Coupled Photocatalysis and Microalgal–Bacterial Synergy System for Continuously Treating Aquaculture Wastewater Containing Real Phthalate Esters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of I-Doped TiO2 and Preparation of I-Doped-TiO2-Coated Beads
2.3. Microalgae and Bacterial Culture
2.4. Coupled PBS design
2.5. Effect of Operation Parameters on PAE Removal Efficiency
2.6. Identification of Bacterial Communities
2.7. Chemical Analysis
3. Results and Discussion
3.1. Effect of Coexisting PAEs on PAE Removal in PBS
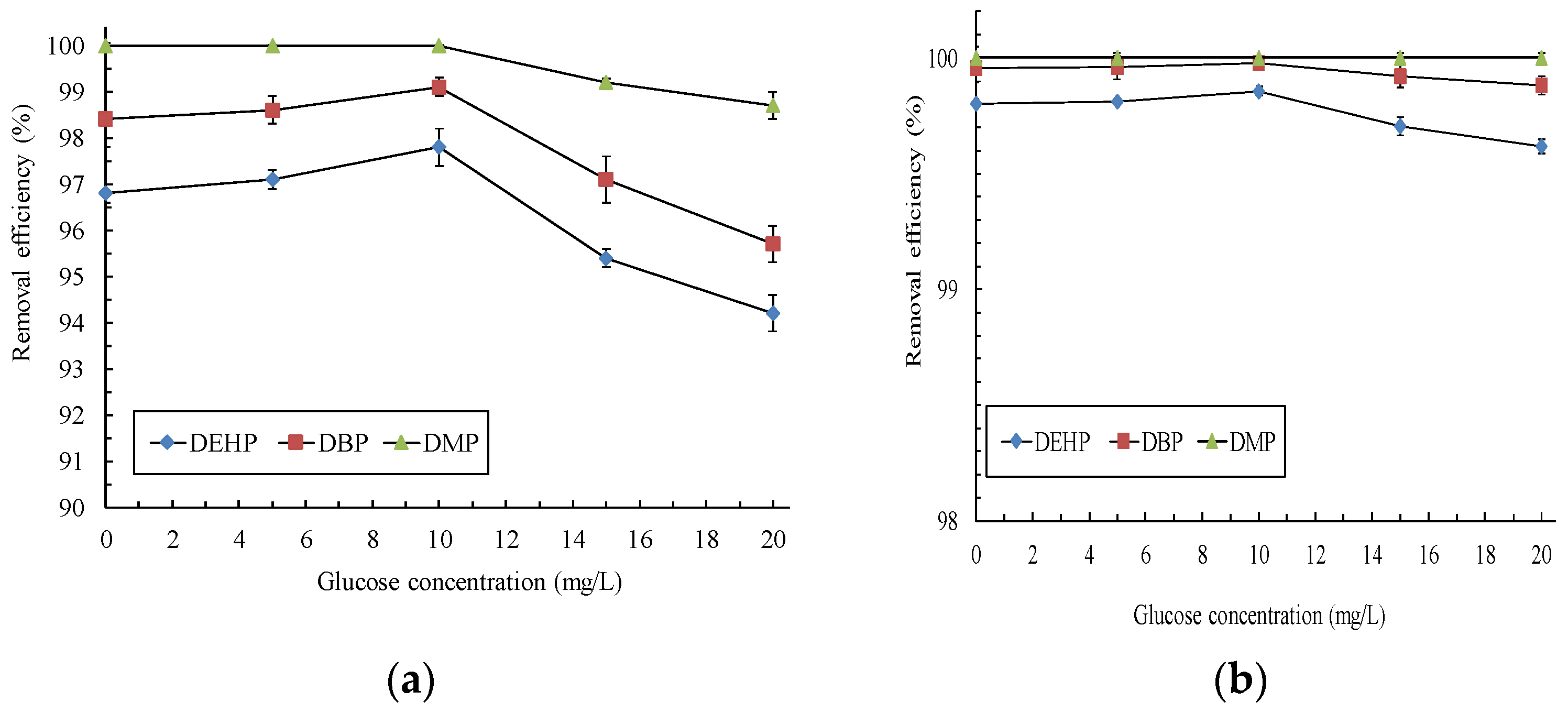
3.2. Effects of Glucose on the Removal Efficiency of PAEs
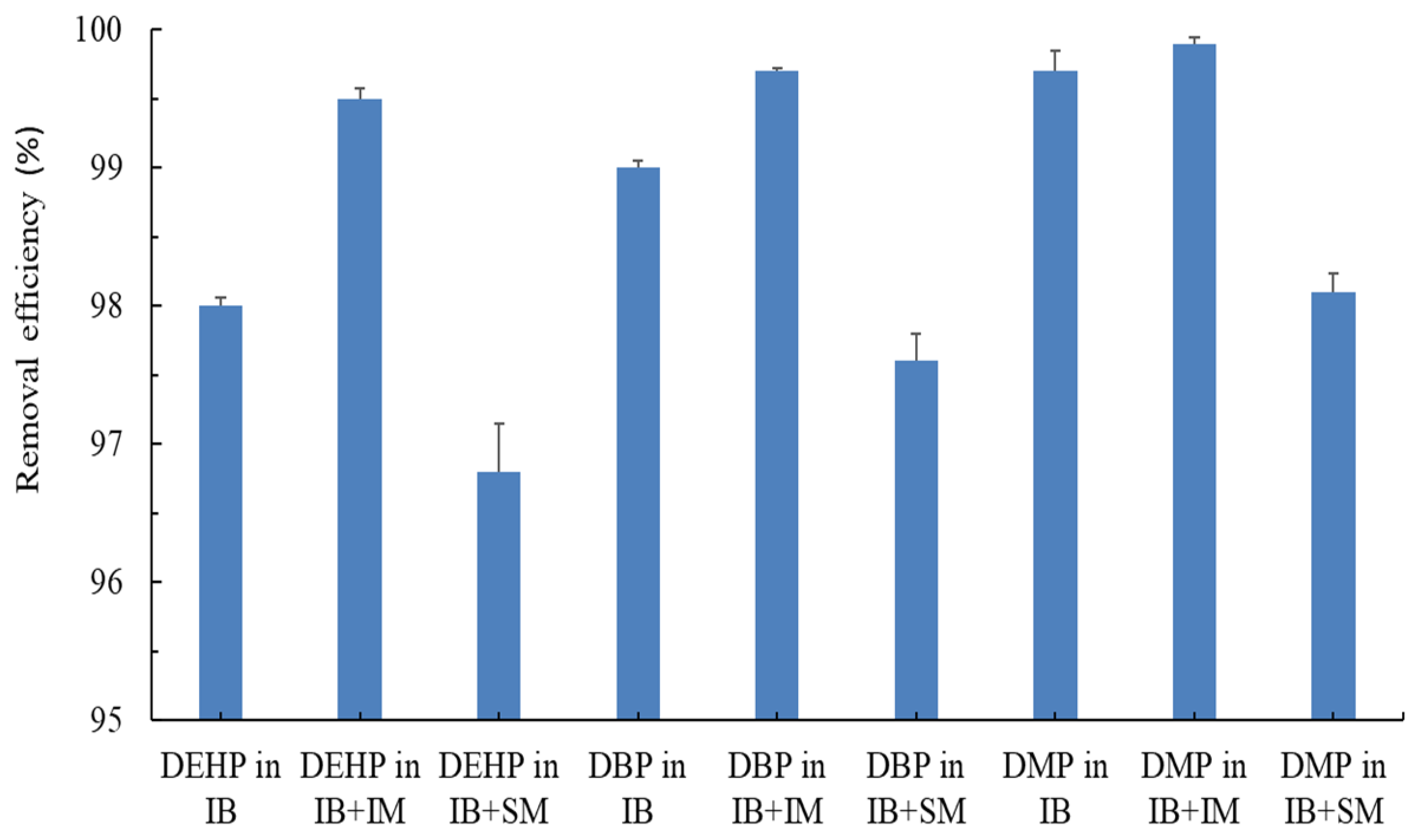
3.3. Comparison of PAE Removal Efficiencies with Different Microbiota Consortia
3.4. Effect of Shock Loading on the PBS
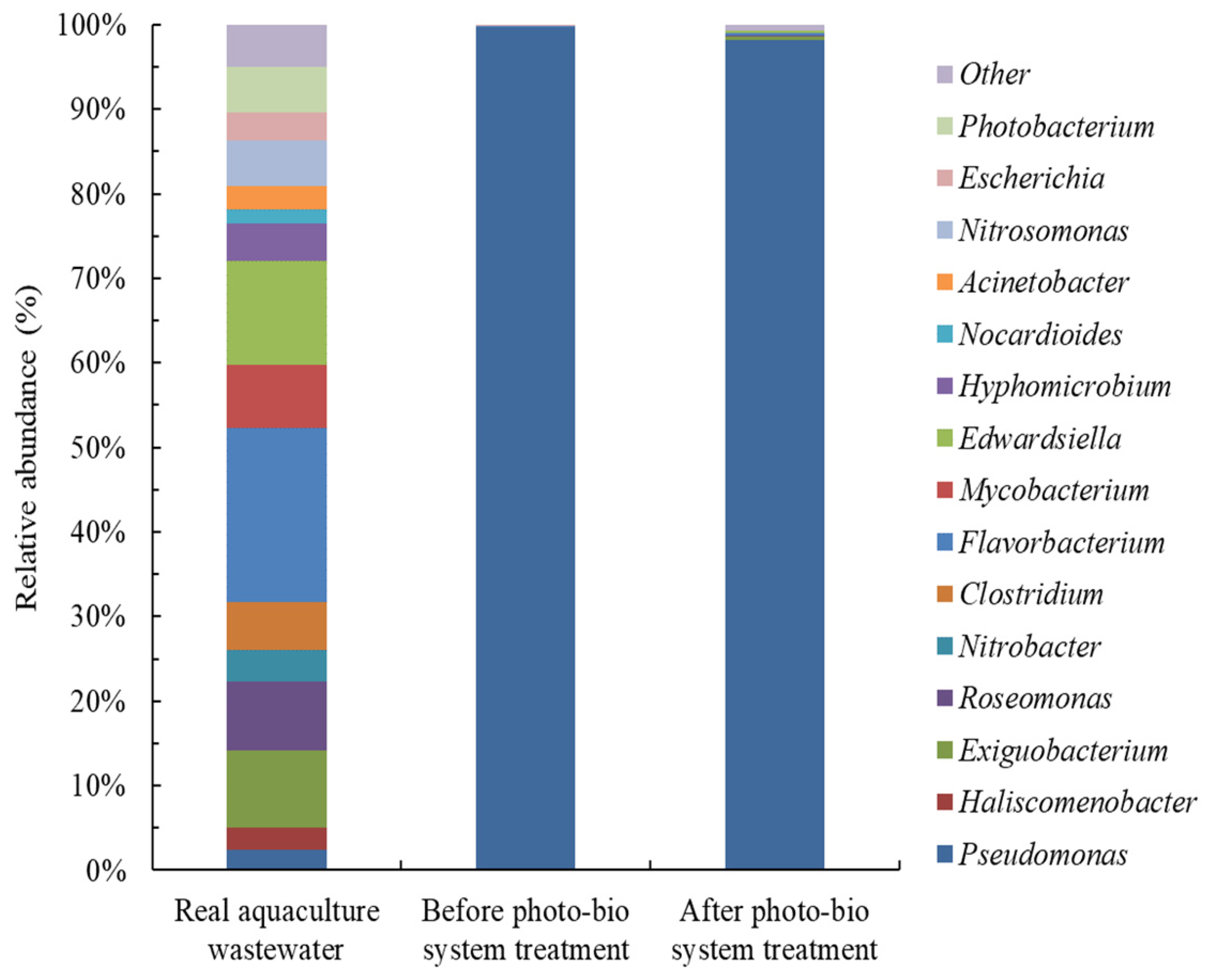
3.5. Changes in Bacterial Communities during the PAE Treatment Process
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pang, X.; Skillen, N.; Gunaratne, N.; Rooney, D.W.; Robertson, P.K. Removal of phthalates from aqueous solution by semiconductor photocatalysis: A review. J. Hazard. Mater. 2021, 402, 123461. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, T.P.; Kumar, M.A. Remediation of phthalate acid esters from contaminated environment—Insights on the bioremedial approaches and future perspectives. Heliyon 2023, 9, e14945. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.F.; Ju, Y.R.; Lim, Y.C.; Wang, M.H.; Patel, A.K.; Singhania, R.R.; Chen, C.W.; Dong, C.D. The effect of heavy rainfall on the exposure risks of sedimentary phthalate esters to aquatic organisms. Chemosphere 2022, 290, 133204. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Wang, G.H.; Chang, Y.J.; Chen, Y.H.; Cheng, C.Y.; Chung, Y.C. Combination of a Highly Efficient Biological System and Visible-Light Photocatalysis Pretreatment System for the Removal of Phthalate Esters from Wastewater. Water 2022, 14, 3139. [Google Scholar] [CrossRef]
- Chang, W.H.; Herianto, S.; Lee, C.C.; Hung, H.; Chen, H.L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, L.; Fang, C.; Chen, L. Effects of di-n-butyl phthalate and di-2-ethylhexyl phthalate on pollutant removal and microbial community during wastewater treatment. Ecotoxicol. Environ. Saf. 2020, 198, 110665. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Li, B.; Geng, J.; Ma, L.; Qi, H.; Zhang, A.; Zhao, Z. The spatiotemporal distribution and potential risk assessment of 19 phthalate acid esters in wastewater treatment plants in China. Environ. Sci. Pollut. Res. 2021, 28, 67280–67291. [Google Scholar] [CrossRef]
- Zhou, X.; Xiong, W.; Li, Y.; Zhang, C.; Xiong, X. A novel simultaneous coupling of memory photocatalysts and microbial communities for alternate removal of dimethyl phthalate and nitrate in water under light/dark cycles. J. Hazard. Mater. 2022, 430, 128395. [Google Scholar] [CrossRef]
- Anandan, S.; Pugazhenthiran, N.; Lana-Villarreal, T.; Lee, G.J.; Wu, J.J. Catalytic degradation of a plasticizer, di-ethylhexyl phthalate, using Nx–TiO2−x nanoparticles synthesized via co-precipitation. Chem. Eng. J. 2013, 231, 182–189. [Google Scholar] [CrossRef]
- Khadka, S.; Nshimiyimana, J.B.; Zou, P.; Koirala, N.; Xiong, L. Biodegradation kinetics of diethyl phthalate by three newly isolated strains of Pseudomonas. Sci. Afr. 2020, 8, e00380. [Google Scholar] [CrossRef]
- Hung, C.H.; Yuan, C.; Li, H.W. Photodegradation of diethyl phthalate with PANi/CNT/TiO2 immobilized on glass plate irradiated with visible light and simulated sunlight—Effect of synthesized method and pH. J. Hazard. Mater. 2017, 322, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Štengl, V.; Grygar, T.M. The simplest way to iodine-doped anatase for photocatalysts activated by visible light. Int. J. Photoenergy 2011, 11, 13. [Google Scholar] [CrossRef]
- Nambiar, K.; Bokil, S. Luxury uptake of nitrogen in flocculating algal-bacterial system. Water Res. 1981, 15, 667–669. [Google Scholar] [CrossRef]
- Del Rio-Chanona, E.A.; Cong, X.; Bradford, E.; Zhang, D.; Jing, K. Review of advanced physical and data-driven models for dynamic bioprocess simulation: Case study of algae–bacteria consortium wastewater treatment. Biotechnol. Bioeng. 2019, 116, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Li, S.N.; Zhang, C.; Li, F.; Ren, N.Q.; Ho, S.H. Recent advances of algae-bacteria consortia in aquatic remediation. Crit. Rev. Environ. Sci. Technol. 2023, 53, 315–339. [Google Scholar] [CrossRef]
- Mujtaba, G.; Rizwan, M.; Lee, K. Removal of nutrients and COD from wastewater using symbiotic co-culture of bacterium Pseudomonas putida and immobilized microalga Chlorella vulgaris. J. Indust. Eng. Chem. 2017, 49, 145–151. [Google Scholar] [CrossRef]
- Wu, L.C.; Tsai, T.H.; Liu, M.H.; Kuo, J.L.; Chang, Y.C.; Chung, Y.C. A green microbial fuel cell-based biosensor for in situ chromium (VI) measurement in electroplating wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef]
- Kanaujiya, D.K.; Sivashanmugam, S.; Pakshirajan, K. Biodegradation and toxicity removal of phthalate mixture by Gordonia sp. in a continuous stirred tank bioreactor system. Environ. Technol. Innov. 2022, 26, 102324. [Google Scholar] [CrossRef]
- Xie, Y.; Guo, X.; Shim, H. Degradation of dibutyl phthalate and diethyl phthalate by indigenous isolate Bacillus sp. MY156. IOP Conf. Ser. Earth Environ. Sci. 2023, 1171, 012057. [Google Scholar] [CrossRef]
- Esmaeli, R.; Hassani, A.; Eslami, A.; Ahmadimoghadam, M.; Safari, A. Di-(2-Ethylhexyl) Phthalate oxidative degradation by Fenton process in synthetic and real petrochemical wastewater. J. Environ. Health Sci. Eng. 2011, 8, 201–206. [Google Scholar]
- Chen, C.Y.; Wu, P.S.; Chung, Y.C. Coupled biological and photo-Fenton pretreatment system for the removal of di-(2-ethylhexyl) phthalate (DEHP) from water. Bioresour. Technol. 2009, 100, 4531–4534. [Google Scholar] [CrossRef]
- Mamlouk, D.; Gullo, M. Acetic acid bacteria: Physiology and carbon sources oxidation. Indian J. Microbiol. 2013, 53, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Xia, Y.; He, X.; Li, W.; Yuan, L.; Wu, X.; Qin, Y.; Yuan, R.; Gong, X. Glucose addition enhanced the advanced treatment of coking wastewater. Water 2021, 13, 3365. [Google Scholar] [CrossRef]
- Hu, X.; Wan, J. Study of biodegradation properties of phthalate esters in aqueous culture conditions. J. Synth. Lubr. 2006, 23, 71–80. [Google Scholar] [CrossRef]
- Bahr, M.; Stams, A.J.; De la Rosa, F.; García-Encina, P.A.; Muñoz, R. Assessing the influence of the carbon oxidation-reduction state on organic pollutant biodegradation in algal–bacterial photobioreactors. Appl. Microbiol. Biotechnol. 2011, 90, 1527–1536. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Ma, H.; Hursthouse, A.S. Removal of manganese (II) from acid mine wastewater: A review of the challenges and opportunities with special emphasis on Mn-oxidizing bacteria and microalgae. Water 2019, 11, 2493. [Google Scholar] [CrossRef]
- Pajoumshariati, S.; Zare, N.; Bonakdarpour, B. Considering membrane sequencing batch reactors for the biological treatment of petroleum refinery wastewaters. J. Membr. Sci. 2017, 523, 542–550. [Google Scholar] [CrossRef]
- Yang, G.C.; Wang, C.L.; Chiu, Y.H. Occurrence and distribution of phthalate esters and pharmaceuticals in Taiwan river sediments. J. Soils Sediments 2015, 15, 198–210. [Google Scholar] [CrossRef]
- Chang, H.Y.; Yang, W.C.; Xue, Y.J.; Tsai, M.Y.; Wang, J.H.; Chang, G.R. Phthalates and organophosphorus insecticide residues in shrimp determined by liquid/gas chromatography–Tandem mass spectrometry and a health risk assessment. Mar. Pollut. Bull. 2019, 144, 140–145. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Pedrazzani, R.; Bellazzi, S.; Carnevale Miino, M.; Caccamo, F.M.; Baldi, M.; Abba, A.; Bertanza, G. Numerical Analysis of a Full-Scale Thermophilic Biological System and Investigation of Nitrate and Ammonia Fates. Appl. Sci. 2022, 12, 6952. [Google Scholar] [CrossRef]
- Li, J.; Fang, L.; Liang, X.F.; Guo, W.; Lv, L.; Li, L. Influence of environmental factors and bacterial community diversity in pond water on health of Chinese perch through Gut Microbiota change. Aquac. Rep. 2021, 20, 100629. [Google Scholar] [CrossRef]
- Alagarsamy, S.; Thampuran, N.; Joseph, T.C. Virulence genes, serobiotypes and antibiotic resistance profile of Escherichia coli strains isolated from aquaculture and other sources. Aquac. Res. 2010, 41, 1003–1014. [Google Scholar]
- Baaloudj, O.; Assadi, I.; Nasrallah, N.; El Jery, A.; Khezami, L.; Assadi, A.A. Simultaneous removal of antibiotics and inactivation of antibiotic-resistant bacteria by photocatalysis: A review. J. Water Process Eng. 2021, 42, 102089. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Du, S.; Yuan, Y.; Zhu, M.; Li, Y.; Zhu, X. Removal of dibutyl phthalate and its effects on bacterial communities in lab-scale constructed wetlands. J. Environ. Account. Manag. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Kido, Y.; Tanaka, T.; Yamada, K.; Hachiyanagi, H.; Baba, H.; Iriguchi, T.; Uyeda, M. Complete degradation of the endocrine-disrupting chemical dimethyl phthalate ester by Flavobacterium sp. J. Health Sci. 2007, 53, 740–744. [Google Scholar] [CrossRef]
- Chikere, C.B.; Fenibo, E.O. Distribution of PAH-ring hydroxylating dioxygenase genes in bacteria isolated from two illegal oil refining sites in the Niger Delta, Nigeria. Sci. Afr. 2018, 1, e00003. [Google Scholar] [CrossRef]
- Lu, J.; Zhang, H.; Pan, L.; Guan, W.; Lou, Y. Environmentally relevant concentrations of triclosan exposure promote the horizontal transfer of antibiotic resistance genes mediated by Edwardsiella piscicida. Environ. Sci. Pollut. Res. 2022, 29, 64622–64632. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, Y.-C.; Chen, C.-Y. Coupled Photocatalysis and Microalgal–Bacterial Synergy System for Continuously Treating Aquaculture Wastewater Containing Real Phthalate Esters. Environments 2023, 10, 215. https://doi.org/10.3390/environments10120215
Chung Y-C, Chen C-Y. Coupled Photocatalysis and Microalgal–Bacterial Synergy System for Continuously Treating Aquaculture Wastewater Containing Real Phthalate Esters. Environments. 2023; 10(12):215. https://doi.org/10.3390/environments10120215
Chicago/Turabian StyleChung, Ying-Chien, and Chih-Yu Chen. 2023. "Coupled Photocatalysis and Microalgal–Bacterial Synergy System for Continuously Treating Aquaculture Wastewater Containing Real Phthalate Esters" Environments 10, no. 12: 215. https://doi.org/10.3390/environments10120215





