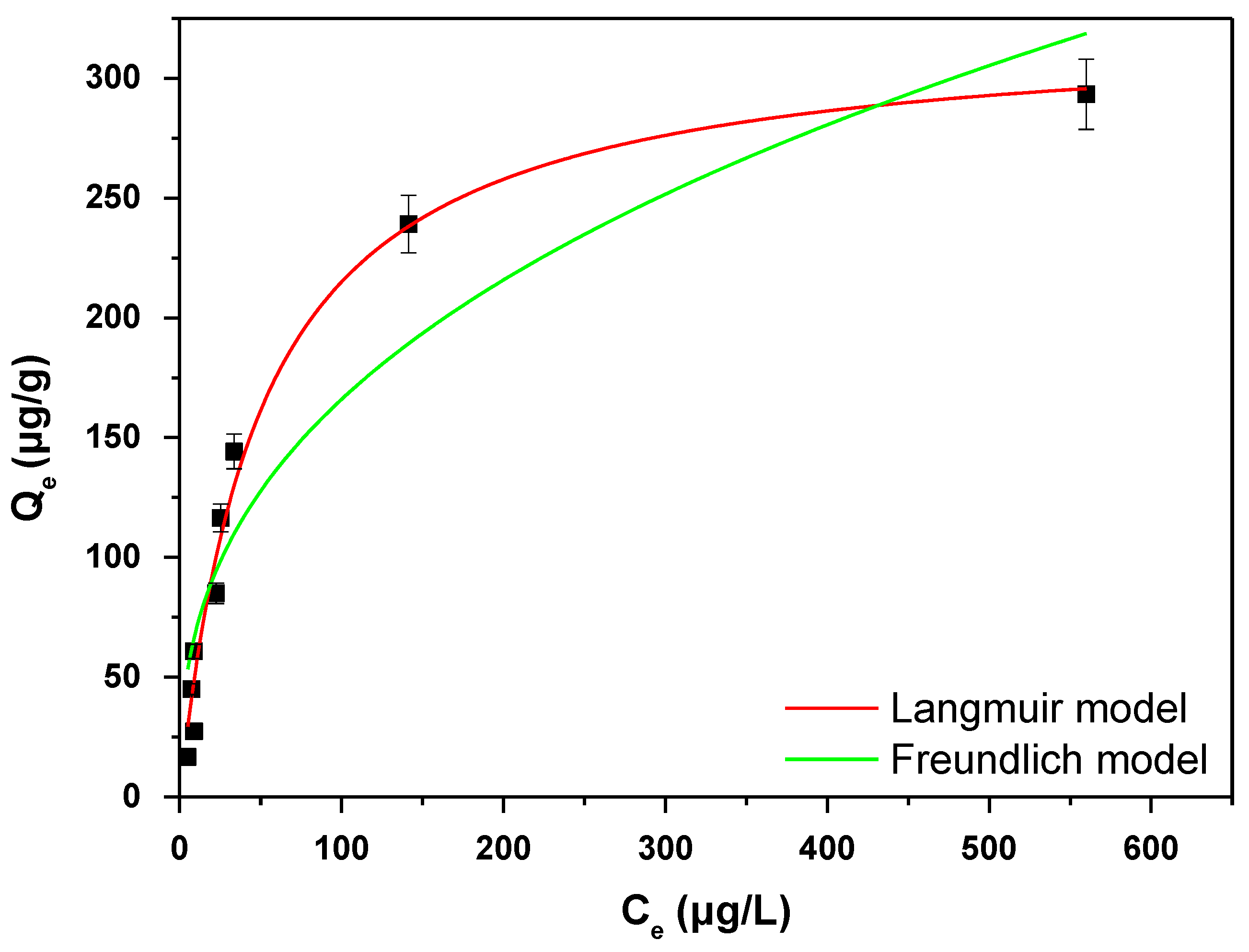
3.4. Adsorption Isotherms
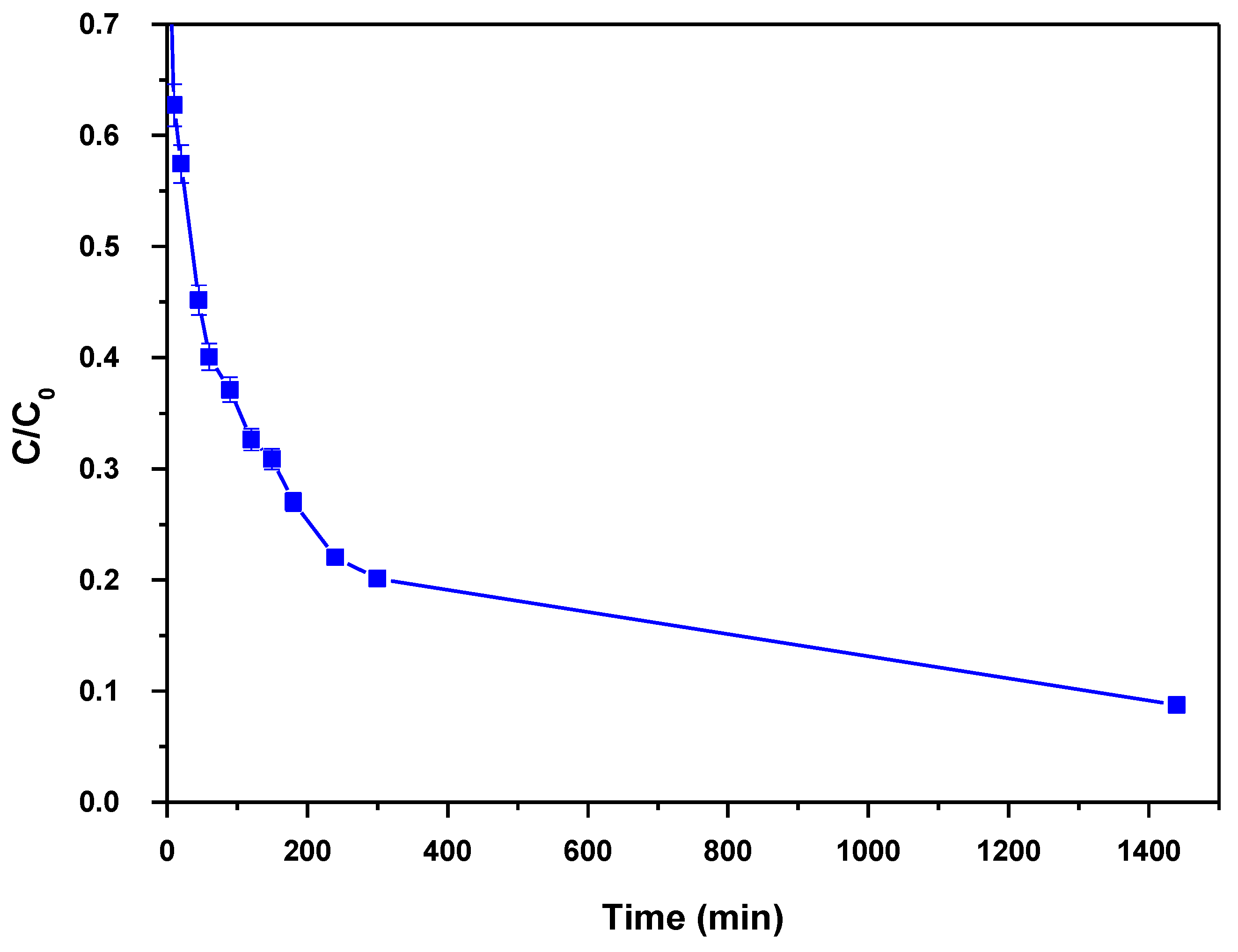
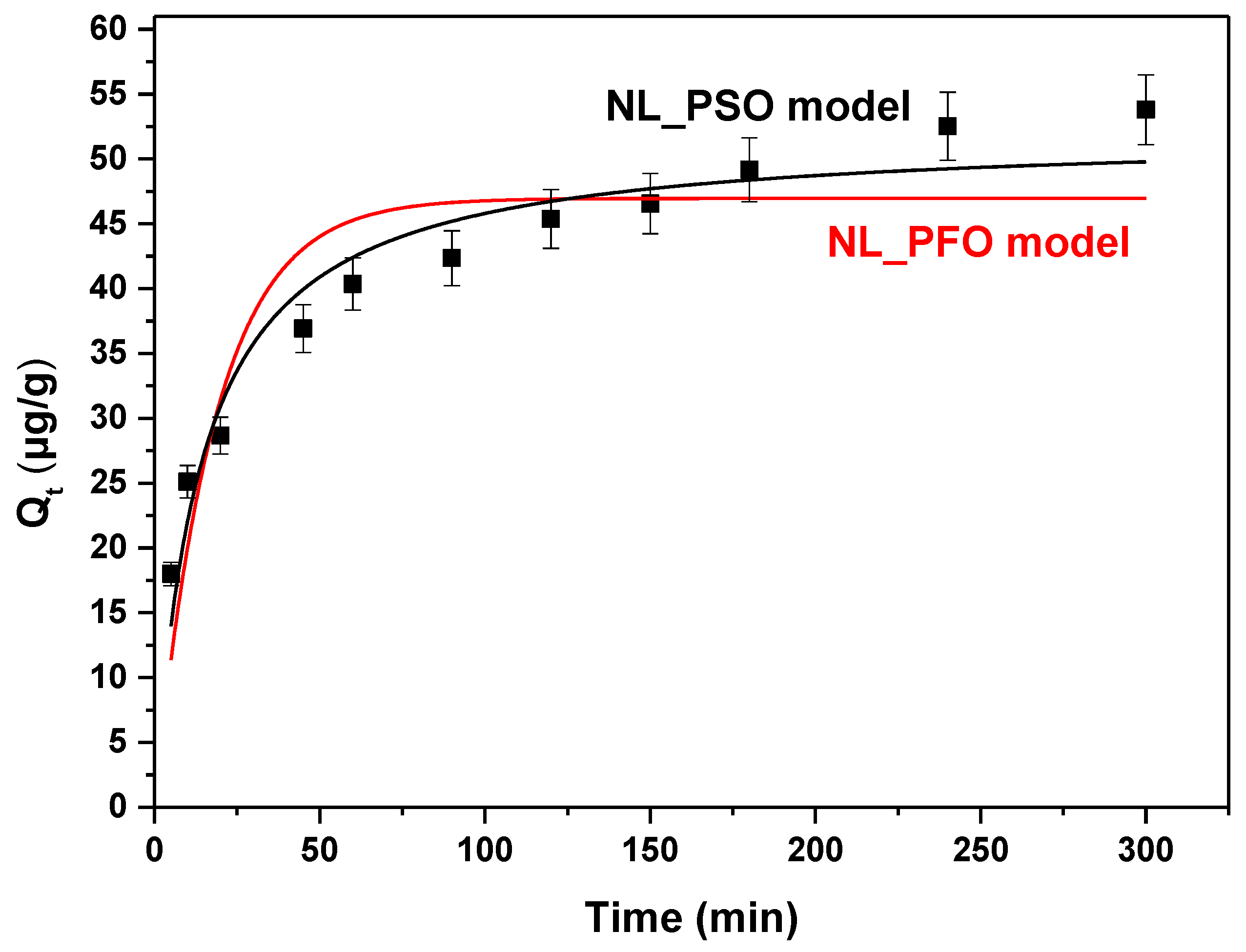
3.5. Effect of Contact Time–Kinetic Models
3.6. Thermodynamics
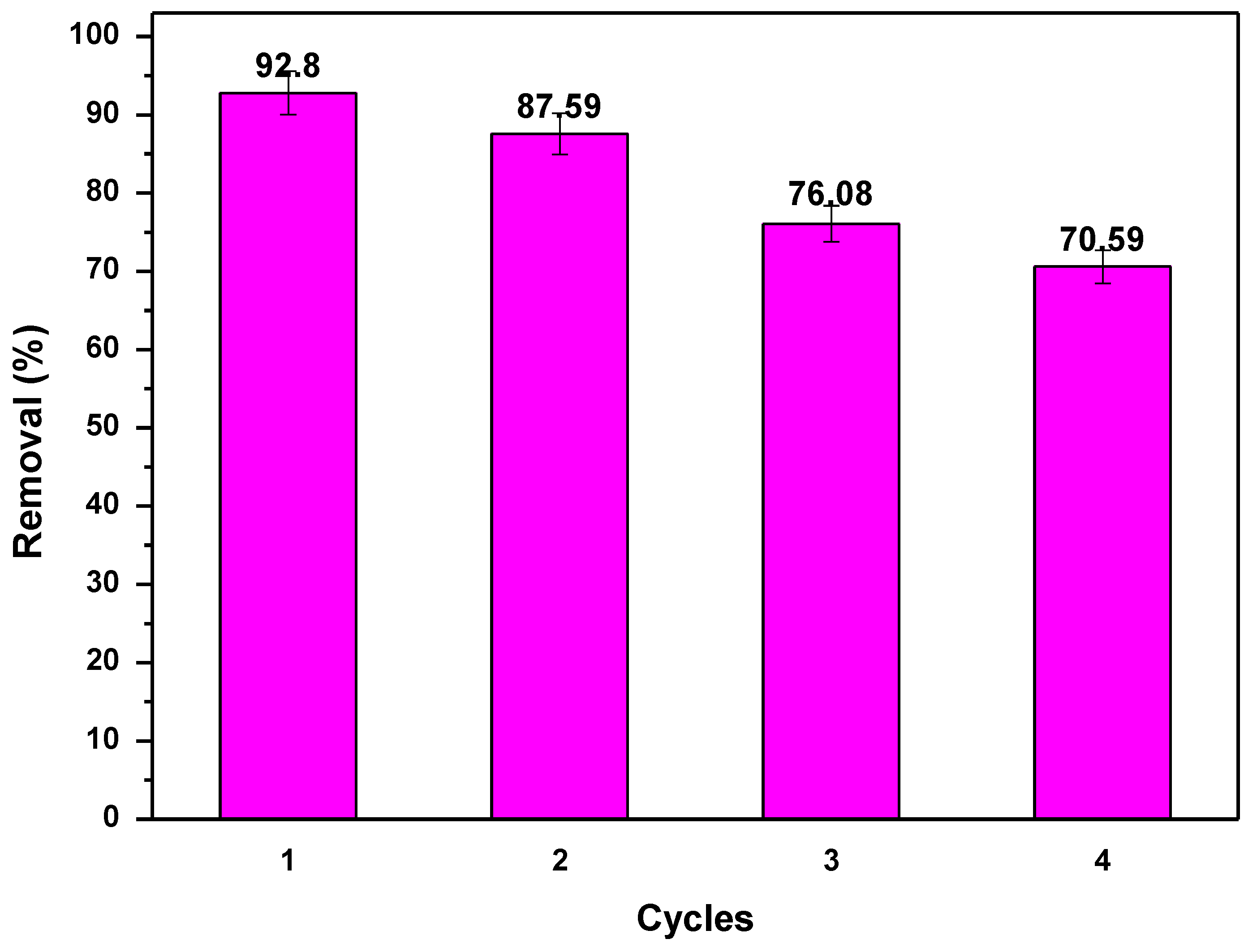
3.7. Regeneration Study
3.8. Comparison with Literature
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, P.; Karunanidhi, D.; Subramani, T.; Srinivasamoorthy, K. Sources and Consequences of Groundwater Contamination. Arch. Environ. Contam. Toxicol. 2021, 80, 1–10. [Google Scholar] [CrossRef]
- Bundschuh, J.; Armienta, M.A.; Morales-Simfors, N.; Alam, M.A.; López, D.L.; Delgado Quezada, V.; Dietrich, S.; Schneider, J.; Tapia, J.; Sracek, O.; et al. Arsenic in Latin America: New Findings on Source, Mobilization and Mobility in Human Environments in 20 Countries Based on Decadal Research 2010–2020. Crit. Rev. Environ. Sci. Technol. 2020, 51, 1727–1865. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Mise, N.; Ichihara, S. Arsenic Contamination in Food Chain in Bangladesh: A Review on Health Hazards, Socioeconomic Impacts and Implications. Hyg. Environ. Health Adv. 2022, 2, 100004. [Google Scholar] [CrossRef]
- McKay, G.; Otterburn, M.S.; Sweeney, A.G. The Removal of Colour from Effluent Using Various Adsorbents: Some Preliminary Economic Considerations. J. Soc. Dye. Colour. 1978, 94, 357–360. [Google Scholar] [CrossRef]
- Shaji, E.; Santosh, M.; Sarath, K.V.; Prakash, P.; Deepchand, V.; Divya, B.V. Arsenic Contamination of Groundwater: A Global Synopsis with Focus on the Indian Peninsula. Geosci. Front. 2021, 12, 101079. [Google Scholar] [CrossRef]
- Ur Rehman, H.; Ahmed, S.; Ur Rahman, M.; Mehmood, M.S. Arsenic Contamination, Induced Symptoms, and Health Risk Assessment in Groundwater of Lahore, Pakistan. Environ. Sci. Pollut. Res. 2022, 29, 49796–49807. [Google Scholar] [CrossRef]
- Subhani, M.; Mustafa, I.; Alamdar, A.; Katsoyiannis, I.A.; Ali, N.; Huang, Q.; Peng, S.; Shen, H.; Eqani, S.A.M.A.S. Arsenic Levels from Different Land-Use Settings in Pakistan: Bio-Accumulation and Estimation of Potential Human Health Risk via Dust Exposure. Ecotoxicol. Environ. Saf. 2015, 115, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Pezeshki, H.; Hashemi, M.; Rajabi, S. Removal of Arsenic as a Potentially Toxic Element from Drinking Water by Filtration: A Mini Review of Nanofiltration and Reverse Osmosis Techniques. Heliyon 2023, 9, e14246. [Google Scholar] [CrossRef]
- Mazumder, D.N.G. Health Effects of Chronic Arsenic Toxicity. In Handbook of Arsenic Toxicology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 151–192. [Google Scholar] [CrossRef]
- Fatoki, J.O.; Badmus, J.A. Arsenic as an Environmental and Human Health Antagonist: A Review of Its Toxicity and Disease Initiation. J. Hazard. Mater. Adv. 2022, 5, 100052. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Arsenic: A Review on a Great Health Issue Worldwide. Appl. Sci. 2022, 12, 6184. [Google Scholar] [CrossRef]
- World Health Organization European Standards for Drinking-Water. Am. J. Med. Sci. 1970, 242, 56.
- Jadhav, S.V.; Bringas, E.; Yadav, G.D.; Rathod, V.K.; Ortiz, I.; Marathe, K.V. Arsenic and Fluoride Contaminated Groundwaters: A Review of Current Technologies for Contaminants Removal. J. Environ. Manag. 2015, 162, 306–325. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Tzollas, N.M.; Tolkou, A.K.; Mitrakas, M.; Ernst, M.; Zouboulis, A.I. Use of Novel Composite Coagulants for Arsenic Removal from Waters-Experimental Insight for the Application of Polyferric Sulfate (PFS). Sustainability 2017, 9, 590. [Google Scholar] [CrossRef]
- Meez, E.; Tolkou, A.K.; Giannakoudakis, D.A.; Katsoyiannis, I.A.; Kyzas, G.Z. Activated Carbons for Arsenic Removal from Natural Waters and Wastewaters: A Review. Water 2021, 13, 2982. [Google Scholar] [CrossRef]
- Cañas Kurz, E.E.; Hellriegel, U.; Figoli, A.; Gabriele, B.; Bundschuh, J.; Hoinkis, J. Small-Scale Membrane-Based Arsenic Removal for Decentralized Applications–Developing a Conceptual Approach for Future Utilization. Water Res. 2021, 196, 116978. [Google Scholar] [CrossRef]
- Worou, C.N.; Chen, Z.L.; Bacharou, T. Arsenic Removal from Water by Nanofiltration Membrane: Potentials and Limitations. Water Pract. Technol. 2021, 16, 291–319. [Google Scholar] [CrossRef]
- Nur, T.; Loganathan, P.; Ahmed, M.B.; Johir, M.A.H.; Nguyen, T.V.; Vigneswaran, S. Removing Arsenic from Water by Coprecipitation with Iron: Effect of Arsenic and Iron Concentrations and Adsorbent Incorporation. Chemosphere 2019, 226, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Zama, E.F.; Li, G.; Tang, Y.T.; Reid, B.J.; Ngwabie, N.M.; Sun, G.X. The Removal of Arsenic from Solution through Biochar-Enhanced Precipitation of Calcium-Arsenic Derivatives. Environ. Pollut. 2022, 292, 118241. [Google Scholar] [CrossRef]
- Dudek, S.; Kołodyńska, D. Arsenate Removal on the Iron Oxide Ion Exchanger Modified with Neodymium(III) Ions. J. Environ. Manag. 2022, 307, 114551. [Google Scholar] [CrossRef]
- Saleh, T.A.; Mustaqeem, M.; Khaled, M. Water Treatment Technologies in Removing Heavy Metal Ions from Wastewater: A Review. Environ. Nanotechnol. Monit. Manag. 2022, 17, 100617. [Google Scholar] [CrossRef]
- Shankar, S.; Shanker, U. Shikha Arsenic Contamination of Groundwater: A Review of Sources, Prevalence, Health Risks, and Strategies for Mitigation. Sci. World J. 2014, 2014, 304524. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Sellaoui, L.; Franco, D.; Dotto, G.L.; Georgin, J.; Bajahzar, A.; Belmabrouk, H.; Ben Lamine, A.; Bonilla-Petriciolet, A.; Li, Z. Adsorption of Crystal Violet on Biomasses from Pecan Nutshell, Para Chestnut Husk, Araucaria Bark and Palm Cactus: Experimental Study and Theoretical Modeling via Monolayer and Double Layer Statistical Physics Models. Chem. Eng. J. 2019, 378, 122101. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Zhang, L.; Pei, L.; Xue, H.; Li, Z. Adsorption of Methylene Blue and Congo Red from Aqueous Solution on 3D MXene/Carbon Foam Hybrid Aerogels: A Study by Experimental and Statistical Physics Modeling. J. Environ. Chem. Eng. 2023, 11, 109206. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Chen, M.; Seliem, M.K.; Mobarak, M.; Diao, Z.; Li, Z. Adsorption of Azo Dyes and Naproxen by Few-Layer MXene Immobilized with Dialdehyde Starch Nanoparticles: Adsorption Properties and Statistical Physics Modeling. Chem. Eng. J. 2023, 473, 145385. [Google Scholar] [CrossRef]
- Bilici Baskan, M.; Pala, A. Removal of Arsenic from Drinking Water Using Modified Natural Zeolite. Desalination 2011, 281, 396–403. [Google Scholar] [CrossRef]
- Losev, V.N.; Didukh-Shadrina, S.L.; Orobyeva, A.S.; Metelitsa, S.I.; Borodina, E.V.; Ondar, U.V.; Nesterenko, P.N.; Maznyak, N.V. A New Method for Highly Efficient Separation and Determination of Arsenic Species in Natural Water Using Silica Modified with Polyamines. Anal. Chim. Acta 2021, 1178, 338824. [Google Scholar] [CrossRef]
- Jacobson, A.T.; Fan, M. Evaluation of Natural Goethite on the Removal of Arsenate and Selenite from Water. J. Environ. Sci. 2019, 76, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Ha, Z.; Lv, C.; Xu, X.; Li, C.; Du, D.; Zhang, T.C.; Chi, R. A Novel Passivator Based on Electrolytic Manganese Residues and Calcite for Arsenic Sorption and Heavy Metal Passivation of Contaminated Soil. J. Clean. Prod. 2023, 414, 137544. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Simultaneous Removal and Oxidation of Arsenic from Water by δ-MnO2 Modified Activated Carbon. J. Environ. Sci. 2020, 94, 147–160. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic Removal from Aqueous Solutions by Adsorption onto Iron Oxide/Activated Carbon Magnetic Composite. J. Environ. Health Sci. Eng. 2014, 12, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.K.; Garg, R. A Comprehensive Review on Removal of Arsenic Using Activated Carbon Prepared from Easily Available Waste Materials. Environ. Sci. Pollut. Res. 2017, 24, 13295–13306. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, M.B.; Niazi, N.K.; Bibi, I.; Murtaza, G.; Kunhikrishnan, A.; Seshadri, B.; Shahid, M.; Ali, S.; Bolan, N.S.; Ok, Y.S.; et al. Remediation of Arsenic-Contaminated Water Using Agricultural Wastes as Biosorbents. Crit. Rev. Environ. Sci. Technol. 2016, 46, 467–499. [Google Scholar] [CrossRef]
- Nagra, M.A.; Natasha, N.; Bibi, I.; Tariq, T.Z.; Naz, R.; Ansar, S.; Shahid, M.; Murtaza, B.; Imran, M.; Khalid, M.S.; et al. Biowaste-Based Sorbents for Arsenic Removal from Aqueous Medium and Risk Assessment. Environ. Geochem. Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Chatzimichailidou, S.; Xanthopoulou, M.; Tolkou, A.K.; Katsoyiannis, I.A. Biochar Derived from Rice By-Products for Arsenic and Chromium Removal by Adsorption: A Review. J. Compos. Sci. 2023, 7, 59. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Rada, E.C.; Torretta, V.; Xanthopoulou, M.; Kyzas, G.Z.; Katsoyiannis, I.A. Removal of Arsenic(III) from Water with a Combination of Graphene Oxide (GO) and Granular Ferric Hydroxide (GFH) at the Optimum Molecular Ratio. C 2023, 9, 10. [Google Scholar] [CrossRef]
- Xanthopoulou, M.; Katsoyiannis, I.A. Enhanced Adsorption of Chromate and Arsenate Ions from Contaminated Water with Emphasis on Polyethylenimine Modified Materials: A Review. Separations 2023, 10, 441. [Google Scholar] [CrossRef]
- Samimi, M.; Zakeri, M.; Alobaid, F.; Aghel, B. A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials 2023, 13, 60. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Wang, L.; Sheng, G.; Liu, J.; Yu, H.; Huang, X.J. Enhanced Arsenic Removal from Water by Hierarchically Porous CeO2-ZrO2 Nanospheres: Role of Surface- and Structure-Dependent Properties. J. Hazard. Mater. 2013, 260, 498–507. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Kyzas, G.Z.; Katsoyiannis, I.A. Arsenic(III) and Arsenic(V) Removal from Water Sources by Molecularly Imprinted Polymers (MIPs): A Mini Review of Recent Developments. Sustainability 2022, 14, 5222. [Google Scholar] [CrossRef]
- Koutzaris, S.; Xanthopoulou, M.; Laskaridis, A.; Katsoyiannis, I.A. H2O2-Enhanced As(III) Removal from Natural Waters by Fe(III) Coagulation at Neutral PH Values and Comparison with the Conventional Fe(II)-H2O2 Fenton Process. Sustainability 2022, 14, 6306. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As(III) Adsorption on Fe-Mn Binary Oxides: Are Fe and Mn Oxides Synergistic or Antagonistic for Arsenic Removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Trikalioti, S.; Makrogianni, O.; Xanthopoulou, M.; Deliyanni, E.A.; Kyzas, G.Z.; Katsoyiannis, I.A. Lanthanum Modified Activated Carbon from Coconut Shells for Chromium (VI) Removal from Water. Nanomaterials 2022, 12, 1067. [Google Scholar] [CrossRef] [PubMed]
- Tolkou, A.K.; Trikalioti, S.; Makrogianni, O.; Katsoyiannis, I.A.; Kyzas, G.Z. Magnesium Modified Activated Carbons Derived from Coconut Shells for the Removal of Fluoride from Water. Sustain. Chem. Pharm. 2023, 31, 100898. [Google Scholar] [CrossRef]
- Sujiono, E.H.; Zabrian, D.; Zharvan, V.; Humairah, N.A. Fabrication and Characterization of Coconut Shell Activated Carbon Using Variation Chemical Activation for Wastewater Treatment Application. Results Chem. 2022, 4, 100291. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Deliyanni, E.A. Modified Activated Carbons from Potato Peels as Green Environmental-Friendly Adsorbents for the Treatment of Pharmaceutical Effluents. Chem. Eng. Res. Des. 2015, 97, 135–144. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Sultana, M.; Rownok, M.H.; Sabrin, M.; Rahaman, M.H.; Alam, S.M.N. A Review on Experimental Chemically Modified Activated Carbon to Enhance Dye and Heavy Metals Adsorption. Clean. Eng. Technol. 2022, 6, 100382. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Guo, T.; Liu, H.; Li, J.; Wang, S.; Li, X.; Wang, X.; Jia, Y. Lanthanum Hydroxide: A Highly Efficient and Selective Adsorbent for Arsenate Removal from Aqueous Solution. Environ. Sci. Pollut. Res. 2020, 27, 42868–42880. [Google Scholar] [CrossRef] [PubMed]
- Sawana, R.; Somasundar, Y.; Iyer, V.S.; Baruwati, B. Ceria Modified Activated Carbon: An Efficient Arsenic Removal Adsorbent for Drinking Water Purification. Appl. Water Sci. 2017, 7, 1223–1230. [Google Scholar] [CrossRef]
- Merodio-Morales, E.E.; Reynel-Ávila, H.E.; Mendoza-Castillo, D.I.; Duran-Valle, C.J.; Bonilla-Petriciolet, A. Lanthanum- and Cerium-Based Functionalization of Chars and Activated Carbons for the Adsorption of Fluoride and Arsenic Ions. Int. J. Environ. Sci. Technol. 2020, 17, 115–128. [Google Scholar] [CrossRef]
- Yanagisawa, H.; Matsumoto, Y.; Machida, M. Adsorption of Zn(II) and Cd(II) Ions onto Magnesium and Activated Carbon Composite in Aqueous Solution. Appl. Surf. Sci. 2010, 256, 1619–1623. [Google Scholar] [CrossRef]
- Choong, C.E.; Ibrahim, S.; Yoon, Y.; Jang, M. Removal of Lead and Bisphenol A Using Magnesium Silicate Impregnated Palm-Shell Waste Powdered Activated Carbon: Comparative Studies on Single and Binary Pollutant Adsorption. Ecotoxicol. Environ. Saf. 2018, 148, 142–151. [Google Scholar] [CrossRef]
- Irunde, R.; Ligate, F.J.; Ijumulana, J.; Ahmad, A.; Maity, J.P.; Hamisi, R.; Philip, J.Y.N.; Kilulya, K.F.; Karltun, E.; Mtamba, J.; et al. The Natural Magnesite Efficacy on Arsenic Extraction from Water and Alkaline Influence on Metal Release in Water. Appl. Geochem. 2023, 155, 105705. [Google Scholar] [CrossRef]
- Sugita, H.; Oguma, T.; Hara, J.; Zhang, M.; Kawabe, Y. Removal of Arsenate from Contaminated Water via Combined Addition of Magnesium-Based and Calcium-Based Adsorbents. Sustainability 2023, 15, 4689. [Google Scholar] [CrossRef]
- Swenson, H.; Stadie, N.P. Langmuir’s Theory of Adsorption: A Centennial Review. Langmuir 2019, 35, 5409–5426. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Phys. Chem. 1907, 57U, 385–470. [Google Scholar] [CrossRef]
- Smith, A.H.; Steinmaus, C.M. Health Effects of Arsenic and Chromium in Drinking Water: Recent Human Findings. Annu. Rev. Public Health 2009, 30, 107–122. [Google Scholar] [CrossRef]
- Sotomayor, F.; Quantatec, A.P.; Sotomayor, F.J.; Cychosz, K.A.; Thommes, M. Characterization of Micro/Mesoporous Materials by Physisorption: Concepts and Case Studies. Acc. Mater. Surf. Res. 2018, 3, 34–50. [Google Scholar]
- Nasri, N.S.; Jibril, M.; Zaini, M.A.A.; Mohsin, R.; Dadum, H.U.; Musa, A.M. Synthesis and Characterization of Bio-Based Porous Carbons by Two Step Physical Activation with CO2. J. Teknol. 2014, 68, 5–9. [Google Scholar] [CrossRef]
- Jais, F.M.; Ibrahim, S.; Yoon, Y.; Jang, M. Enhanced Arsenate Removal by Lanthanum and Nano-Magnetite Composite Incorporated Palm Shell Waste-Based Activated Carbon. Sep. Purif. Technol. 2016, 169, 93–102. [Google Scholar] [CrossRef]
- Dudek, S.; Kołodyńska, D. Arsenic(V) Removal on the Lanthanum-Modified Ion Exchanger with Quaternary Ammonium Groups Based on Iron Oxide. J. Mol. Liq. 2022, 347, 117985. [Google Scholar] [CrossRef]
- Mehanathan, S.; Jaafar, J.; Nasir, A.M.; Ismail, A.F.; Matsuura, T.; Othman, M.H.D.; Rahman, M.A.; Yusof, N. Magnesium Oxide Nanoparticles for the Adsorption of Pentavalent Arsenic from Water: Effects of Calcination. Membranes 2023, 13, 475. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W. Removal of Fluoride from Groundwater Using MnO2 Bentonite-Smectite Rich Clay Soils Composite. Groundw. Sustain. Dev. 2021, 14, 100623. [Google Scholar] [CrossRef]
- Tolkou, A.K.; Trikkaliotis, D.G.; Kyzas, G.Z.; Katsoyiannis, I.A.; Deliyanni, E.A. Simultaneous Removal of As(III) and Fluoride Ions from Water Using Manganese Oxide Supported on Graphene Nanostructures (GO-MnO2). Sustainability 2023, 15, 1179. [Google Scholar] [CrossRef]
- Katsoyiannis, I.A.; Voegelin, A.; Zouboulis, A.I.; Hug, S.J. Enhanced As(III) Oxidation and Removal by Combined Use of Zero Valent Iron and Hydrogen Peroxide in Aerated Waters at Neutral PH Values. J. Hazard. Mater. 2015, 297, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, K.; Nour, S.; Selbie, M.; Prevost, M.; Blumenschein, C.D.; Chen, H.; Amy, G.L. Optimization of Process Parameters for Arsenic Treatment with Granular Ferric Hydroxide; American Water Works Association: Denver, CO, USA, 2003. [Google Scholar]
- Banerjee, S.; Chattopadhyaya, M.C. Adsorption Characteristics for the Removal of a Toxic Dye, Tartrazine from Aqueous Solutions by a Low Cost Agricultural by-Product. Arab. J. Chem. 2017, 10, S1629–S1638. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of Adsorption Technology. Interface Sci. Technol. 2021, 33, 1–70. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, T.; Luo, H.; Geng, J.; Yu, W.; Jiang, Z. Adsorption of Arsenic by Activated Charcoal Coated Zirconium-Manganese Nanocomposite: Performance and Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 318–328. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Christodoulou, E.; Bikiaris, D.N. Basic Dye Removal with Sorption onto Low-Cost Natural Textile Fibers. Processes 2018, 6, 166. [Google Scholar] [CrossRef]
- Chen, W.; Luan, J.; Yu, X.; Wang, X.; Ke, X. Preparation of Core-Shell Structured Polystyrene @ Graphene Oxide Composite Microspheres with High Adsorption Capacity and Its Removal of Dye Contaminants. Environ. Technol. 2021, 42, 3840–3851. [Google Scholar] [CrossRef]
- Sahu, N.; Singh, J.; Koduru, J.R. Removal of Arsenic from Aqueous Solution by Novel Iron and Iron–Zirconium Modified Activated Carbon Derived from Chemical Carbonization of Tectona Grandis Sawdust: Isotherm, Kinetic, Thermodynamic and Breakthrough Curve Modelling. Environ. Res. 2021, 200, 111431. [Google Scholar] [CrossRef]
- Koohzad, E.; Jafari, D.; Esmaeili, H. Adsorption of Lead and Arsenic Ions from Aqueous Solution by Activated Carbon Prepared from Tamarix Leaves. ChemistrySelect 2019, 4, 12356–12367. [Google Scholar] [CrossRef]
- Esmaeili, H.; Mousavi, S.M.; Hashemi, S.A.; Chiang, W.H.; Ahmadpour Abnavi, S. Activated Carbon@MgO@Fe3O4 as an Efficient Adsorbent for As (III) Removal. Carbon Lett. 2021, 31, 851–862. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Hazardous As(III) Removal Using Nanoporous Activated Carbon of Waste Garlic Stem as Adsorbent: Kinetic and Mass Transfer Mechanisms. Korean J. Chem. Eng. 2019, 36, 1900–1914. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, Z.; Zhou, J.; Sun, T.; Liang, Z. Enhanced Adsorption of As(III) on Chemically Modified Activated Carbon Fibers. Appl. Water Sci. 2019, 9, 41. [Google Scholar] [CrossRef]
- Chuc, P.N.; Bac, N.Q.; Thao, D.T.P.; Kien, N.T.; Chi, N.T.H.; Noi, N.V.; Nguyen, V.T.; Bich, N.T.H.; Nhiem, D.N.; Khieu, D.Q. Highly Efficient Adsorption of Arsenite from Aqueous by Zirconia Modified Activated Carbon. Environ. Eng. Res. 2024, 29, 230070–230076. [Google Scholar] [CrossRef]


| Parameters | Mg-Si-La@AC |
|---|---|
| BET Surface area, SBET (m2/g) | 271.46 |
| Micropore volume, Vmicro (cm3/g) | 0.006 |
| Total pore volume, VT (cm3/g) | 0.521 |
| Element | Mg-Si-La@AC | Mg-Si-La@AC_As |
|---|---|---|
| Content% (w/w) | ||
| La | 3.59 | 2.46 |
| Mg | 6.07 | 4.79 |
| C | 12.64 | 9.04 |
| Si | 8.26 | 7.56 |
| P | 0.79 | 1.04 |
| O | 68.65 | 74.14 |
| As | 0 | 0.96 |
| Langmuir Isotherm Model | ||
|---|---|---|
| Qm (μg/g) | KL (L/μg) | R2 |
| 322 | 0.020 | 0.9898 |
| Freundlich isotherm model | ||
| 1/n | KF (μg/g)(L/μg)1/n | R2 |
| 0.3785 | 29.04 | 0.8780 |
| Pseudo-First-Order Kinetic Model (PFO) | |||
|---|---|---|---|
| Qe.exp (μg/g) | K1 (L/μg∙min) | Qe.cal (μg/g) | R2 |
| 61.47 | 0.0554 | 46.96 | 0.8008 |
| Pseudo-second order kinetic model (PSO) | |||
| Qe.exp (μg/g) | K2 (L/μg∙min) | Qe.cal (μg/g) | R2 |
| 61.47 | 0.0014 | 52.04 | 0.9318 |
| T (K) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (kJ/mol∙K) | R2 |
|---|---|---|---|---|
| 298 | −1.969 | 55.114 | 0.1916 | 0.9684 |
| 308 | −3.884 | |||
| 318 | −5.800 | |||
| 338 | −9.631 |
| Adsorbent | [As]0 (mg/L) | Dosage (g/L) | pHinit | Adsorption Capacity (mg/g) | Removal % | Cycles | Ref. |
|---|---|---|---|---|---|---|---|
| Fe–Zr@AC 1 | 1.0 | 3.0 | 7.0 | 1.206 | 86 | 5 | [73] |
| AC–Tamarix 2 | 10.0 | 3.0 | 7.0 | 37.313 | 96 | - | [74] |
| MgO/AC/Fe3O4 3 | 6.0 | 0.13 | 7.0 | 1.166 | 97 | 5 | [75] |
| AGSC 4 | 0.4 | 5.0 | 6.0 | 0.192 | 93 | 5 | [76] |
| ACF 5 | 2.0 | 0.5 | 6.0 | 8.651 | 74 | - | [77] |
| ZrO2/AC 6 | 10.0 | 50.0 | 5.0 | 64.0 | 90 | 4 | [78] |
| Mg-Si-La@AC 7 | 0.1 | 1.5 | 7.0 | 0.322 | 93 | 4 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tolkou, A.K.; Kyzas, G.Z. Magnesium/Silica/Lanthanum@Activated Carbon for the Remediation of As(III) from Water. Environments 2023, 10, 171. https://doi.org/10.3390/environments10100171
Tolkou AK, Kyzas GZ. Magnesium/Silica/Lanthanum@Activated Carbon for the Remediation of As(III) from Water. Environments. 2023; 10(10):171. https://doi.org/10.3390/environments10100171
Chicago/Turabian StyleTolkou, Athanasia K., and George Z. Kyzas. 2023. "Magnesium/Silica/Lanthanum@Activated Carbon for the Remediation of As(III) from Water" Environments 10, no. 10: 171. https://doi.org/10.3390/environments10100171







