The Effect of Foam-Recycle on Ammonium Removal by Aerobic Denitrification Using Alcaligenes faecalis No. 4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism Used
2.2. Medium
2.3. Aerated Batch Cultivation
2.4. Shaking-Flask Cultivation
2.5. Activities of Hydroxylamine Oxidoreductase (HAO) and Nitrite Reductase (NIR)
2.6. Analytical Methods
3. Results and Discussion
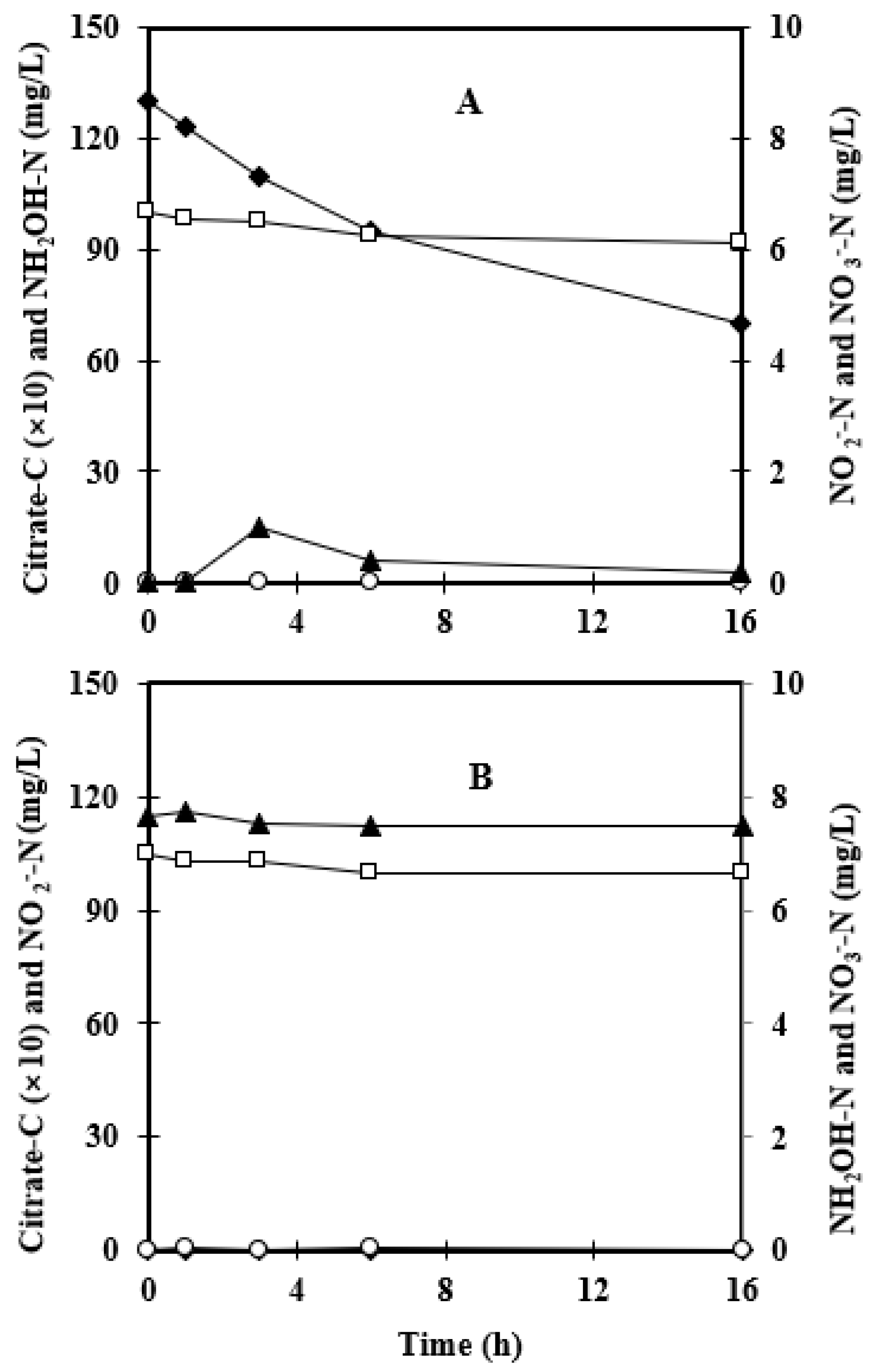
3.1. Effect of Foam-Recycle
3.2. Consumption of Other Nitrogen Sources
3.3. Enzyme Activity
3.4. Effect of Initial Ammonium Load and Airflow Rate
3.5. Effect of pH
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- McCatry, P.L. What is the Best Biological Process for Nitrogen Removal: When and Why? Environ. Sci. Technol. 2018, 52, 3835–3841. [Google Scholar] [CrossRef]
- Hooper, A.B.; Vannelli, T.; Bergmann, D.J.; Arociero, D.M. Enzymology of the oxidation of ammonia to nitrite by bacteria. Antonie Van Leeuwenhoek 1997, 71, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Van Loosdrecht, M.C.M.; Jetten, M.S.M. Microbiological conversion in nitrogen removal. Water Sci. Technol. 1998, 38, 1–7. [Google Scholar] [CrossRef]
- Itokawa, H.; Hanaki, K.; Matsuo, T. Nitrous oxide production in high-loading biological nitrogen removal process under low COD/N ratio condition. Water Res. 2001, 35, 657–664. [Google Scholar] [CrossRef]
- Wrage, N.; Velthof, G.L.; van Beusichem, M.L.; Oenema, O. Role of nitrifer denitrification in the production of nitrous oxide. Soil Biol. Biochem. 2001, 33, 1723–1732. [Google Scholar] [CrossRef]
- Carrera, J.; Baeza, J.A.; Vicent, T.; Lafuente, J. Biological nitrogen removal of high-strength ammonium industrial wastewater with two-sludge system. Water Res. 2003, 37, 4211–4221. [Google Scholar] [CrossRef]
- Jetten, M.S.M.; Logemann, S.; Muyzer, G.; Robertson, L.A.; de Vries, S.; van Loosdrecht, M.C.M.; Kuenen, J.G. Novel principles in the microbial conversion of nitrogen compounds. Antonie Van Leeuwenhoek 1997, 71, 75–93. [Google Scholar] [CrossRef]
- Gupta, A.B.; Gupta, S.K. Simultaneous carbon and nitrogen removal in mixed culture aerobic RBC biofilm. Water Res. 1999, 33, 556–561. [Google Scholar] [CrossRef]
- Gupta, A.B.; Gupta, S.K. Simultaneous carbon and nitrogen removal from high strength domestic wastewater in an aerobic RBC biofilm. Water Res. 2001, 35, 1714–1722. [Google Scholar] [CrossRef]
- Schmidt, I.; Sliekers, O.; Schmid, M.; Bock, E.; Fuerst, J.; Kuenen, J.G.; Jetten, M.S.M.; Strous, M. New concepts of microbial treatment processes for the nitrogen removal in wastewater. FEMS Microbiol. Rev. 2003, 772, 481–492. [Google Scholar] [CrossRef]
- Khin, T.; Annachhatre, A.P. Novel microbial nitrogen removal processes. Biotechnol. Adv. 2004, 22, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, L.; Pan, D.; Lin, H.; Ren, Y.; Zhang, J.; Zhou, B.; Lin, J.; Lin, J. Heterotrophic nitrification and related functional gene expression characteristics of Alcaligenes faecalis SDU20 with the potential use in swine wastewater treatment. Bioprocess Biosyst. Eng. 2021, 44, 2035–2050. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xu, J.; Zhang, S.; Liu, F.; Peng, J.; Peng, Y.; Wu, J. Nitrogen removal characteristics of a novel heterotrophic nitrification and aerobic denitrification bacteria, Alcaligenes faecalis WT14. J. Environ. Manag. 2021, 282, 111961. [Google Scholar] [CrossRef] [PubMed]
- Jetten, M.S.M.; de Bruijn, P.; Kuenen, G. Hydroxylamine metabolism in Pseudomonas PB16: Involvement of a novel hydroxylamine oxidoreductase. Antonie Van Leeuwenhoek 1997, 71, 69–74. [Google Scholar] [CrossRef]
- Wang, Y.; Singh, R.P.; Geng, C.; Fu, D. Carbon to nitrogen ratio influence on the performance of bioretention for wastewater treatment. Environ. Sci. Pollut. Res. 2020, 27, 17652–17660. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Characteristics of ammonium removal by hetetrophic nitrification-aerobic denitrification by Alcaligenes faecalis No. 4. J. Biosci. Bioeng. 2005, 100, 184–191. [Google Scholar] [CrossRef]
- Joo, H.S.; Hirai, M.; Shoda, M. Piggery wastewater treatment using Alcaligenes faecalis strain No. 4 with heterotrophic nitrification and aerobic denitrification. Water Res. 2006, 40, 3029–3036. [Google Scholar] [CrossRef]
- Yang, S.F.; Tay, J.H.; Liu, Y. Inhibition of free ammonia to the formation of aerobic granules. Biochem. Eng. J. 2004, 17, 41–48. [Google Scholar] [CrossRef]
- Liu, Y.; Ngo, H.H.; Guo, W.; Peng, L.; Wnag, D.; Ni, B. The roles of free ammonia (FA) in biological wastewater treatment processes: A review. Environ. Int. 2019, 123, 10–19. [Google Scholar] [CrossRef]
- Liu, Y.; Capdeville, B. Response pattern of nitrifying biofilm reactor to shock loading. Biotechnol. Lett. 1994, 16, 655–660. [Google Scholar] [CrossRef]
- Kim, N.J.; Sugano, Y.; Hirai, M.; Shoda, M. Removal of a high load of ammonia gas by a marine bacterium, Vibrio alginolyticus. J. Biosci. Bioeng. 2000, 90, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Uemoto, H.; Ando, A.; Saiki, H. Effect of oxygen concentration on nitrogen removal by Nitrosomonas europaea and Paracoccus denitrificans immobilized within tubular polymeric gel. J. Biosci. Bioeng. 2000, 6, 654–660. [Google Scholar] [CrossRef]
- Su, J.J.; Liu, B.Y.; Liu, C.Y. Comparison of aerobic denitrification under high oxygen atmosphere by Thiosphaera pantotropha ATCC 35512 and Pseudomonas stutzeri SU2 newly isolated from the activated sludge of a piggery wastewater treatment system. J. Appl. Microbiol. 2001, 90, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Obbard, J.P. Ammonia removal from prawn aquaculture water using immobilized nitrifying bacteria. Appl. Microbiol. Biotechnol. 2001, 57, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, E.; Nomura, N.; Nakajima-Kambe, T.; Okada, N.; Nakahara, T. A simple screening procedure for heterotrophic nitrifying bacteria with oxygen-tolerant denitrification activity. J. Biosci. Bioeng. 2003, 95, 409–411. [Google Scholar] [CrossRef]
- Obaja, D.; Mace, S.; Sans, C.C.; Alvarez, J.M. Nitrification, denitrification and biological phosphorus removal in piggery wastewater using a sequencing batch reactor. Bioresour. Technol. 2003, 87, 103–111. [Google Scholar] [CrossRef]
- Du, X.; Wang, J.; Jegatheesan, V.; Shi, G. Dissolved Oxygen Control in Activated Sludge Process Using a Neural Network-Based Adaptive PID Algorithm. Appl. Sci. 2018, 8, 261. [Google Scholar] [CrossRef]
- Padhi, S.K.; Tripathy, S.; Mohanty, S.; Maiti, N.K. Aerobic and heterotrophic nitrogen removal by Enterobacter cloacae CF-S27 with efficient utilization of hydroxylamine. Bioresour. Technol. 2017, 232, 285–296. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, E.; Huang, S. Heterotrophic nitrogen removal in Bacillus sp. K5: Involvement of a novel hydroxylamine oxidase. Water Sci. Technol. 2017, 76, 3461–3467. [Google Scholar] [CrossRef]
- Liu, Y.; Ni, G.-M.; Wu, M.-R.; Li, S.-S.; Miao, L.-L.; Liu, Z.-P. Photobacterium sp. NNA4, an efficient hydroxylamine-transforming heterotrophic nitrifier/aerobic denitrifier. J. Biosci. Bioeng. 2019, 128, 64–71. [Google Scholar] [CrossRef]
- Huang, F.; Pan, L.; He, Z.; Zhang, M.; Zhang, M. Identification, interactions, nitrogen removal pathways and performances of culturable heterotrophic nitrification-aerobic denitrification bacteria from mariculture water by using cell culture and metagenomics. Sci. Total Environ. 2020, 732, 13268. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, A.; Yao, Q.; Wu, Q.; Zhu, H. Nitrogen removal characteristics of a versatile heterotrophic nitrifying-aerobic denitrifying bacterium, Pseudomonas bauzanensis DN13-1, isolated from deep-sea sediment. Bioresour. Technol. 2020, 305, 122626. [Google Scholar] [CrossRef] [PubMed]
- Otte, S.; Schalk, J.; Kuenen, J.G.; Jetten, M.S.M. Hydroxylamine oxidation and subsequent nitrous oxide production by the heterotrophic ammonia oxidizer Alcaligenes faecalis. Appl. Microbiol. Biotechnol. 1999, 51, 255–261. [Google Scholar] [CrossRef]
- Zhao, B.; An, Q.; Liang, H.Y.; Guo, J.S. N2O and N2 production during heterotrophic nitrification by Alcaligenes faecalis strain NR. Bioresour. Technol. 2012, 116, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Liu, Y.-X.; Ren, R.-P.; Lv, Y.-K. Effect of temperature, salinity, heavy metals, ammonium concentration, pH and dissolved oxygen on ammonium removal by an aerobic nitrifier. RCS Adv. 2015, 5, 79988–79996. [Google Scholar] [CrossRef]
- Suruga, K.; Murakami, K.; Taniyama, Y.; Hama, T.; Chida, H.; Satoh, T.; Yamada, S.; Hakamata, W.; Kawachi, R.; Isogai, Y.; et al. A novel microperoxidase activity: Methyl viologen-linked nitrite reducing activity of microperoxidase. Biochem. Biophys. Res. Commun. 2004, 315, 815–822. [Google Scholar] [CrossRef]
- Da Silva, S.; Cosnier, S.; Almeida, M.G.; Moura, J.J.G. An efficient poly(pyrrole-viologen)-nitrite reductase biosensor for the mediated detection of nitrite. Electrochem. Commun. 2004, 6, 404–408. [Google Scholar] [CrossRef]
- The Indophenol Blue Absorptiometric Method (JIS) k-0102, Japanese Industrial Standard; Japanese Standard Association: Tokyo, Japan, 1991; Volume 42, pp. 134–145. Available online: https://www.jsa.or.jp/en/ (accessed on 23 July 2023).
- Frear, D.S.; Burrell, R.C. Spectrophotometric methods for determining hydroxylamine reductase activity in higher plants. Anal. Chem. 1955, 27, 1664–1665. [Google Scholar] [CrossRef]
- Wako, R. Removal Characteristics of Ammonium by Heterotrophic Nitrifier Alcaligenes faecalis No. 4; Tokyo Institute of Technology: Yokohama, Japan, 2002. [Google Scholar]
- Hooper, A.B.; Balny, C. Reaction of oxygen with hydroxylamine oxidoreductase of Nitrosomonas fast kinetics. FEBS Lett. 1982, 144, 229–303. [Google Scholar] [CrossRef]
- Sasaki, S.; Karube, I.; Hirota, N.; Arikawa, Y.; Nishiyama, M.; Kukimoto, M.; Horinouchi, S.; Beppu, T. Application of nitrite reductase from Alcaligenes faecalis S-6 for nitrite measurement. Biosens. Bioelectron. 1998, 13, 1–5. [Google Scholar] [CrossRef]
- Song, B.; Ward, B.B. Nitrite Reductase genes in halobenzoate degrading denitrifying bacteria. FEMS Microbiol. Ecol. 2003, 43, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, X.; Cheng, Y.; Jiang, M.; Li, X.; Xue, G. Iron Robustly Stimulates Simultaneous Nitrification and Denitrification Under Aerobic Conditions. Environ. Sci. Technol. 2018, 52, 1404–1412. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.S.; Lee, K.; Shoda, M.; Mwangi, N.P. Application of Alcaligenes faecalis No. 4 for treatment of high-strength ammonium wastewater. Environ. Eng. Manag. J. 2020, 19, 589–598. Available online: http://www.eemj.icpm.tuiasi.ro/ (accessed on 21 June 2023).
- Bueno, E.; Sit, B.; Waldor, M.K.; Cava, F. Anaerobic nitrate reduction divergently governs population expansion of the enteropathogen Vibrio cholerae. Nat. Microbiol. 2018, 3, 1346–1353. [Google Scholar] [CrossRef] [PubMed]
- Polcyn, W.; Lucinski, R. Aerobic and anaerobic nitrate and nitrite reduction in free-living cells of Bradyrhizobium sp. (Lupinus). FEMS Microbiol. Lett. 2003, 226, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Mohammed-Nour, A.; Al-Sewailem, M.; El-Naggar, A.H. The Influence of Alkalization and Temperature on Ammonia Recovery from Cow Manure and the Chemical Properties of the Effluents. Sustainability 2019, 1, 2441. [Google Scholar] [CrossRef]
- Beline, F.; Martinez, J.; Marol, C.; Guiraud, G. Application of the 15N technique to determine the contributions of nitrification and denitrification to the flux of nitrous oxide from aerated pig slurry. Water Res. 2001, 35, 2774–2778. [Google Scholar] [CrossRef]
- Pollice, A.; Tandoi, V.; Lestingi, C. Influence of aeration and sludge retention time on ammonium oxidation to nitrite and nitrate. Water Res. 2002, 36, 2541–2546. [Google Scholar] [CrossRef]
- Uygur, A.; Kargi, F. Biological nutrient removal from pre-treated landfill leachate in a sequencing batch reactor. J. Environ. Manag. 2004, 71, 9–14. [Google Scholar] [CrossRef]
- Obaja, D.; Mace, S.; Alvarez, J.M. Biological nutrient removal by a sequencing batch reactor (SBR) using an internal organic carbon source in digested piggery wastewater. Bioresour. Technol. 2005, 96, 7–14. [Google Scholar] [CrossRef]
- Neerackal, G.M.; Ndegwa, P.M.; Joo, H.S.J.; Harrison, H. Manure-pH management for ammonia emission from dairy barns and liquid manure storage. Appl. Eng. Agric. 2017, 33, 235–242. [Google Scholar] [CrossRef]
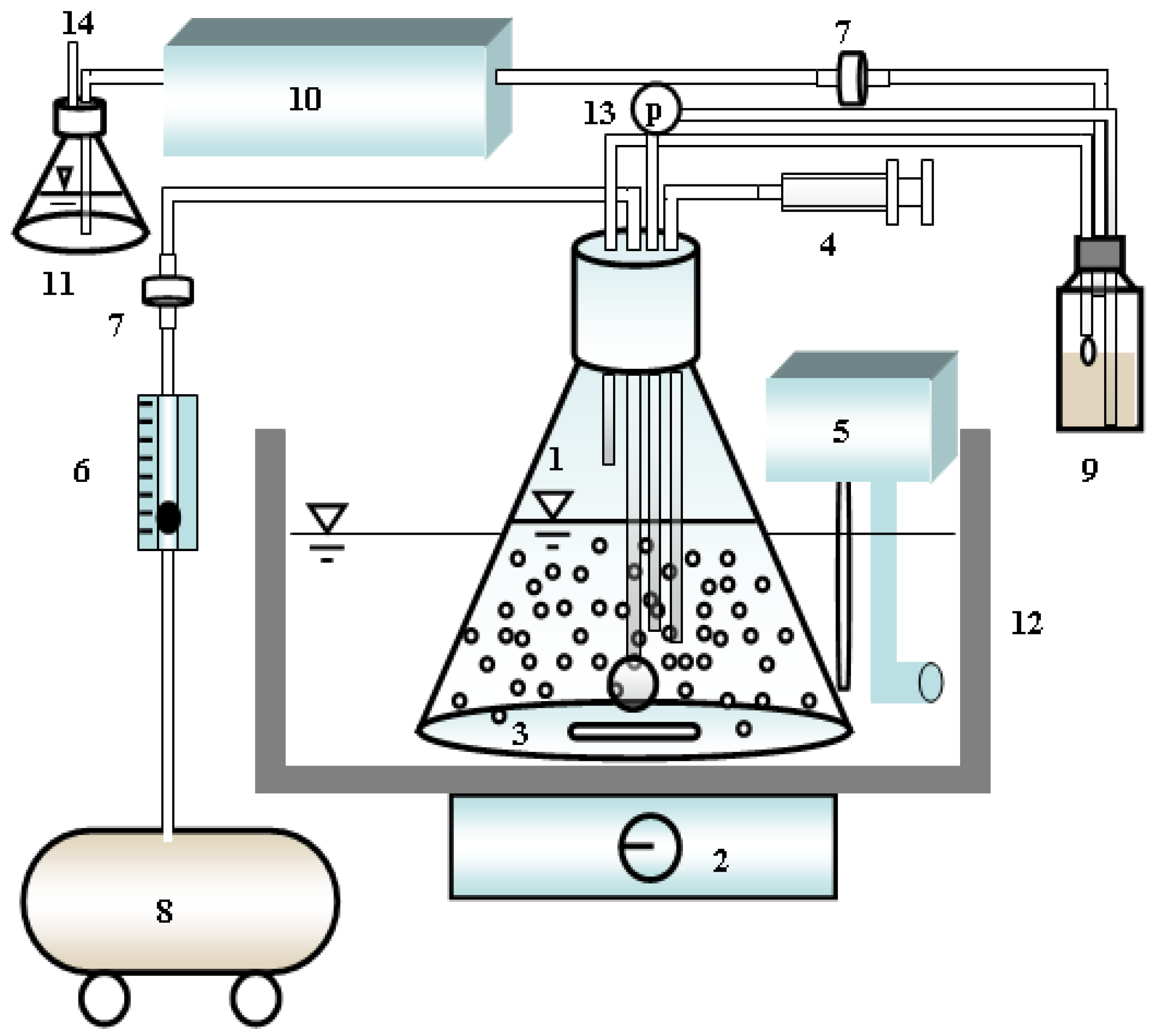
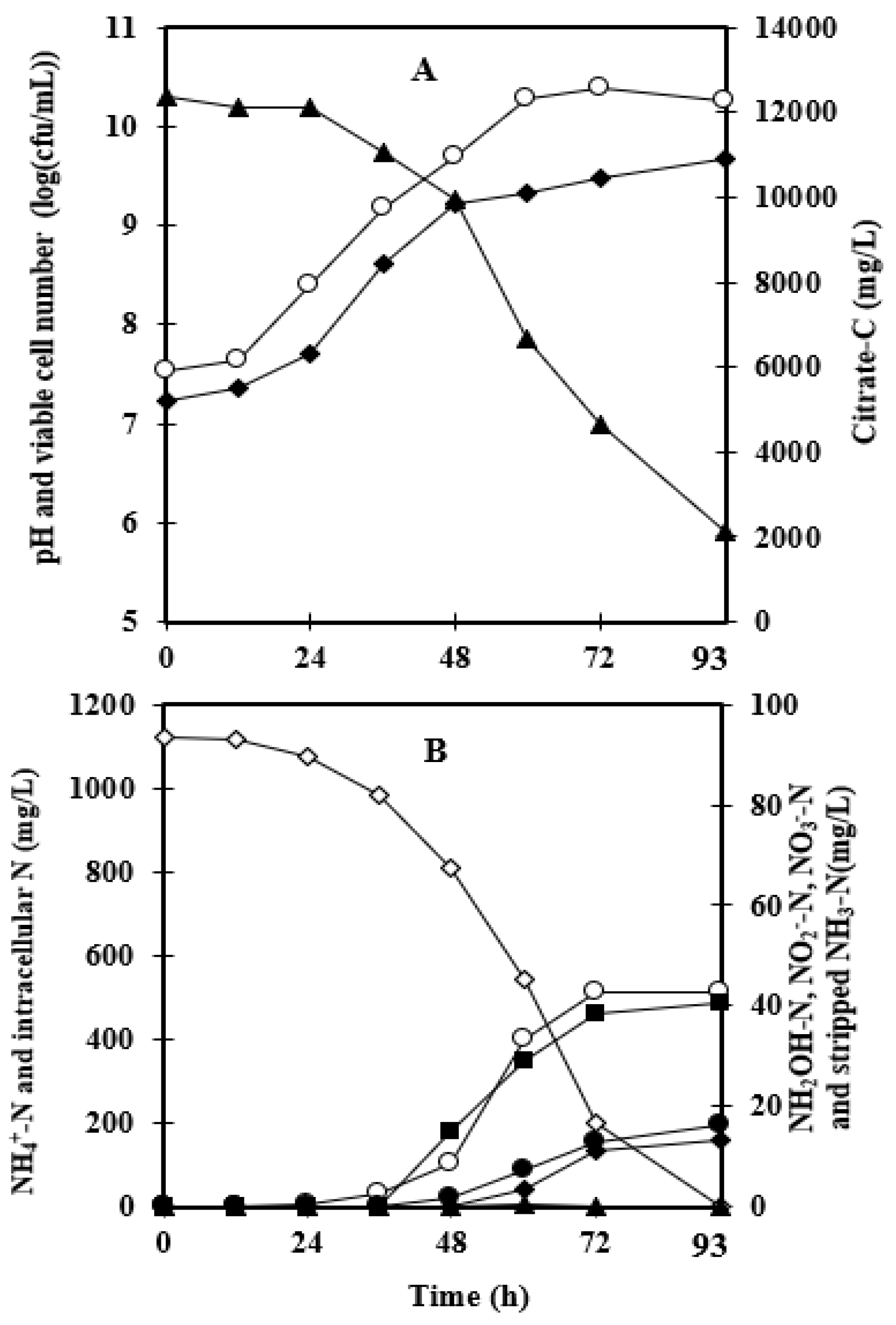
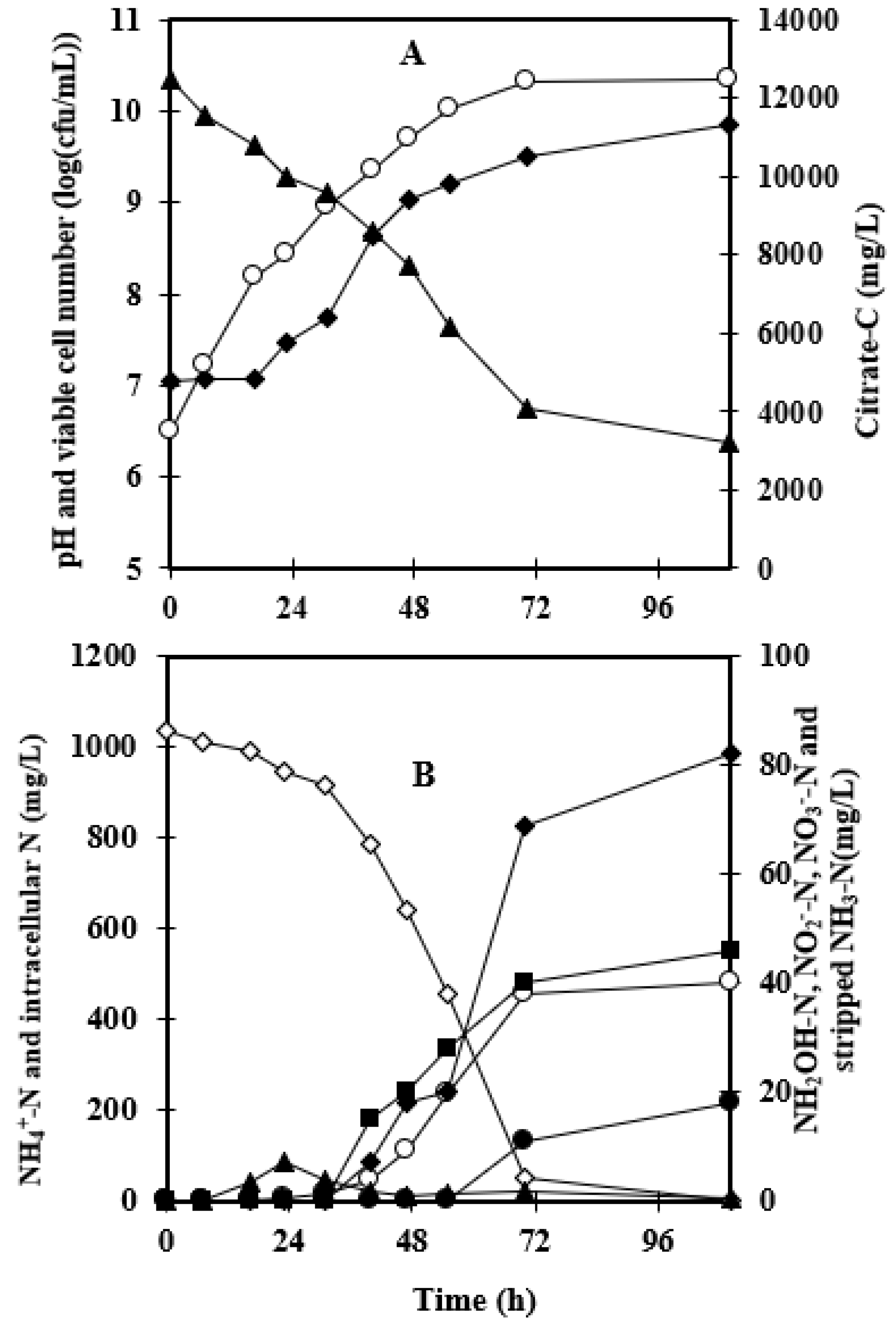
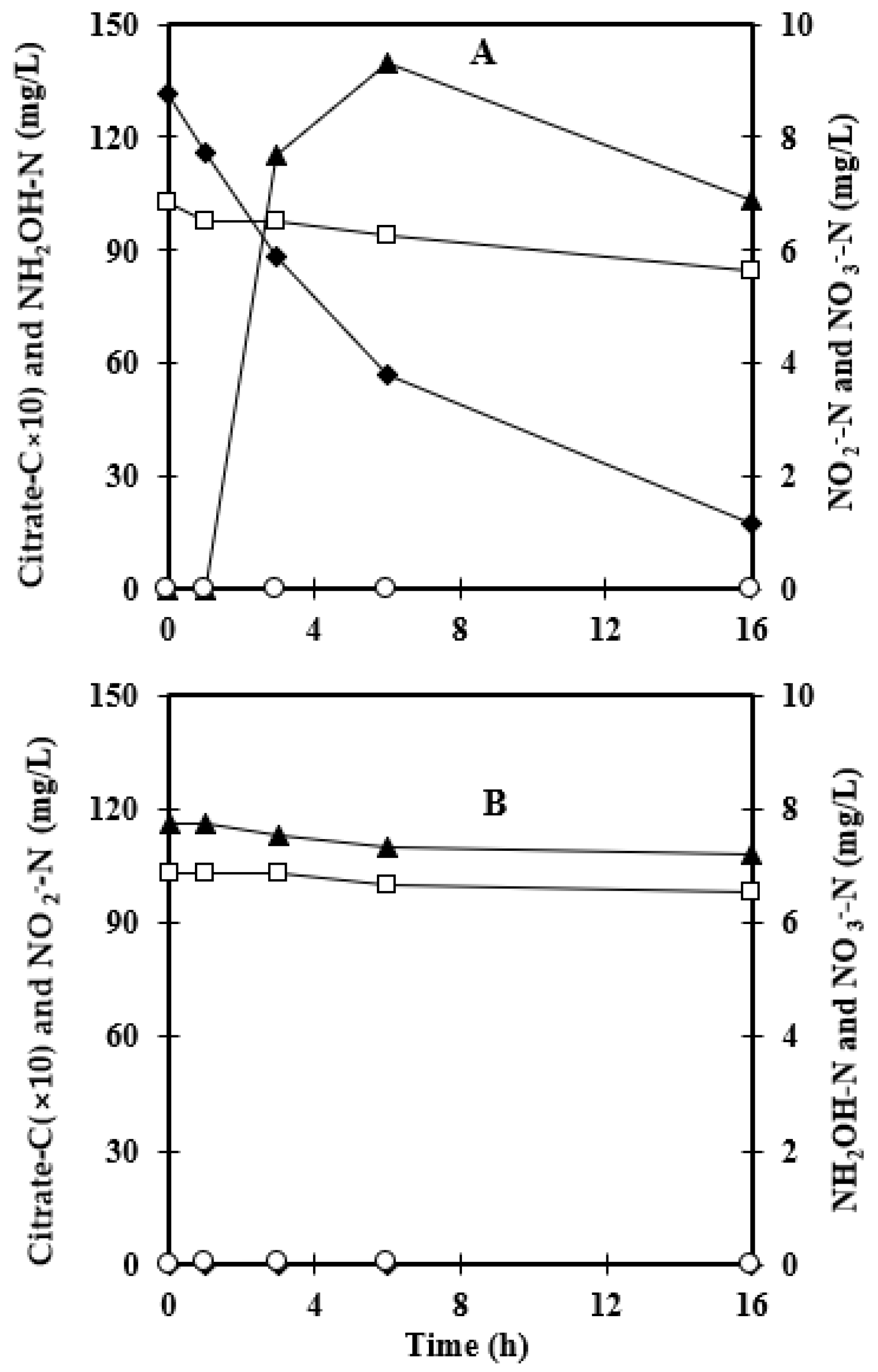



| Without Foam Recycling | Foam Recycling | |
|---|---|---|
| Initial NH4+-N (mg/L) | 1037 ± 22 | 1122 ± 5.5 |
| Removed N (mg/L) | 1037 ± 22 | 1122 ± 5.5 |
| Intracellular N (mg/L) | 481 ± 9.8 | 509 ± 13 |
| Nitrification products (mg-N/L) | 101 ± 8.7 | 30 ± 4.3 |
| Stripped N (mg/L) | 46 ± 3.6 | 41 ± 4.3 |
| Denitrification ratio (%) * | 39 ± 1.1 | 48 ± 0.4 |
| Maximum ammonium removal rate (mg-N/L·h) | 26 ± 0.9 | 25 ± 1.2 |
| Foam | Culture of No. 4 | |
|---|---|---|
| Cell number (cfu/mL) | 2.1 ± 2.1 × 1010 | 4.0 ± 1.2 × 109 |
| Activity (U/mL) * | 34.3 ± 3.2 | 1.25 ± 0.1 |
| Specific Activity (U/cfu) | 1.5 × 10−9 | 3.3 × 10−10 |
| Airflow Rate (L/min) | Initial Ammonium Level (mg-N/L) | |||
|---|---|---|---|---|
| 3000 | 1000 | 250 | ||
| Initial N (mg/L) Removed N (mg/L) (%) Intracellular N (mg/L) (%) * Nitrification N (mg/L) (%) * Stripped N (mg/L) (%) * Denitrification ratio (%) * Maximum ammonium removal rate (mg-N/L·h) | 0.2 | 2965 | 1167 | 217 |
| 990 (33) | 617 (53) | 217 (100) | ||
| 378 (38) | 318 (52) | 110 (51) | ||
| 19 (2) | 14 (2) | 13 (6) | ||
| 289 (29) | 40 (6) | 2.5 (1) | ||
| 31 | 40 | 42 | ||
| 13 | 11 | 11 | ||
| Initial N (mg/L) Removed N (mg/L) (%) Intracellular N (mg/L) (%) * Nitrification N (mg/L (%) * Stripped N (mg/L) (%) * Denitrification ratio (%) * Maximum ammonium removal rate (mg-N/L·h) | 0.5 | 2975 | 1190 | 218 |
| 2697 (91) | 1063 (89) | 218 (100) | ||
| 546 (20) | 397 (37) | 124 (57) | ||
| 39 (2) | 29 (3) | 21 (10) | ||
| 333 (12) | 59 (6) | 5 (2) | ||
| 66 | 54 | 31 | ||
| 28 | 19 | 13 | ||
| Initial N (mg/L) Removed N (mg/L) (%) Intracellular N (mg/L) (%) * Nitrification N (mg/L) (%) * Stripped N (mg/L) (%) * Denitrification ratio (%) * Maximum ammonium removal rate (mg-N/L·h) | 0.8 | 2967 | 1177 | 225 |
| 2967 (100) | 1104 (94) | 225 (100) | ||
| 668 (23) | 404 (37) | 128 (57) | ||
| 56 (2) | 38 (3) | 24 (11) | ||
| 354 (12) | 72 (6) | 4 (2) | ||
| 64 | 54 | 31 | ||
| 39 | 21 | 15 | ||
| pH 6 | pH 7 | pH 8 | |
|---|---|---|---|
| Initial NH4+-N (mg/L) | 1186 | 1122 | 1169 |
| Removed N (mg/L) | 1165 | 1122 | 1159 |
| Intracellular N (mg/L) | 424 | 509 | 521 |
| Nitrification N (mg/L) | 25 | 30 | 21 |
| Stripped N (mg/L) | 79 | 41 | 170 |
| Denitrification ratio (%) * | 55 | 48 | 39 |
| Maximum ammonium removal rate (mg-N/L) | 20 | 25 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.; Joo, H.-S. The Effect of Foam-Recycle on Ammonium Removal by Aerobic Denitrification Using Alcaligenes faecalis No. 4. Environments 2023, 10, 184. https://doi.org/10.3390/environments10100184
Lee K, Joo H-S. The Effect of Foam-Recycle on Ammonium Removal by Aerobic Denitrification Using Alcaligenes faecalis No. 4. Environments. 2023; 10(10):184. https://doi.org/10.3390/environments10100184
Chicago/Turabian StyleLee, Kwanyong, and Hung-Soo Joo. 2023. "The Effect of Foam-Recycle on Ammonium Removal by Aerobic Denitrification Using Alcaligenes faecalis No. 4" Environments 10, no. 10: 184. https://doi.org/10.3390/environments10100184






