Leaching Performance of Nanotechnology-Induced High-Arsenic-Bearing Tooeleite-like Mineral Nanowaste
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mineralized Nanowaste Formation
2.2.1. ZnS Precursor Synthesis
2.2.2. One-Step Arsenic Bearing Waste Generation
2.3. Short-Term Stability Test
2.4. Long-Term Stability Test
2.5. Characterization of Samples
3. Results and Discussion
3.1. Characteristics of Mineralized Nanowaste
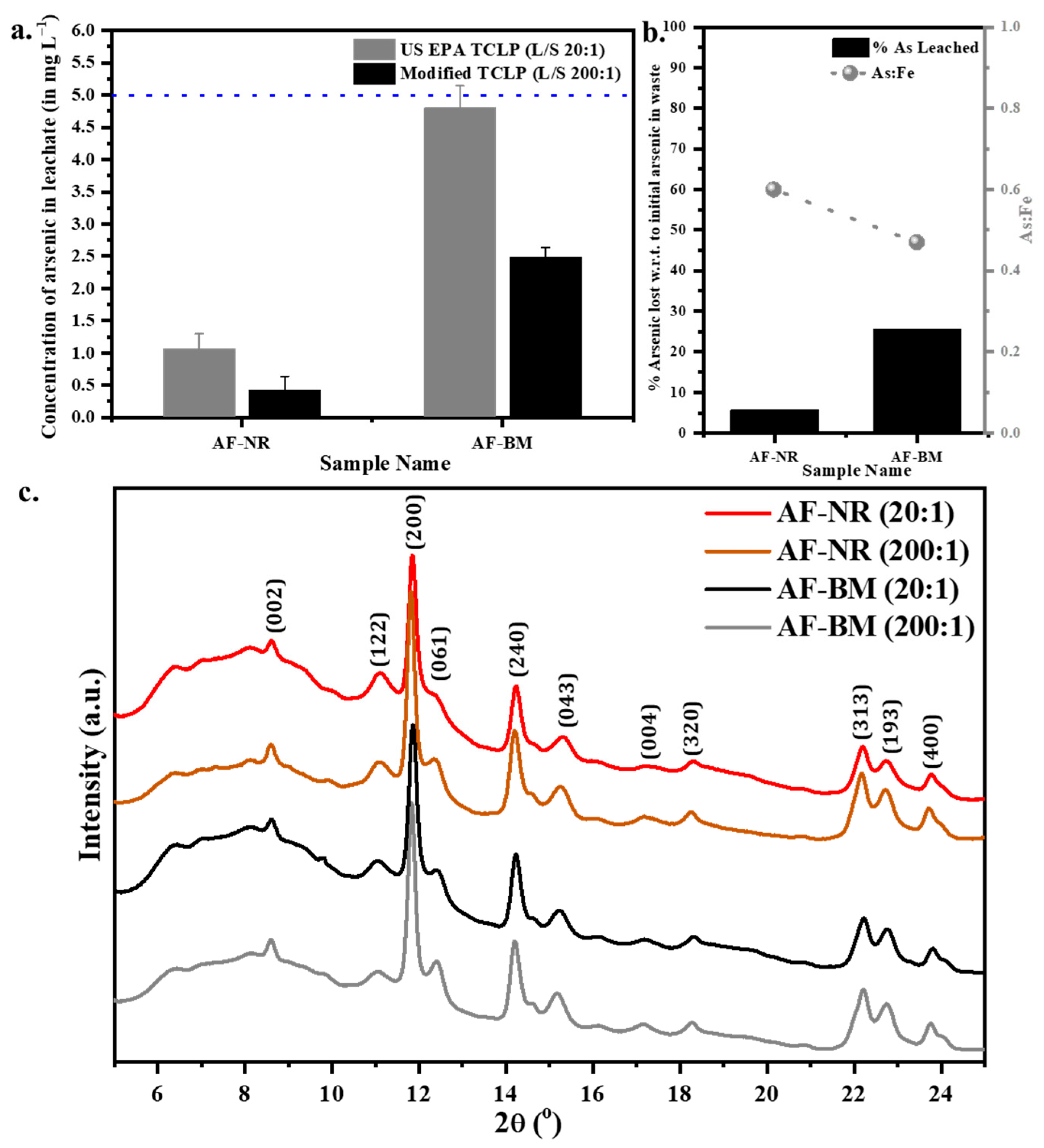
3.2. Short-Term Stability Test
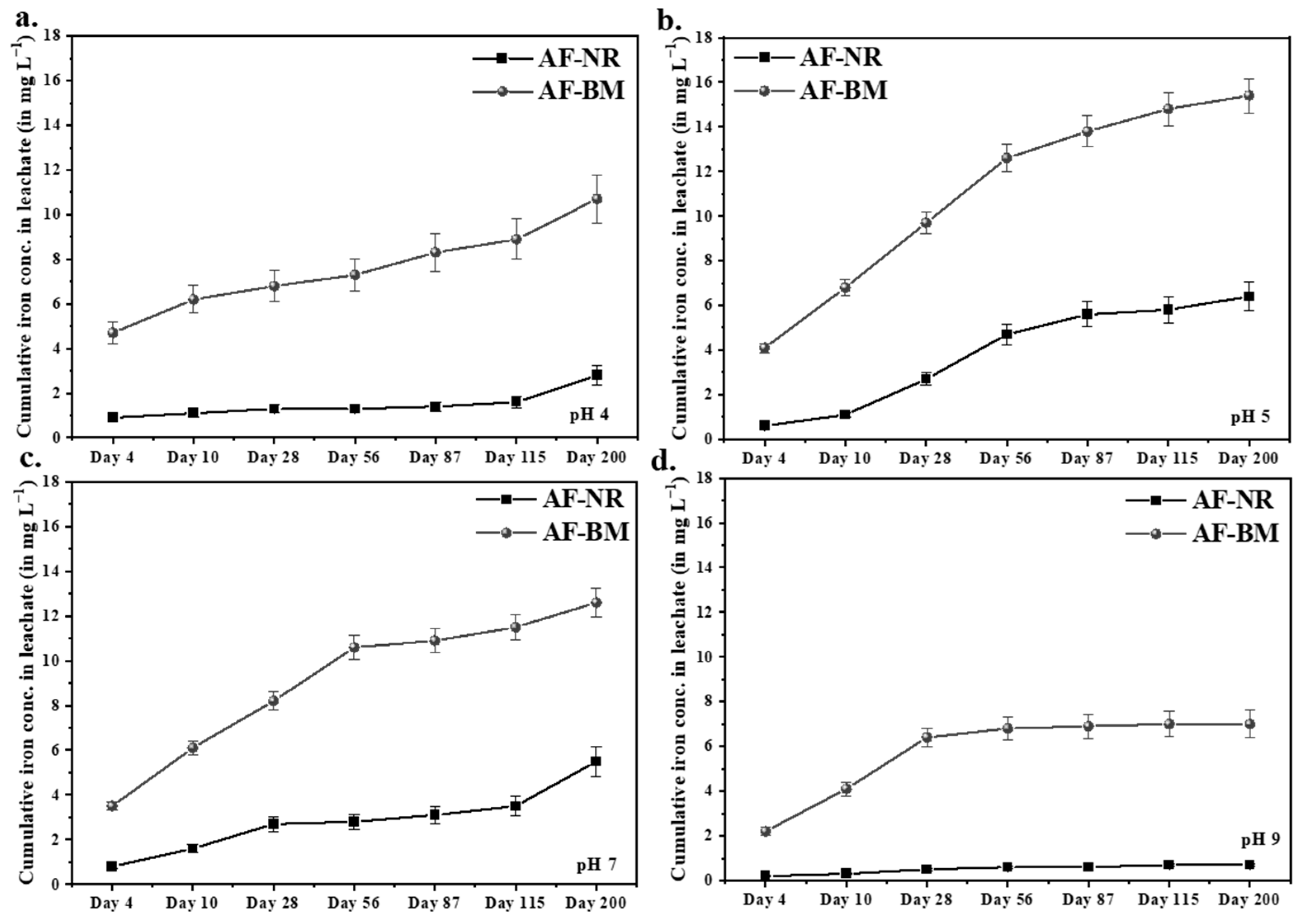
3.3. Long-Term Stability Test
3.3.1. Arsenic Leaching Using Inorganic Acid
3.3.2. Iron Leaching Using Inorganic Acid
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Malakar, A.; Islam, S.; Ali, M.A.; Ray, S. Rapid decadal evolution in the groundwater arsenic content of Kolkata, India and its correlation with the practices of her dwellers. Environ. Monit. Assess. 2016, 188, 584. [Google Scholar] [CrossRef]
- Population, H. Arsenic Contamination in Groundwater: A Global Perspective with Emphasis on the Asian Scenario. J. Health Popul. Nutr. 2006, 24, 142–163. [Google Scholar]
- Somani, M.; Datta, M.; Ramana, G.V.; Sreekrishnan, T.R. Leachate Characteristics of Aged Soil-Like Material from MSW Dumps: Sustainability of Landfill Mining. J. Hazard. Toxic Radioact. Waste 2019, 23, 04019014. [Google Scholar] [CrossRef]
- Sengupta, M.K.; Mukherjee, S.C.; Pati, S.; Mukherjeel, A.; Rahman, M.M.; Hossain, M.A.; Das, B.; Nayakl, B.; Pal, A.; Zafar, A.; et al. An eight-year study report on arsenic contamination in groundwater and health effects in Eruani village, Bangladesh and an approach for its mitigation. J. Health Popul. Nutr. 2006, 24, 129–141. [Google Scholar]
- Farooq, M.A.; Islam, F.; Ali, B.; Najeeb, U.; Mao, B.; Gill, R.A.; Yan, G.; Siddique, K.H.M.; Zhou, W. Arsenic toxicity in plants: Cellular and molecular mechanisms of its transport and metabolism. Environ. Exp. Bot. 2016, 132, 42–52. [Google Scholar] [CrossRef]
- Meng, X.; Korfiatis, G.P.; Jing, C.; Christodoulatos, C. Redox transformations of arsenic and iron in water treatment sludge during aging and TCLP extraction. Environ. Sci. Technol. 2001, 35, 3476–3481. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Loganathan, P.; Vigneswaran, S.; Krupanidhi, S.; Pham, T.T.N.; Ngo, H.-H. Arsenic waste from water treatment systems: Characteristics, treatments and its disposal. Water Sci. Technol. Water Supply 2014, 14, 939–950. [Google Scholar] [CrossRef]
- Kundu, S.; Gupta, A.K. Immobilization and leaching characteristics of arsenic from cement and/or lime solidified/stabilized spent adsorbent containing arsenic. J. Hazard. Mater. 2008, 153, 434–443. [Google Scholar] [CrossRef]
- U.S. EPA. Arsenic Treatment Technology Evaluation Handbook for Small Systems; U.S. EPA: Washington, DC, USA, 2003. [Google Scholar]
- Malakar, A.; Singh, R.; Westrop, J.; Weber, K.A.; Elofson, C.N.; Kumar, M.; Snow, D.D. Occurrence of arsenite in surface and groundwater associated with a perennial stream located in Western Nebraska, USA. J. Hazard. Mater. 2021, 416, 126170. [Google Scholar] [CrossRef]
- Malakar, A.; Das, B.; Islam, S.; Meneghini, C.; De Giudici, G.; Merlini, M.; Kolen’ko, Y.V.; Iadecola, A.; Aquilanti, G.; Acharya, S.; et al. Efficient artificial mineralization route to decontaminate Arsenic(III) polluted water—The Tooeleite Way. Sci. Rep. 2016, 6, 26031. [Google Scholar] [CrossRef]
- Neumann, R.B.; Ashfaque, K.N.; Badruzzaman, A.B.M.; Ashraf Ali, M.; Shoemaker, J.K.; Harvey, C.F. Anthropogenic influences on groundwater arsenic concentrations in Bangladesh. Nat. Geosci. 2010, 3, 46–52. [Google Scholar] [CrossRef]
- Rawson, J.; Prommer, H.; Siade, A.; Carr, J.; Berg, M.; Davis, J.A.; Fendorf, S. Numerical Modeling of Arsenic Mobility during Reductive Iron-Mineral Transformations. Environ. Sci. Technol. 2016, 50, 2459–2467. [Google Scholar] [CrossRef]
- Qi, P.; Pichler, T. Closer look at As(III) and As(V) adsorption onto ferrihydrite under competitive conditions. Langmuir 2014, 30, 11110–11116. [Google Scholar] [CrossRef]
- Febrianto, J.; Natasia, A.; Sunarso, J.; Ju, Y.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef]
- Leist, M.; Casey, R.J.; Caridi, D. The management of arsenic wastes: Problems and prospects. J. Hazard. Mater. 2000, 76, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Sáez, A.E.; Ela, W. Effect of pH, competitive anions and NOM on the leaching of arsenic from solid residuals. Sci. Total Environ. 2006, 363, 46–59. [Google Scholar] [CrossRef]
- Bhatt, A.; Rajagopal, C.; Chopra, R.; Gupta, A.K. Journal of Environmental Chemical Engineering Stabilisation of heavy metals in polymeric matrices: Studies on leaching behaviour. Biochem. Pharmacol. 2014, 2, 1474–1479. [Google Scholar]
- Shaw, J.K.; Fathordoobadi, S.; Zelinski, B.J.; Ela, W.P.; Sáez, A.E. Stabilization of arsenic-bearing solid residuals in polymeric matrices. J. Hazard. Mater. 2008, 152, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.W.; Chai, L.Y.; Peng, B.; Liang, Y.J.; He, Y.; Yan, Z.H. Arsenic vitrification by copper slag based glass: Mechanism and stability studies. J. Non. Cryst. Solids 2017, 466–467, 21–28. [Google Scholar] [CrossRef]
- Houng Aloune, S.; Hiroyoshi, N.; Ito, M. Stability of As(V)-sorbed schwertmannite under porphyry copper mine conditions. Miner. Eng. 2015, 74, 51–59. [Google Scholar] [CrossRef]
- Raghav, M.; Shan, J.; Sáez, A.E.; Ela, W.P. Scoping candidate minerals for stabilization of arsenic-bearing solid residuals. J. Hazard. Mater. 2013, 263, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Yue, M.; Yang, J.; Wang, Q.; Li, Q.; Liu, H. Formation of tooeleite and the role of direct removal of As(III) from high-arsenic acid wastewater. J. Hazard. Mater. 2016, 320, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Belman, N.; Israelachvili, J.N.; Li, Y.; Safinya, C.R.; Ezersky, V.; Rabkin, A.; Sima, O.; Golan, Y. Hierarchical superstructure of alkylamine-coated ZnS nanoparticle assemblies. Phys. Chem. Chem. Phys. 2011, 13, 4974. [Google Scholar] [CrossRef] [PubMed]
- Malakar, A.; Das, B.; Sengupta, S.; Acharya, S.; Ray, S. ZnS nanorod as an efficient heavy metal ion extractor from water. J. Water Process Eng. 2014, 3, 74–81. [Google Scholar] [CrossRef]
- Pathak, C.S.; Mishra, D.D.; Agarwala, V.; Mandal, M.K. Materials Science in Semiconductor Processing Optical properties of ZnS nanoparticles prepared by high energy ball milling. Mater. Sci. Semicond. Process. 2013, 16, 525–529. [Google Scholar] [CrossRef]
- Yang, L.; Donahoe, R.J.; Redwine, J.C. In situ chemical fixation of arsenic-contaminated soils: An experimental study. Sci. Total Environ. 2007, 387, 28–41. [Google Scholar] [CrossRef]
- Liu, D.D.; Miao, D.R.; Liu, F. Evaluation of the As, Cu and Pb Immobilizing Efficiency by Tessier, TCLP and SBET Method. Adv. Mater. Res. 2014, 878, 520–531. [Google Scholar] [CrossRef]
- Clancy, T.M.; Hayes, K.F.; Raskin, L. Arsenic Waste Management: A Critical Review of Testing and Disposal of Arsenic-Bearing Solid Wastes Generated during Arsenic Removal from Drinking Water. Environ. Sci. Technol. 2013, 47, 10799–10812. [Google Scholar] [CrossRef]
- Ghosh, A.; Mukiibi, M.; Ela, W. TCLP underestimates leaching of arsenic from solid residuals under landfill conditions. Environ. Sci. Technol. 2004, 38, 4677–4682. [Google Scholar] [CrossRef]
- Lim, M.; Han, G.C.; Ahn, J.W.; You, K.S.; Kim, H.S. Leachability of arsenic and heavy metals from mine tailings of abandoned metal mines. Int. J. Environ. Res. Public Health 2009, 6, 2865–2879. [Google Scholar] [CrossRef]
- Sullivan, C.; Tyrer, M.; Cheeseman, C.R.; Graham, N.J.D. Disposal of water treatment wastes containing arsenic—A review. Sci. Total Environ. 2010, 408, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Opio, F.K. Investigation of Fe (III)-As (III) Bearing Phase. Ph.D. Thesis, Queen’s University, Kingston, ON, Canada, 2013. Available online: https://qspace.library.queensu.ca/bitstream/1974/7798/1/Opio_Faith_K_201301_PhD.pdf (accessed on 12 July 2019).
- Nishimura, T.; Robins, R.G. Confirmation that tooeleite is a ferric arsenite sulfate hydrate, and is relevant to arsenic stabilisation. Miner. Eng. 2008, 21, 246–251. [Google Scholar] [CrossRef]
- Yang, J.; Yan, Y.; Hu, K.; Zhang, G.; Jiang, D.; Li, Q.; Ye, B.; Chai, L.; Wang, Q.; Liu, H.; et al. Structural substitution for SO4 group in tooeleite crystal by As(V) and As(III) oxoanions and the environmental implications. Chemosphere 2018, 213, 305–313. [Google Scholar] [CrossRef]
- Weatherill, J.S.; Morris, K.; Bots, P.; Stawski, T.M.; Janssen, A.; Abrahamsen, L.; Blackham, R.; Shaw, S. Ferrihydrite formation: The role of Fe13 Keggin clusters. Environ. Sci. Technol. 2016, 50, 9333–9342. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Marhaba, T.F.; Rachakornkij, M. Leaching behaviors of arsenic from arsenic-iron hydroxide sludge during TCLP. J. Environ. Eng. 2008, 134, 671–682. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malakar, A.; Das, S.; Islam, S.; Singh, R.; Ray, S. Leaching Performance of Nanotechnology-Induced High-Arsenic-Bearing Tooeleite-like Mineral Nanowaste. Environments 2023, 10, 185. https://doi.org/10.3390/environments10100185
Malakar A, Das S, Islam S, Singh R, Ray S. Leaching Performance of Nanotechnology-Induced High-Arsenic-Bearing Tooeleite-like Mineral Nanowaste. Environments. 2023; 10(10):185. https://doi.org/10.3390/environments10100185
Chicago/Turabian StyleMalakar, Arindam, Sanjit Das, Samirul Islam, Rajneesh Singh, and Sugata Ray. 2023. "Leaching Performance of Nanotechnology-Induced High-Arsenic-Bearing Tooeleite-like Mineral Nanowaste" Environments 10, no. 10: 185. https://doi.org/10.3390/environments10100185




