Anthropogenic Microparticles Abundance in Sandy Beach Sediments along the Tetouan Coast (Morocco Mediterranean)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Sample Collection
2.2. AMs Extraction
2.3. Visual Identification
2.4. Quality Control
2.5. Polymer Characterization
2.6. Impact Assessment
2.7. Data Analysis
3. Results
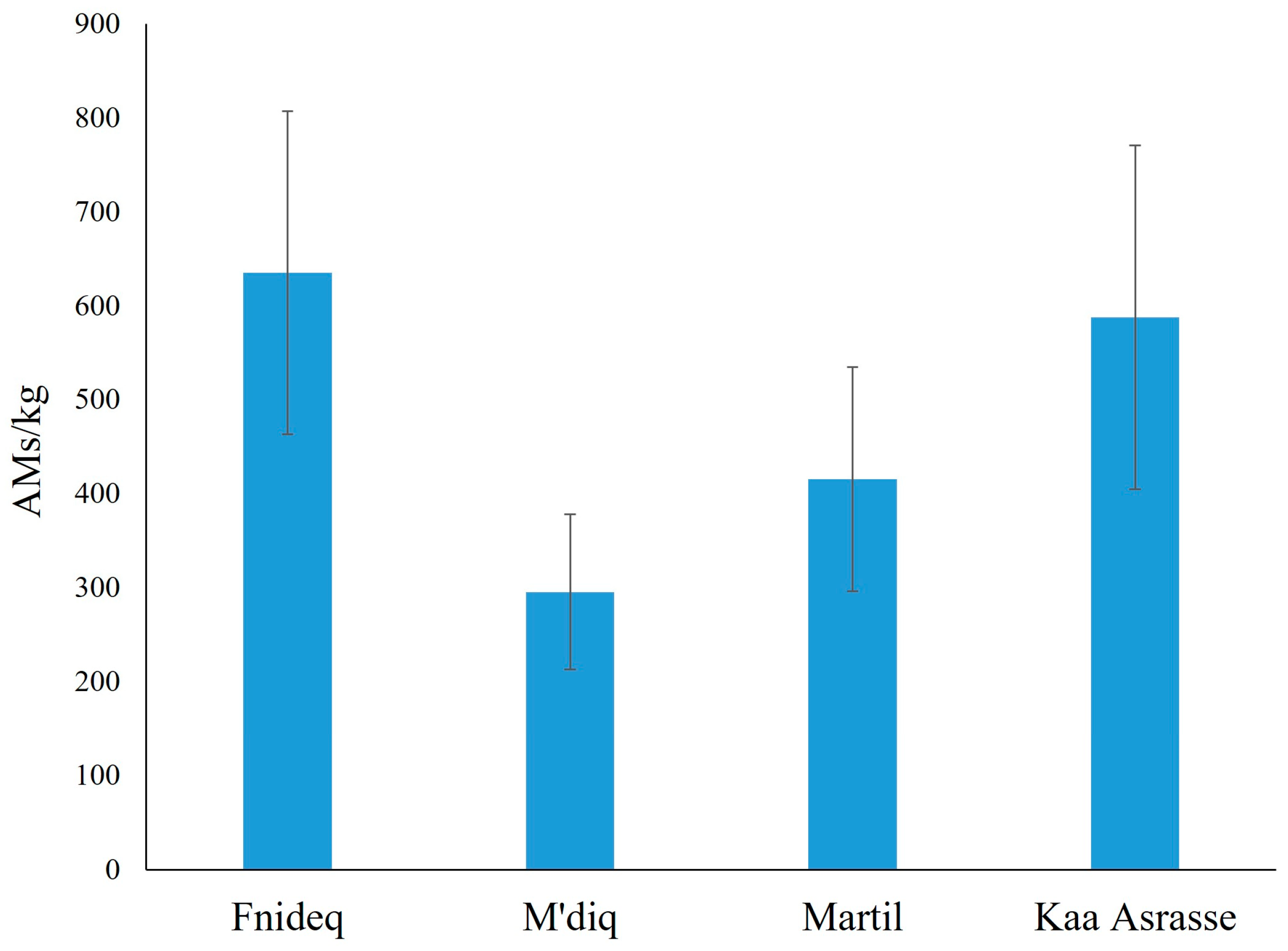
3.1. Abundance of AMs in Beach Sediments
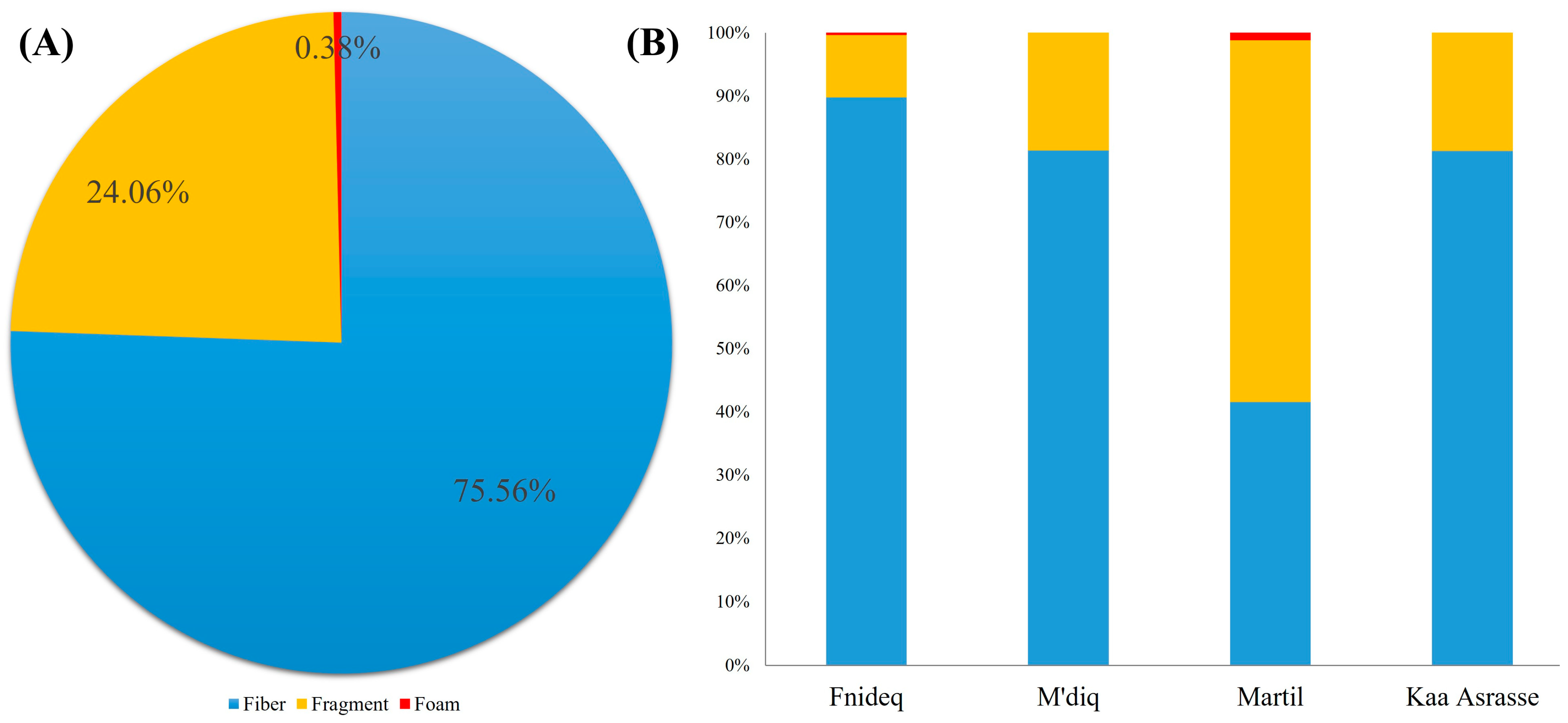
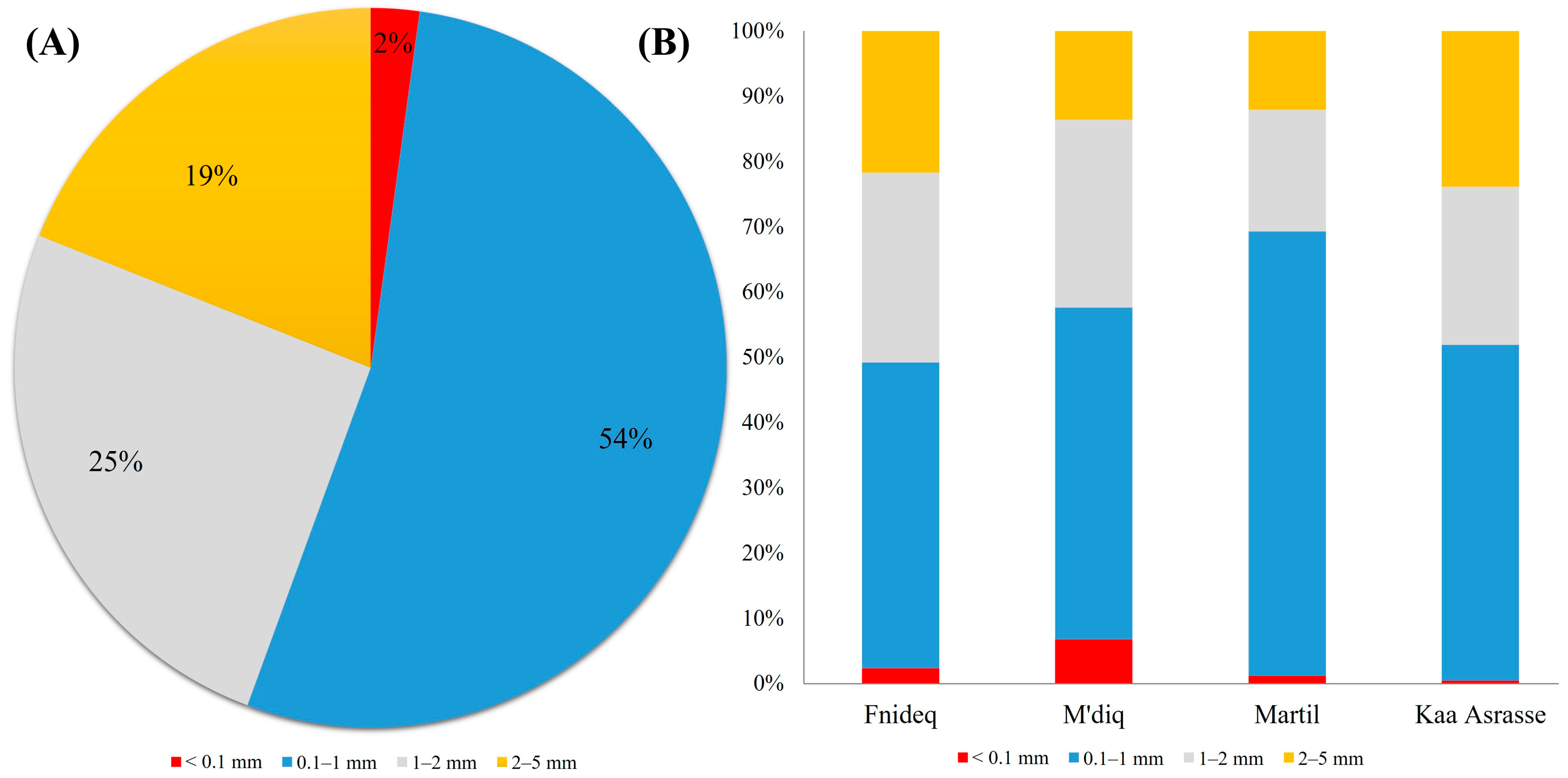
3.2. Morphological Characteristics of AMs in Beach Sediment
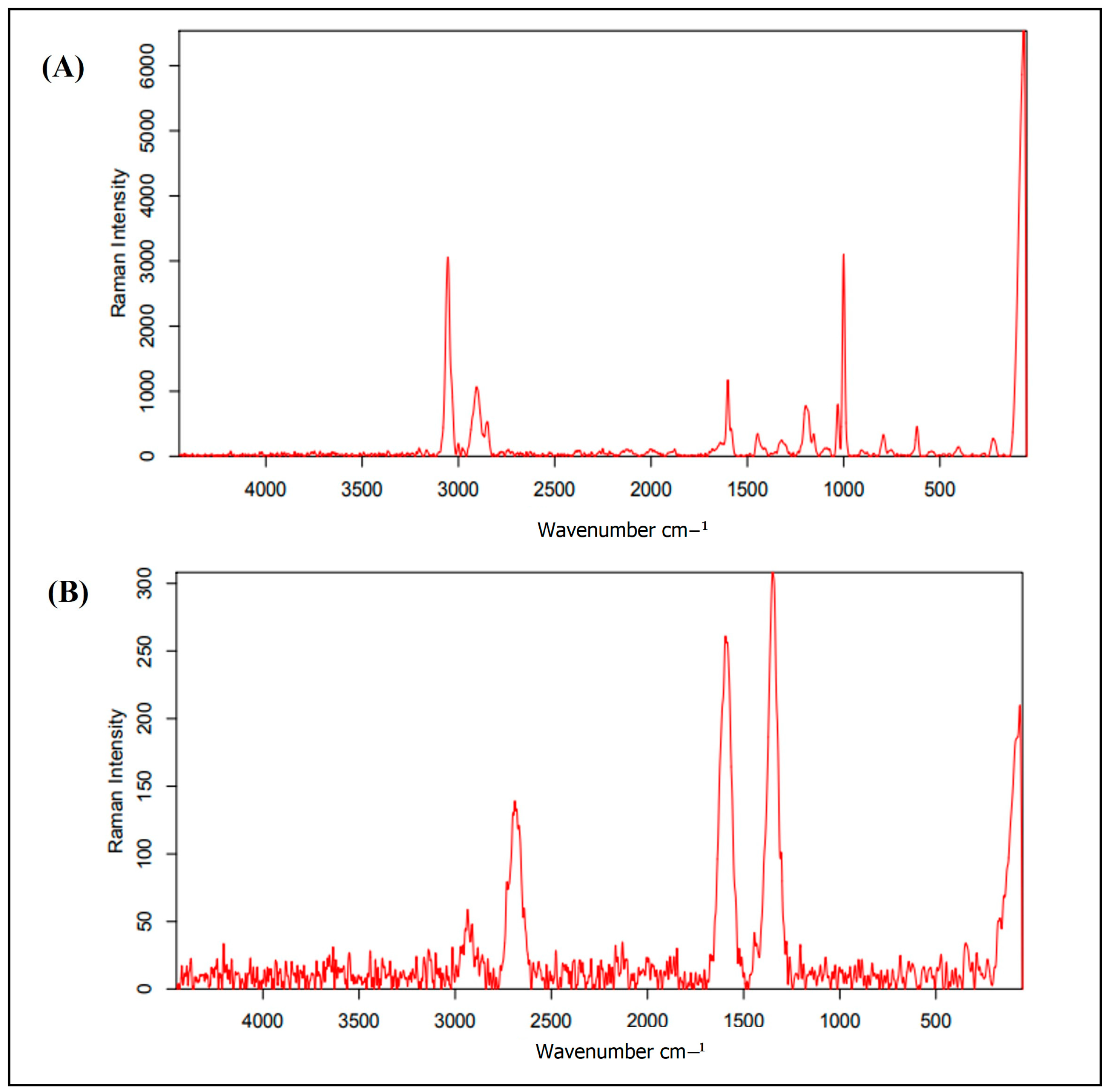
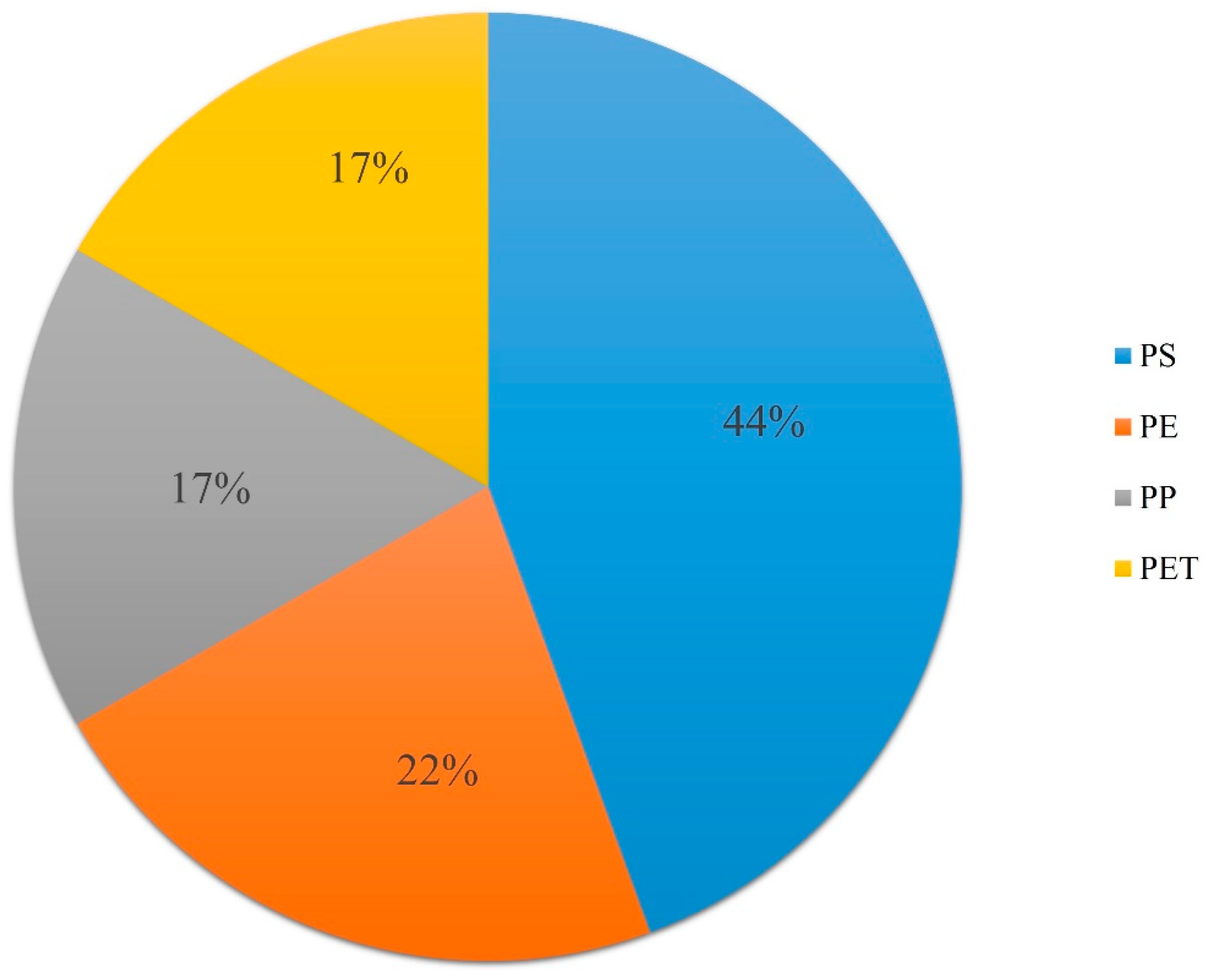
3.3. Polymer Identification
3.4. Beach Quality Assessment
4. Discussion
4.1. Abundance of AMs in Beach Sediments
4.2. Morphological Characteristics of AMs in Beach Sediment
4.3. Polymer Identification
4.4. Beach Quality Assessment
4.5. Research Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Evans, D.M.; Parsons, R.; Jackson, P.; Greenwood, S.; Ryan, A. Understanding Plastic Packaging: The Co-Evolution of Materials and Society. Glob. Environ. Chang. 2020, 65, 102166. [Google Scholar] [CrossRef]
- PlasticEurope. Plastics—The Facts 2020. Available online: https://www.plasticseurope.org/application/files/5716/0752/4286/AF_Plastics_the_facts-WEB-2020-ING_FINAL.Pdf (accessed on 1 February 2024).
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating Scenarios toward Zero Plastic Pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- Li, M.; Pan, Y.; Hou, Z.; Wu, Z.; Zeng, Z.; Wang, B. Plastic or Plastic-Free Life: From Formation to Removal. Sci. Total Environ. 2023, 890, 164359. [Google Scholar] [CrossRef]
- Boucher, J.; Billard, C. Mediterranean: Mare Plasticum; IUCN: Gland, Switzerland, 2020; p. 62. [Google Scholar]
- Galgani, F.; Brien, A.S.; Weis, J.; Ioakeimidis, C.; Schuyler, Q.; Makarenko, I.; Griffiths, H.; Bondareff, J.; Vethaak, D.; Deidun, A.; et al. Are Litter, Plastic and Microplastic Quantities Increasing in the Ocean? Microplastics Nanoplastics 2021, 1, 2. [Google Scholar] [CrossRef]
- Gunasekaran, K.; Mghili, B.; Saravanakumar, A. Personal Protective Equipment (PPE) Pollution Driven by the COVID-19 Pandemic in Coastal Environment, Southeast Coast of India. Mar. Pollut. Bull. 2022, 180, 113769. [Google Scholar] [CrossRef]
- De-la-Torre, G.E.; Dioses-Salinas, D.C.; Pizarro-Ortega, C.I.; Forero López, A.D.; Fernández Severini, M.D.; Rimondino, G.N.; Malanca, F.E.; Dobaradaran, S.; Aragaw, T.A.; Mghili, B.; et al. Plastic and Paint Debris in Marine Protected Areas of Peru. Sci. Total Environ. 2023, 901, 165788. [Google Scholar] [CrossRef]
- Okuku, E.O.; Kombo, M.; Mwalugha, C.; Owato, G.; Otieno, K.; Mbuche, M.; Chepkemboi, P.; Kiteresi, L.I.; Wanjeri, V. Are Tropical Mangroves a Sink for Litter Leaking from Land-and Sea-Based Sources? Evidence from Selected Kenyan Mangroves. Mar. Pollut. Bull. 2023, 187, 114590. [Google Scholar] [CrossRef]
- Soares, M.O.; Rizzo, L.; Ximenes Neto, A.R.; Barros, Y.; Martinelli Filho, J.E.; Giarrizzo, T.; Rabelo, E.F. Do Coral Reefs Act as Sinks for Microplastics? Environ. Pollut. 2023, 337, 122509. [Google Scholar] [CrossRef]
- Browne, M.A.; Crump, P.; Niven, S.J.; Teuten, E.; Tonkin, A.; Galloway, T.; Thompson, R. Accumulation of Microplastic on Shorelines Woldwide: Sources and Sinks. Environ. Sci. Technol. 2011, 45, 9175–9179. [Google Scholar] [CrossRef]
- Akdogan, Z.; Guven, B. Microplastics in the Environment: A Critical Review of Current Understanding and Identification of Future Research Needs. Environ. Pollut. 2019, 254, 113011. [Google Scholar] [CrossRef]
- Mohan, K.; Lakshmanan, V.R. A Critical Review of the Recent Trends in Source Tracing of Microplastics in the Environment. Environ. Res. 2023, 239, 117394. [Google Scholar] [CrossRef]
- Pedrotti, M.L.; Petit, S.; Eyheraguibel, B.; Kerros, M.E.; Elineau, A.; Ghiglione, J.F.; Loret, J.F.; Rostan, A.; Gorsky, G. Pollution by Anthropogenic Microfibers in North-West Mediterranean Sea and Efficiency of Microfiber Removal by a Wastewater Treatment Plant. Sci. Total Environ. 2021, 758, 144195. [Google Scholar] [CrossRef]
- Santini, S.; De Beni, E.; Martellini, T.; Sarti, C.; Randazzo, D.; Ciraolo, R.; Scopetani, C.; Cincinelli, A. Occurrence of Natural and Synthetic Micro-Fibers in the Mediterranean Sea: A Review. Toxics 2022, 10, 391. [Google Scholar] [CrossRef]
- Gavigan, J.; Kefela, T.; Macadam-Somer, I.; Suh, S.; Geyer, R. Synthetic Microfiber Emissions to Land Rival Those to Waterbodies and Are Growing. PLoS ONE 2020, 15, e0237839. [Google Scholar] [CrossRef]
- Manshoven, S.; Smeets, A.; Malarciuc, C.; Tenhunen-Lunkka, A.; Mortensen, L.F. Microplastic Pollution from Textile Consumption in Europe. Available online: https://www.eionet.europa.eu/etcs/etc-ce/products/etc-ce-products/etc-ce-report-1-2022-microplastic-pollution-from-textile-consumption-in-europe (accessed on 1 February 2024).
- Mishra, S.; Dash, D.; Das, A.P. Detection, Characterization and Possible Biofragmentation of Synthetic Microfibers Released from Domestic Laundering Wastewater as an Emerging Source of Marine Pollution. Mar. Pollut. Bull. 2022, 185, 114254. [Google Scholar] [CrossRef]
- Mishra, S.; Singh, R.P.; Rath, C.C.; Das, A.P. Synthetic Microfibers: Source, Transport and Their Remediation. J. Water Process Eng. 2020, 38, 101612. [Google Scholar] [CrossRef]
- Suran, M. A Planet Too Rich in Fibre. EMBO Rep. 2018, 19, e46701. [Google Scholar] [CrossRef]
- Athey, S.N.; Erdle, L.M. Are We Underestimating Anthropogenic Microfiber Pollution? A Critical Review of Occurrence, Methods, and Reporting. Environ. Toxicol. Chem. 2022, 41, 822–837. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of Textile Dyes on Health and the Environment and Bioremediation Potential of Living Organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Remy, F.; Collard, F.; Gilbert, B.; Compère, P.; Eppe, G.; Lepoint, G. When Microplastic Is Not Plastic: The Ingestion of Artificial Cellulose Fibers by Macrofauna Living in Seagrass Macrophytodetritus. Environ. Sci. Technol. 2015, 49, 11158–11166. [Google Scholar] [CrossRef]
- Oehlmann, J.; Schulte-Oehlmann, U.; Kloas, W.; Jagnytsch, O.; Lutz, I.; Kusk, K.O.; Wollenberger, L.; Santos, E.M.; Paull, G.C.; Van Look, K.J.W.; et al. A Critical Analysis of the Biological Impacts of Plasticizers on Wildlife. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2047–2062. [Google Scholar] [CrossRef]
- Ryan, P.G.; Suaria, G.; Perold, V.; Pierucci, A.; Bornman, T.G.; Aliani, S. Sampling Microfibres at the Sea Surface: The Effects of Mesh Size, Sample Volume and Water Depth. Environ. Pollut. 2020, 258, 113413. [Google Scholar] [CrossRef]
- Adams, J.K.; Dean, B.Y.; Athey, S.N.; Jantunen, L.M.; Bernstein, S.; Stern, G.; Diamond, M.L.; Finkelstein, S.A. Anthropogenic Particles (Including Microfibers and Microplastics) in Marine Sediments of the Canadian Arctic. Sci. Total Environ. 2021, 784, 147155. [Google Scholar] [CrossRef]
- Bottari, T.; Nibali, V.C.; Branca, C.; Grotti, M.; Savoca, S.; Romeo, T.; Spanò, N.; Azzaro, M.; Greco, S.; D’Angelo, G.; et al. Anthropogenic Microparticles in the Emerald Rockcod Trematomus Bernacchii (Nototheniidae) from the Antarctic. Sci. Rep. 2022, 12, 17214. [Google Scholar] [CrossRef]
- Sánchez-Guerrero-Hernández, M.J.; González-Fernández, D.; Sendra, M.; Ramos, F.; Yeste, M.P.; González-Ortegón, E. Contamination from Microplastics and Other Anthropogenic Particles in the Digestive Tracts of the Commercial Species Engraulis encrasicolus and Sardina pilchardus. Sci. Total Environ. 2023, 860, 160451. [Google Scholar] [CrossRef]
- Fossi, M.C.; Coppola, D.; Baini, M.; Giannetti, M.; Guerranti, C.; Marsili, L.; Panti, C.; de Sabata, E.; Clò, S. Large Filter Feeding Marine Organisms as Indicators of Microplastic in the Pelagic Environment: The Case Studies of the Mediterranean Basking Shark (Cetorhinus maximus) and Fin Whale (Balaenoptera physalus). Mar. Environ. Res. 2014, 100, 17–24. [Google Scholar] [CrossRef]
- Caron, A.G.M.; Thomas, C.R.; Berry, K.L.E.; Motti, C.A.; Ariel, E.; Brodie, J.E. Ingestion of Microplastic Debris by Green Sea Turtles (Chelonia mydas) in the Great Barrier Reef: Validation of a Sequential Extraction Protocol. Mar. Pollut. Bull. 2018, 127, 743–751. [Google Scholar] [CrossRef]
- Gérigny, O.; Pedrotti, M.-L.; El Rakwe, M.; Brun, M.; Pavec, M.; Henry, M.; Mazeas, F.; Maury, J.; Garreau, P.; Galgani, F. Characterization of Floating Microplastic Contamination in the Bay of Marseille (French Mediterranean Sea) and Its Impact on Zooplankton and Mussels. Mar. Pollut. Bull. 2022, 175, 113353. [Google Scholar] [CrossRef]
- Bottari, T.; Mancuso, M.; Pedà, C.; De Domenico, F.; Laface, F.; Schirinzi, G.F.; Battaglia, P.; Consoli, P.; Spanò, N.; Greco, S.; et al. Microplastics in the Bogue, Boops boops: A Snapshot of the Past from the Southern Tyrrhenian Sea. J. Hazard. Mater. 2022, 424, 127669. [Google Scholar] [CrossRef]
- Rivers-Auty, J.; Bond, A.L.; Grant, M.L.; Lavers, J.L. The One-Two Punch of Plastic Exposure: Macro- and Micro-Plastics Induce Multi-Organ Damage in Seabirds. J. Hazard. Mater. 2023, 442, 130117. [Google Scholar] [CrossRef]
- Armellini, A.; Ferri, G.; Lauteri, C.; De Camillis, A.; Pennisi, L. Microplastics in Sepia officinalis Caught on the Central Adriatic Coast: Preliminary Results. Ital. J. Food Saf. 2023, 12, 9971. [Google Scholar] [CrossRef]
- Morgana, S.; Estévez-Calvar, N.; Gambardella, C.; Faimali, M.; Garaventa, F. A Short-Term Swimming Speed Alteration Test with Nauplii of Artemia franciscana. Ecotoxicol. Environ. Saf. 2018, 147, 558–564. [Google Scholar] [CrossRef]
- Wright, S.L.; Rowe, D.; Thompson, R.C.; Galloway, T.S. Microplastic Ingestion Decreases Energy Reserves in Marine Worms. Curr. Biol. 2013, 23, R1031–R1033. [Google Scholar] [CrossRef]
- De Sá, L.C.; Oliveira, M.; Ribeiro, F.; Rocha, T.L.; Futter, M.N. Studies of the Effects of Microplastics on Aquatic Organisms: What Do We Know and Where Should We Focus Our Efforts in the Future? Sci. Total Environ. 2018, 645, 1029–1039. [Google Scholar] [CrossRef]
- Mallik, A.; Xavier, K.A.M.; Naidu, B.C.; Nayak, B.B. Ecotoxicological and Physiological Risks of Microplastics on Fish and Their Possible Mitigation Measures. Sci. Total Environ. 2021, 779, 146433. [Google Scholar] [CrossRef]
- Ahrendt, C.; Perez-Venegas, D.J.; Urbina, M.; Gonzalez, C.; Echeveste, P.; Aldana, M.; Pulgar, J.; Galbán-Malagón, C. Microplastic Ingestion Cause Intestinal Lesions in the Intertidal Fish Girella laevifrons. Mar. Pollut. Bull. 2020, 151, 110795. [Google Scholar] [CrossRef]
- Contino, M.; Ferruggia, G.; Indelicato, S.; Pecoraro, R.; Scalisi, E.M.; Salvaggio, A.; Brundo, M.V. Sublethal Effects of Polystyrene Nanoplastics on the Embryonic Development of Artemia salina (Linnaeus, 1758). Animals 2023, 13, 3152. [Google Scholar] [CrossRef]
- Miranda, M.N.; Lado Ribeiro, A.R.; Silva, A.M.T.; Pereira, M.F.R. Can Aged Microplastics Be Transport Vectors for Organic Micropollutants?—Sorption and Phytotoxicity Tests. Sci. Total Environ. 2022, 850, 158073. [Google Scholar] [CrossRef]
- Tumwesigye, E.; Felicitas Nnadozie, C.; C Akamagwuna, F.; Siwe Noundou, X.; William Nyakairu, G.; Odume, O.N. Microplastics as Vectors of Chemical Contaminants and Biological Agents in Freshwater Ecosystems: Current Knowledge Status and Future Perspectives. Environ. Pollut. 2023, 330, 121829. [Google Scholar] [CrossRef]
- Amelia, T.S.M.; Khalik, W.M.A.W.M.; Ong, M.C.; Shao, Y.T.; Pan, H.-J.; Bhubalan, K. Marine Microplastics as Vectors of Major Ocean Pollutants and Its Hazards to the Marine Ecosystem and Humans. Prog. Earth Planet. Sci. 2021, 8, 12. [Google Scholar] [CrossRef]
- Bouzekry, A.; Mghili, B.; Aksissou, M. Addressing the Challenge of Marine Plastic Litter in the Moroccan Mediterranean: A Citizen Science Project with Schoolchildren. Mar. Pollut. Bull. 2022, 184, 114167. [Google Scholar] [CrossRef]
- Mghili, B.; Keznine, M.; Hasni, S.; Aksissou, M. Abundance, Composition and Sources of Benthic Marine Litter Trawled-up in the Fishing Grounds on the Moroccan Mediterranean Coast. Reg. Stud. Mar. Sci. 2023, 63, 103002. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Mghili, B.; Ben-Haddad, M.; Zerrad, O.; Rangel-Buitrago, N.; Aksissou, M. Tackling Marine Plastic Pollution in Morocco: A Review of Current Research, Regulatory Measures, and Future Challenges. Reg. Stud. Mar. Sci. 2024, 69, 103286. [Google Scholar] [CrossRef]
- Mghili, B.; Benhardouze, W.; Aksissou, M.; Tiwari, M. Sea Turtle Strandings along the Northwestern Moroccan Coast: Spatio-Temporal Distribution and Main Threats. Ocean. Coast. Manag. 2023, 237, 106539. [Google Scholar] [CrossRef]
- Alshawafi, A.; Analla, M.; Alwashali, E.; Aksissou, M. Assessment of Marine Debris on the Coastal Wetland of Martil in the North-East of Morocco. Mar. Pollut. Bull. 2017, 117, 302–310. [Google Scholar] [CrossRef]
- Mghili, B.; Analla, M.; Aksissou, M.; Aissa, C. Marine Debris in Moroccan Mediterranean Beaches: An Assessment of Their Abundance, Composition and Sources. Mar. Pollut. Bull. 2020, 160, 111692. [Google Scholar] [CrossRef]
- Masura, J.; Baker, J.E.; Foster, G.D.; Arthur, C.; Herring, C. Laboratory Methods for the Analysis of Microplastics in the Marine Environment: Recommendations for Quantifying Synthetic Particles in Waters and Sediments; NOAA Marine Debris Division: Silver Spring, MD, USA, 2015.
- Hidalgo-Ruz, V.; Gutow, L.; Thompson, R.C.; Thiel, M. Microplastics in the Marine Environment: A Review of the Methods Used for Identification and Quantification. Environ. Sci. Technol. 2012, 46, 3060–3075. [Google Scholar] [CrossRef]
- Bouzekry, A.; Mghili, B.; Bouadil, O.; Mancuso, M.; Ben-Haddad, M.; Bottari, T.; Aksissou, M. First Report of Microplastic Ingestion in Edible Fish along Moroccan Mediterranean Coasts. Sustainability 2023, 15, 16313. [Google Scholar] [CrossRef]
- Rangel-Buitrago, N.; Arroyo-Olarte, H.; Trilleras, J.; Arana, V.A.; Mantilla-Barbosa, E.; Gracia, A.; Mendoza, A.V.; Neal, W.J.; Williams, A.T.; Micallef, A. Microplastics Pollution on Colombian Central Caribbean Beaches. Mar. Pollut. Bull. 2021, 170, 112685. [Google Scholar] [CrossRef]
- Langford, E. Quartiles in Elementary Statistics. J. Stat. Educ. 2006, 14, 1–6. [Google Scholar] [CrossRef]
- Constant, M.; Kerhervé, P.; Mino-Vercellio-Verollet, M.; Dumontier, M.; Sànchez Vidal, A.; Canals, M.; Heussner, S. Beached Microplastics in the Northwestern Mediterranean Sea. Mar. Pollut. Bull. 2019, 142, 263–273. [Google Scholar] [CrossRef]
- Tata, T.; Belabed, B.E.; Bououdina, M.; Bellucci, S. Occurrence and Characterization of Surface Sediment Microplastics and Litter from North African Coasts of Mediterranean Sea: Preliminary Research and First Evidence. Sci. Total Environ. 2020, 713, 136664. [Google Scholar] [CrossRef]
- Sayed, A.E.-D.H.; Hamed, M.; Badrey, A.E.A.; Ismail, R.F.; Osman, Y.A.A.; Osman, A.G.M.; Soliman, H.A.M. Microplastic Distribution, Abundance, and Composition in the Sediments, Water, and Fishes of the Red and Mediterranean Seas, Egypt. Mar. Pollut. Bull. 2021, 173, 112966. [Google Scholar] [CrossRef]
- Abdel Ghani, S.A.; El-Sayed, A.A.M.; Ibrahim, M.I.A.; Ghobashy, M.M.; Shreadah, M.A.; Shabaka, S. Characterization and Distribution of Plastic Particles along Alexandria Beaches, Mediterranean Coast of Egypt, Using Microscopy and Thermal Analysis Techniques. Sci. Total Environ. 2022, 834, 155363. [Google Scholar] [CrossRef]
- Azaaouaj, S.; Nachite, D.; Anfuso, G.; Er-Ramy, N. Abundance and Distribution of Microplastics on Sandy Beaches of the Eastern Moroccan Mediterranean Coast. Mar. Pollut. Bull. 2024, 200, 116144. [Google Scholar] [CrossRef]
- Abidli, S.; Antunes, J.C.; Ferreira, J.L.; Lahbib, Y.; Sobral, P.; Trigui El Menif, N. Microplastics in Sediments from the Littoral Zone of the North Tunisian Coast (Mediterranean Sea). Estuar. Coast. Shelf Sci. 2018, 205, 1–9. [Google Scholar] [CrossRef]
- Abdelkader, N.; Ben Ismail, S.; Zakhama-Sraieb, R. Macro-, Meso- and Microplastic Debris in Three Sandy Beaches of North-Eastern Tunisian Coasts. Reg. Stud. Mar. Sci. 2023, 67, 103229. [Google Scholar] [CrossRef]
- Taïbi, N.-E.; Bentaallah, M.E.A.; Alomar, C.; Compa, M.; Deudero, S. Micro- and Macro-Plastics in Beach Sediment of the Algerian Western Coast: First Data on Distribution, Characterization, and Source. Mar. Pollut. Bull. 2021, 165, 112168. [Google Scholar] [CrossRef]
- Grini, H.; Metallaoui, S.; González-Fernández, D.; Bensouilah, M. First Evidence of Plastic Pollution in Beach Sediments of the Skikda Coast (Northeast of Algeria). Mar. Pollut. Bull. 2022, 181, 113831. [Google Scholar] [CrossRef]
- Bentaallah, M.E.A.; Baghdadi, D.; Gündoğdu, S.; Megharbi, A.; Taibi, N.-E.; Büyükdeveci, F. Assessment of Microplastic Abundance and Impact on Recreational Beaches along the Western Algerian Coastline. Mar. Pollut. Bull. 2024, 199, 116007. [Google Scholar] [CrossRef]
- Godoy, V.; Prata, J.C.; Blázquez, G.; Almendros, A.I.; Duarte, A.C.; Rocha-Santos, T.; Calero, M.; Martín-Lara, M.Á. Effects of Distance to the Sea and Geomorphological Characteristics on the Quantity and Distribution of Microplastics in Beach Sediments of Granada (Spain). Sci. Total Environ. 2020, 746, 142023. [Google Scholar] [CrossRef]
- Expósito, N.; Rovira, J.; Sierra, J.; Folch, J.; Schuhmacher, M. Microplastics Levels, Size, Morphology and Composition in Marine Water, Sediments and Sand Beaches. Case Study of Tarragona Coast (Western Mediterranean). Sci. Total Environ. 2021, 786, 147453. [Google Scholar] [CrossRef]
- Bayo, J.; Rojo, D.; Olmos, S. Weathering Indices of Microplastics along Marine and Coastal Sediments from the Harbor of Cartagena (Spain) and Its Adjoining Urban Beach. Mar. Pollut. Bull. 2022, 178, 113647. [Google Scholar] [CrossRef]
- Chouchene, K.; Prata, J.C.; da Costa, J.; Duarte, A.C.; Rocha-Santos, T.; Ksibi, M. Microplastics on Barra Beach Sediments in Aveiro, Portugal. Mar. Pollut. Bull. 2021, 167, 112264. [Google Scholar] [CrossRef]
- Korez, Š.; Gutow, L.; Saborowski, R. Microplastics at the Strandlines of Slovenian Beaches. Mar. Pollut. Bull. 2019, 145, 334–342. [Google Scholar] [CrossRef]
- Munari, C.; Scoponi, M.; Mistri, M. Plastic Debris in the Mediterranean Sea: Types, Occurrence and Distribution along Adriatic Shorelines. Waste Manag. 2017, 67, 385–391. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Ben-Haddad, M.; Rangel-Buitrago, N.; Hajji, S.; El Alem, N.; Ait Alla, A. Microplastics Pollution along the Central Atlantic Coastline of Morocco. Mar. Pollut. Bull. 2022, 174, 113190. [Google Scholar] [CrossRef]
- Ben-Haddad, M.; Abelouah, M.R.; Hajji, S.; Rangel-Buitrago, N.; Hamadi, F.; Alla, A.A. Microplastics Pollution in Sediments of Moroccan Urban Beaches: The Taghazout Coast as a Case Study. Mar. Pollut. Bull. 2022, 180, 113765. [Google Scholar] [CrossRef]
- Lots, F.A.E.; Behrens, P.; Vijver, M.G.; Horton, A.A.; Bosker, T. A Large-Scale Investigation of Microplastic Contamination: Abundance and Characteristics of Microplastics in European Beach Sediment. Mar. Pollut. Bull. 2017, 123, 219–226. [Google Scholar] [CrossRef]
- Guerranti, C.; Cannas, S.; Scopetani, C.; Fastelli, P.; Cincinelli, A.; Renzi, M. Plastic Litter in Aquatic Environments of Maremma Regional Park (Tyrrhenian Sea, Italy): Contribution by the Ombrone River and Levels in Marine Sediments. Mar. Pollut. Bull. 2017, 117, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Vianello, A.; Boldrin, A.; Guerriero, P.; Moschino, V.; Rella, R.; Sturaro, A.; Da Ros, L. Microplastic Particles in Sediments of Lagoon of Venice, Italy: First Observations on Occurrence, Spatial Patterns and Identification. Estuar. Coast. Shelf Sci. 2013, 130, 54–61. [Google Scholar] [CrossRef]
- Compa, M.; Alomar, C.; Morató, M.; Álvarez, E.; Deudero, S. Spatial Distribution of Macro- and Micro-Litter Items along Rocky and Sandy Beaches of a Marine Protected Area in the Western Mediterranean Sea. Mar. Pollut. Bull. 2022, 178, 113520. [Google Scholar] [CrossRef] [PubMed]
- Uddin, S.; Fowler, S.W.; Uddin, M.F.; Behbehani, M.; Naji, A. A Review of Microplastic Distribution in Sediment Profiles. Mar. Pollut. Bull. 2021, 163, 111973. [Google Scholar] [CrossRef] [PubMed]
- Devereux, R.; Ayati, B.; Westhead, E.K.; Jayaratne, R.; Newport, D. “The Great Source” Microplastic Abundance and Characteristics along the River Thames. Mar. Pollut. Bull. 2023, 191, 114965. [Google Scholar] [CrossRef] [PubMed]
- Simon-Sánchez, L.; Grelaud, M.; Franci, M.; Ziveri, P. Are Research Methods Shaping Our Understanding of Microplastic Pollution? A Literature Review on the Seawater and Sediment Bodies of the Mediterranean Sea. Environ. Pollut. 2022, 292, 118275. [Google Scholar] [CrossRef] [PubMed]
- Gedik, K.; Eryaşar, A.R.; Öztürk, R.Ç.; Mutlu, E.; Karaoğlu, K.; Şahin, A.; Özvarol, Y. The Broad-Scale Microplastic Distribution in Surface Water and Sediments along Northeastern Mediterranean Shoreline. Sci. Total Environ. 2022, 843, 157038. [Google Scholar] [CrossRef] [PubMed]
- de Smit, J.C.; Anton, A.; Martin, C.; Rossbach, S.; Bouma, T.J.; Duarte, C.M. Habitat-Forming Species Trap Microplastics into Coastal Sediment Sinks. Sci. Total Environ. 2021, 772, 145520. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Banik, P.; Nur, A.-A.U.; Rahman, T. Abundance and Characteristics of Microplastics in Sediments from the World’s Longest Natural Beach, Cox’s Bazar, Bangladesh. Mar. Pollut. Bull. 2021, 163, 111956. [Google Scholar] [CrossRef]
- Sorasan, C.; Edo, C.; González-Pleiter, M.; Fernández-Piñas, F.; Leganés, F.; Rodríguez, A.; Rosal, R. Ageing and Fragmentation of Marine Microplastics. Sci. Total Environ. 2022, 827, 154438. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating Microplastic Trophic Transfer in Marine Top Predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Sari Erkan, H.; Takatas, B.; Ozturk, A.; Gündogdu, S.; Aydın, F.; Koker, L.; Ozdemir, O.K.; Albay, M.; Onkal Engin, G. Spatio-Temporal Distribution of Microplastic Pollution in Surface Sediments along the Coastal Areas of Istanbul, Turkey. Mar. Pollut. Bull. 2023, 195, 115461. [Google Scholar] [CrossRef] [PubMed]
- De Sá, L.C.; Luís, L.G.; Guilhermino, L. Effects of Microplastics on Juveniles of the Common Goby (Pomatoschistus microps): Confusion with Prey, Reduction of the Predatory Performance and Efficiency, and Possible Influence of Developmental Conditions. Environ. Pollut. 2015, 196, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Bouadil, O.; Benomar, M.; El Ouarghi, H.; Aboulhassan, M.A.; Benbrahim, S. Identification and Quantification of Microplastics in Surface Water of a Southwestern Mediterranean Bay (Al Hoceima, Morocco). Waste Manag. Bull. 2024, 2, 142–151. [Google Scholar] [CrossRef]
- Bošković, N.; Joksimović, D.; Perošević-Bajčeta, A.; Peković, M.; Bajt, O. Distribution and Characterization of Microplastics in Marine Sediments from the Montenegrin Coast. J. Soils Sediments 2022, 22, 2958–2967. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, P.; Zhang, G.; Wang, S.; Cai, Y.; Wang, H. Vertical Microplastic Distribution in Sediments of Fuhe River Estuary to Baiyangdian Wetland in Northern China. Chemosphere 2021, 280, 130800. [Google Scholar] [CrossRef]
- Pervez, R.; Lai, Y.; Song, Y.; Li, X.; Lai, Z. Impact of Microplastic Pollution on Coastal Ecosystems Using Comprehensive Beach Quality Indices. Mar. Pollut. Bull. 2023, 194, 115304. [Google Scholar] [CrossRef] [PubMed]
- Krikech, I.; Oliveri Conti, G.; Pulvirenti, E.; Rapisarda, P.; Castrogiovanni, M.; Maisano, M.; Le Pennec, G.; Leermakers, M.; Ferrante, M.; Cappello, T.; et al. Microplastics (≤10 μm) Bioaccumulation in Marine Sponges along the Moroccan Mediterranean Coast: Insights into Species-Specific Distribution and Potential Bioindication. Environ. Res. 2023, 235, 116608. [Google Scholar] [CrossRef]
- Thacharodi, A.; Hassan, S.; Meenatchi, R.; Bhat, M.A.; Hussain, N.; Arockiaraj, J.; Ngo, H.H.; Sharma, A.; Nguyen, H.T.; Pugazhendhi, A. Mitigating Microplastic Pollution: A Critical Review on the Effects, Remediation, and Utilization Strategies of Microplastics. J. Environ. Manag. 2024, 351, 119988. [Google Scholar] [CrossRef]




| Beach | Coordinates | Type | Port/Harbor | Wastewater Treatment Plant (WWTP) | River |
|---|---|---|---|---|---|
| Fnideq | 35°50′46″ N/5°21′07″ W | Urban | Artisanal fishing | Yes | Yes |
| M’diq | 35°41′34″ N/5°18′12″ W | Urban | Industrial and Artisanal | No | No |
| Martil | 35°37′26″ N/5°16′88″ W | Urban | Artisanal fishing | Yes | Yes |
| Kaa Asrasse | 35°25′24″ N/5°2′12″ W | Rural | Artisanal fishing | No | Yes |
| Site Name | AMPI Fiber | AMPI Type | AMPI Fragment | AMPI Type | CAMI-Fiber | CAMI Type | CAMI Fragment | CAMI Type |
|---|---|---|---|---|---|---|---|---|
| Fnideq | 114 | Very High Abundance | 12.5 | Moderate Abundance | 0.89 | Extreme | 0.09 | Minimum |
| M’diq | 48 | Very High Abundance | 11 | Moderate Abundance | 0.81 | Extreme | 0.18 | Average |
| Martil | 34.5 | Very High Abundance | 47.5 | Very High Abundance | 0.41 | Average | 0.57 | Maximum |
| Kaa Asrasse | 95.5 | Very High Abundance | 22 | High Abundance | 0.82 | Extreme | 0.17 | Average |
| AMPI (Fibers) | |||||||
|---|---|---|---|---|---|---|---|
| Very Low Abundance | Low Abundance | Moderate Abundance | High Abundance | Very High Abundance | |||
| CAMI (Fibers) | Minimum | 0 | |||||
| Average | 1 | 1 | |||||
| Maximum | 0 | ||||||
| Extreme | 3 | 3 | |||||
| 0 | 0 | 0 | 0 | 4 | 4 | ||
| AMPI (Fragments) | |||||||
| Very low abundance | Low abundance | Moderate abundance | High abundance | Very high abundance | |||
| CAMI (Fragments) | Minimum | 1 | 1 | ||||
| Average | 1 | 1 | 2 | ||||
| Maximum | 1 | 1 | |||||
| Extreme | 0 | ||||||
| 0 | 0 | 2 | 1 | 1 | 4 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzekry, A.; Mghili, B.; Mancuso, M.; Bouadil, O.; Bottari, T.; Aksissou, M. Anthropogenic Microparticles Abundance in Sandy Beach Sediments along the Tetouan Coast (Morocco Mediterranean). Environments 2024, 11, 83. https://doi.org/10.3390/environments11040083
Bouzekry A, Mghili B, Mancuso M, Bouadil O, Bottari T, Aksissou M. Anthropogenic Microparticles Abundance in Sandy Beach Sediments along the Tetouan Coast (Morocco Mediterranean). Environments. 2024; 11(4):83. https://doi.org/10.3390/environments11040083
Chicago/Turabian StyleBouzekry, Assia, Bilal Mghili, Monique Mancuso, Oumayma Bouadil, Teresa Bottari, and Mustapha Aksissou. 2024. "Anthropogenic Microparticles Abundance in Sandy Beach Sediments along the Tetouan Coast (Morocco Mediterranean)" Environments 11, no. 4: 83. https://doi.org/10.3390/environments11040083







