Plant-Wide Models for Optimizing the Operation and Maintenance of BTEX-Contaminated Wastewater Treatment and Reuse
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mathematical Model Development
2.1.1. Biodegradation
2.1.2. Gas–Liquid Transfer
2.1.3. Adsorption on Granular Activated Carbon
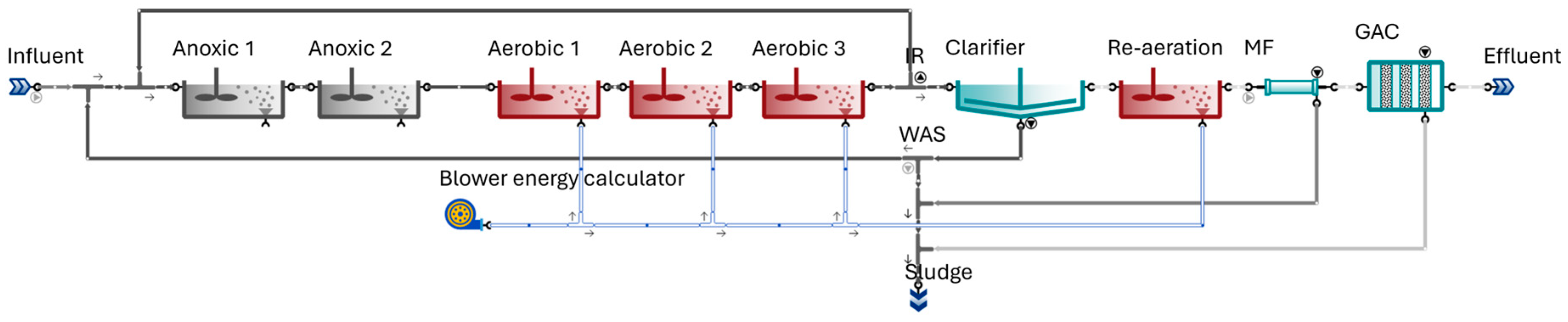
2.2. Model Configuration
3. Results and Discussion
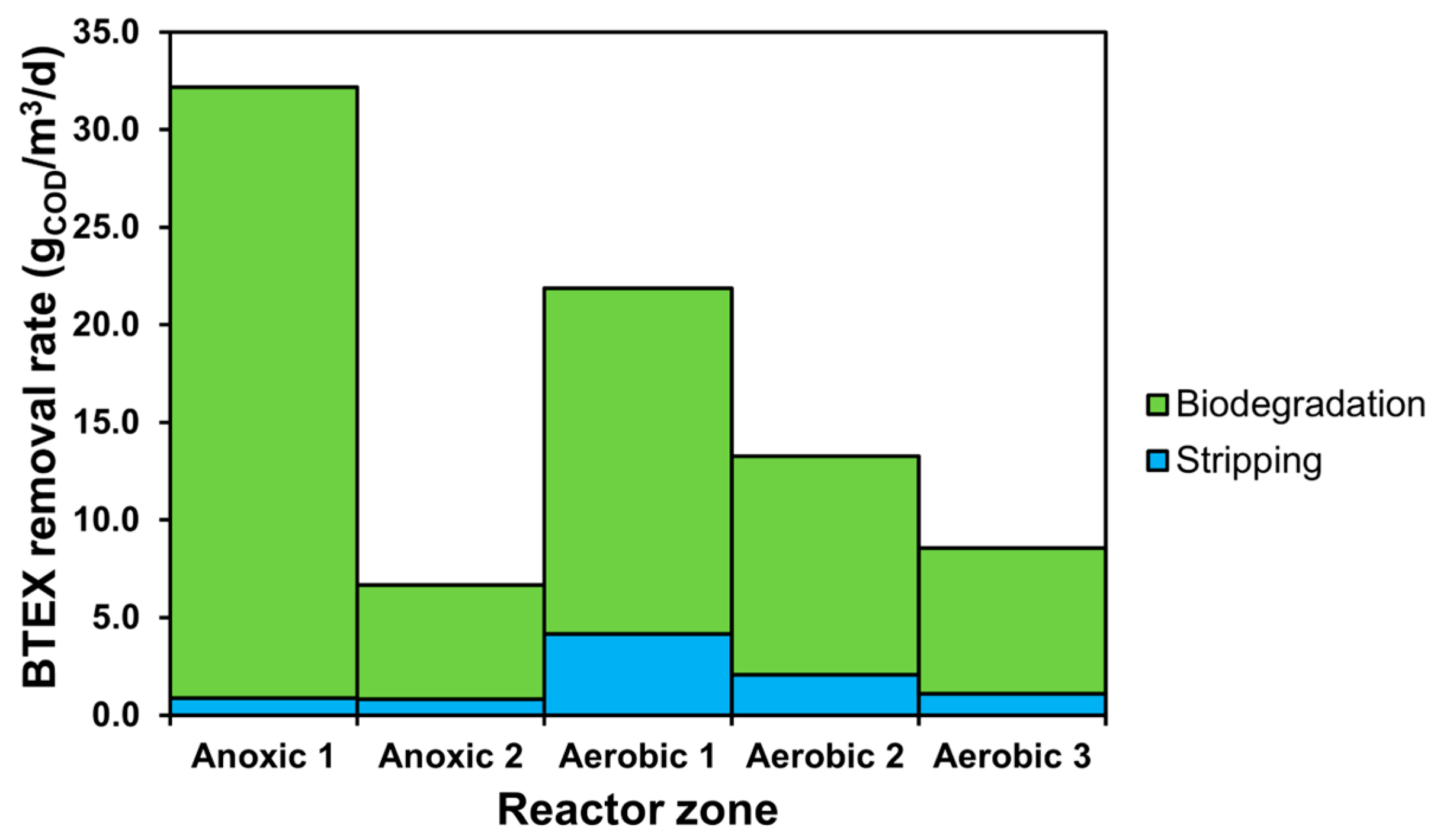
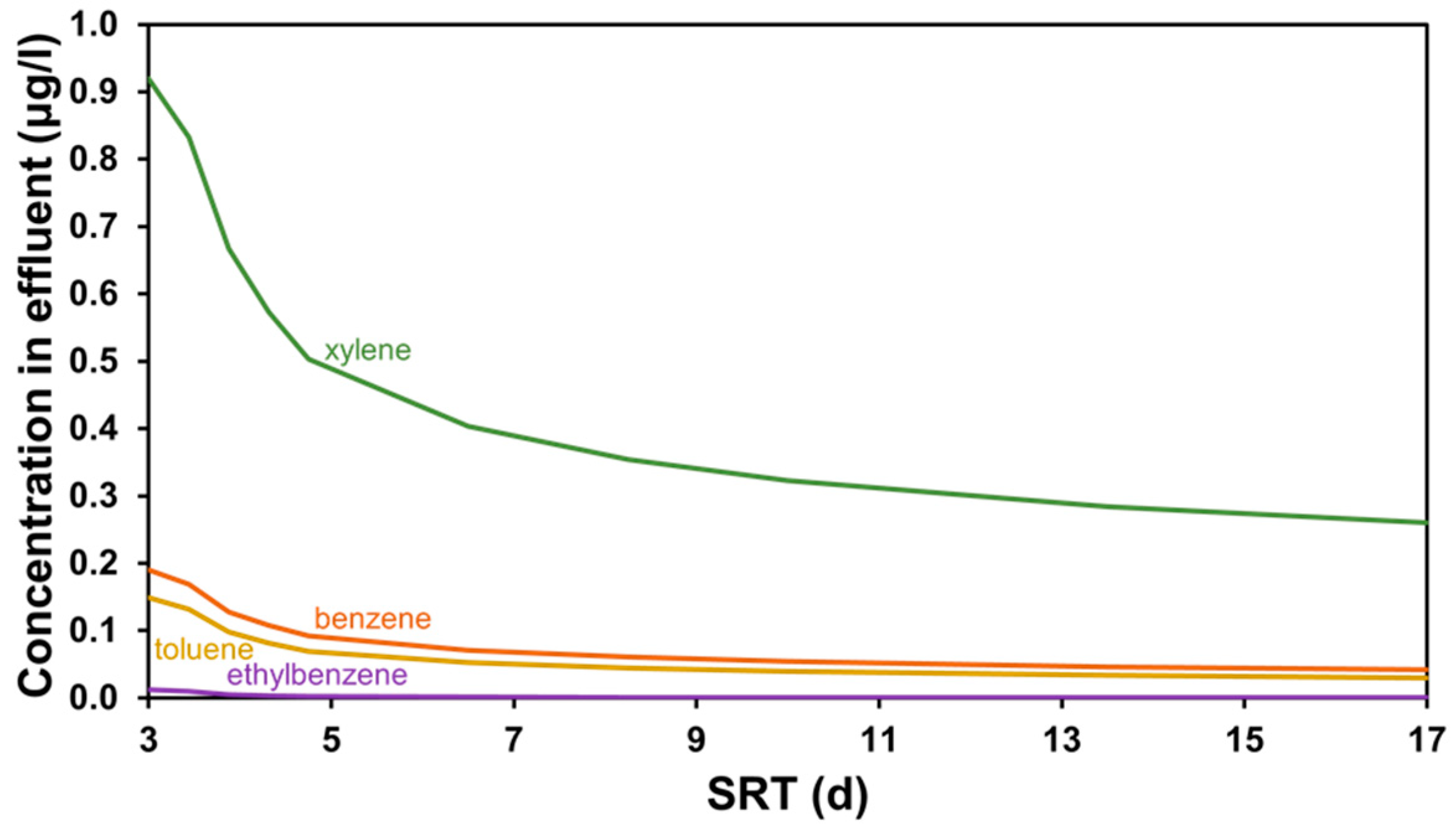
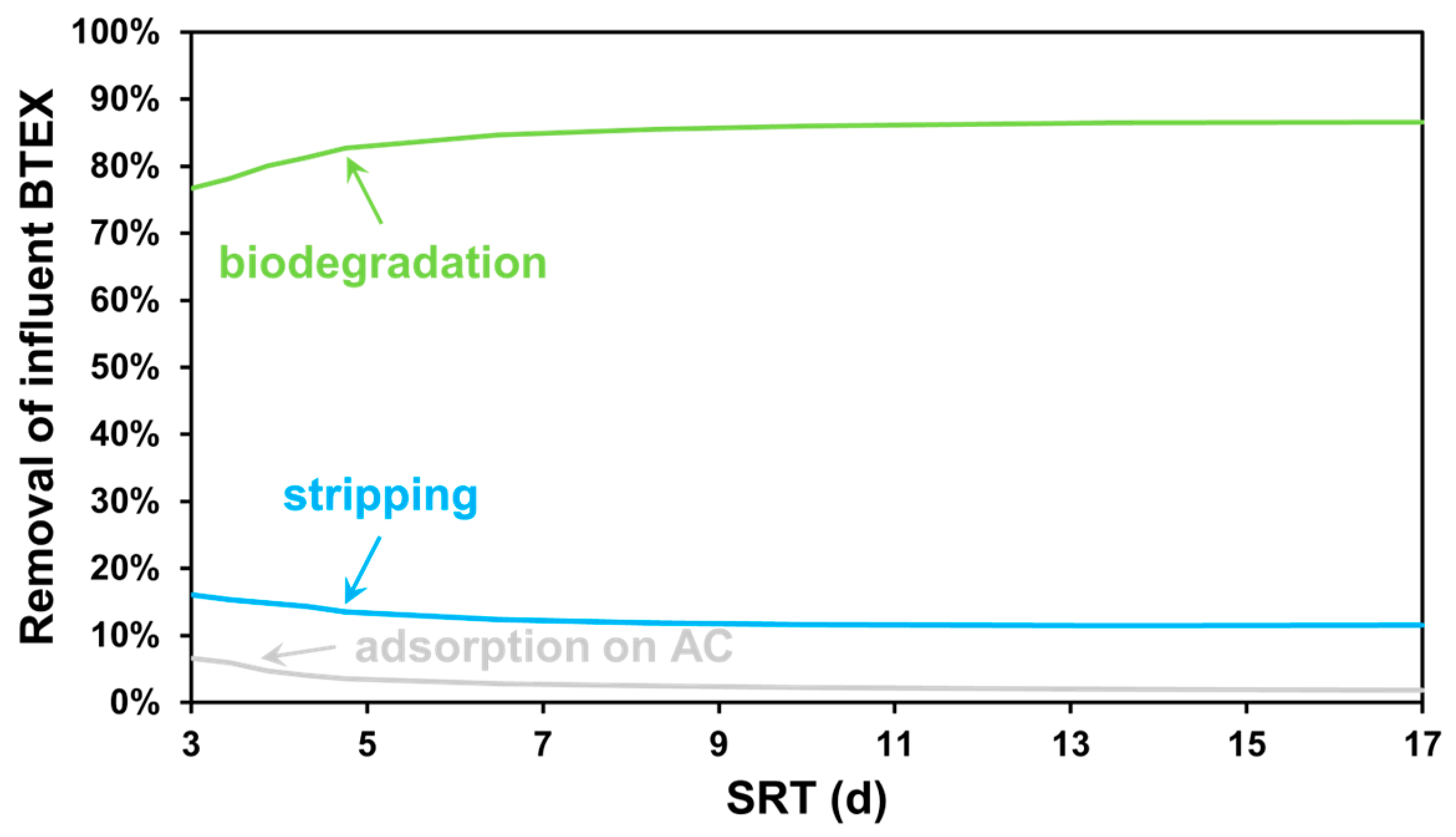
3.1. SRT-Based Scenarios
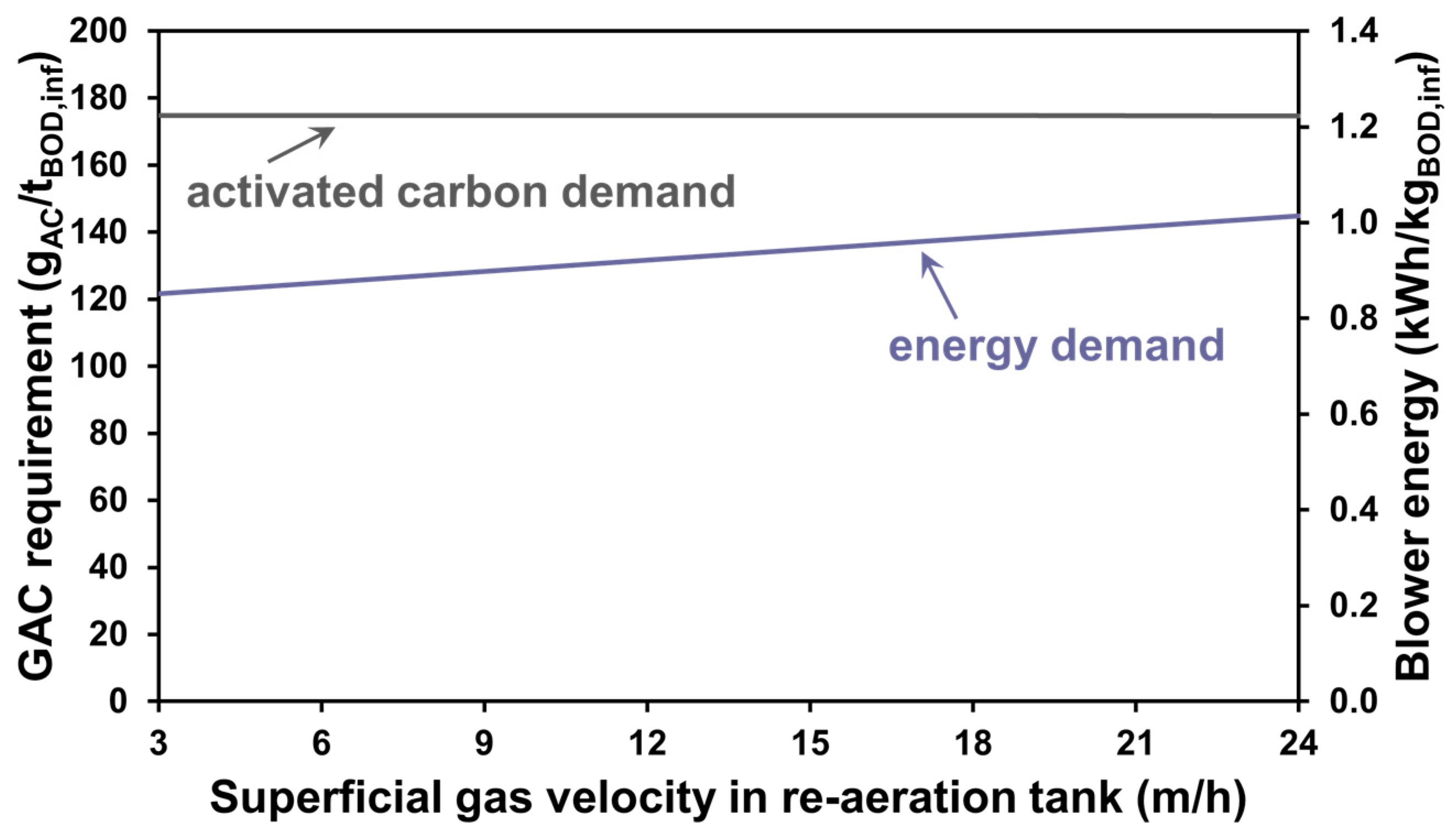
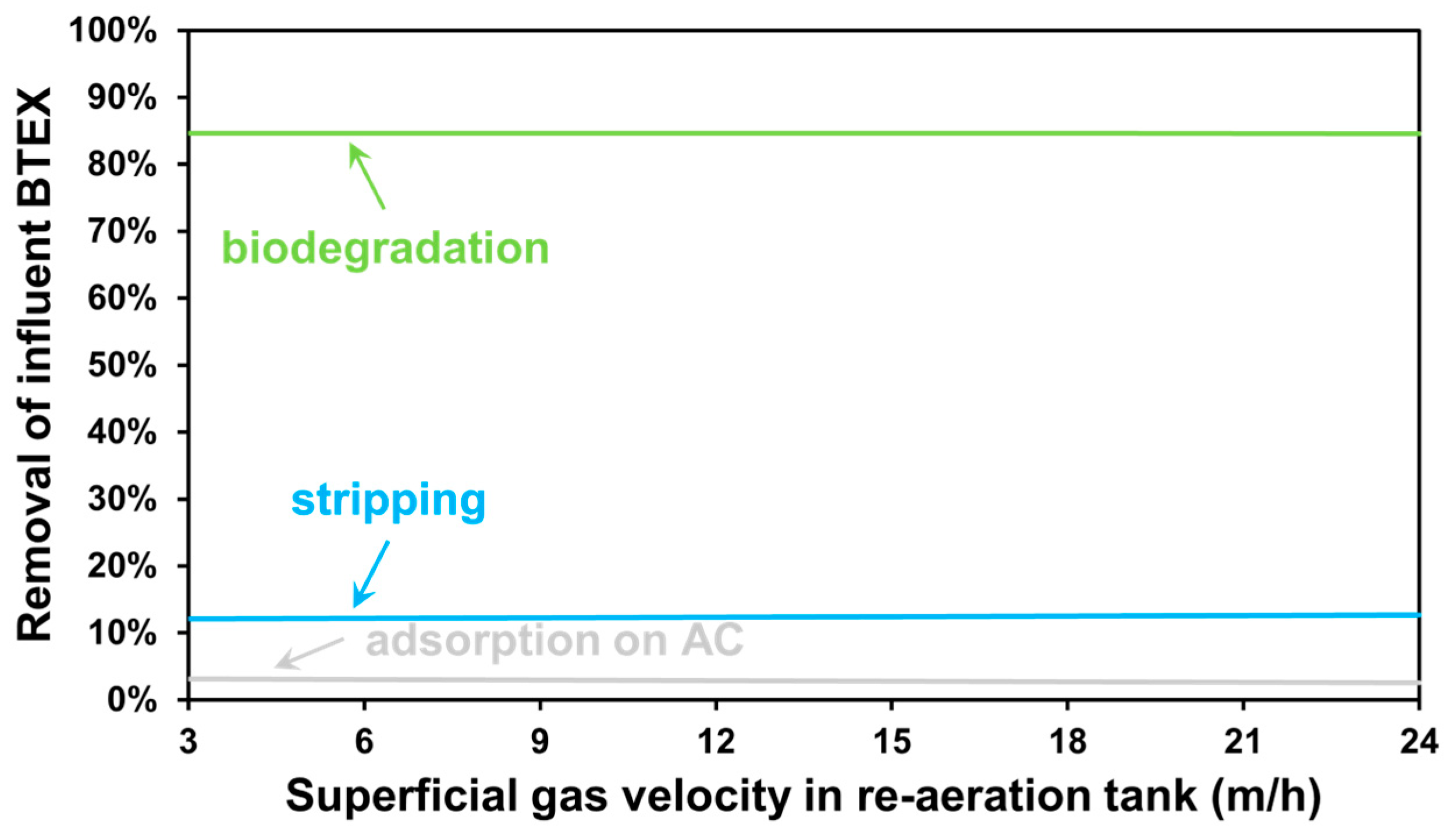
3.2. The Effect of Aeration Intensity
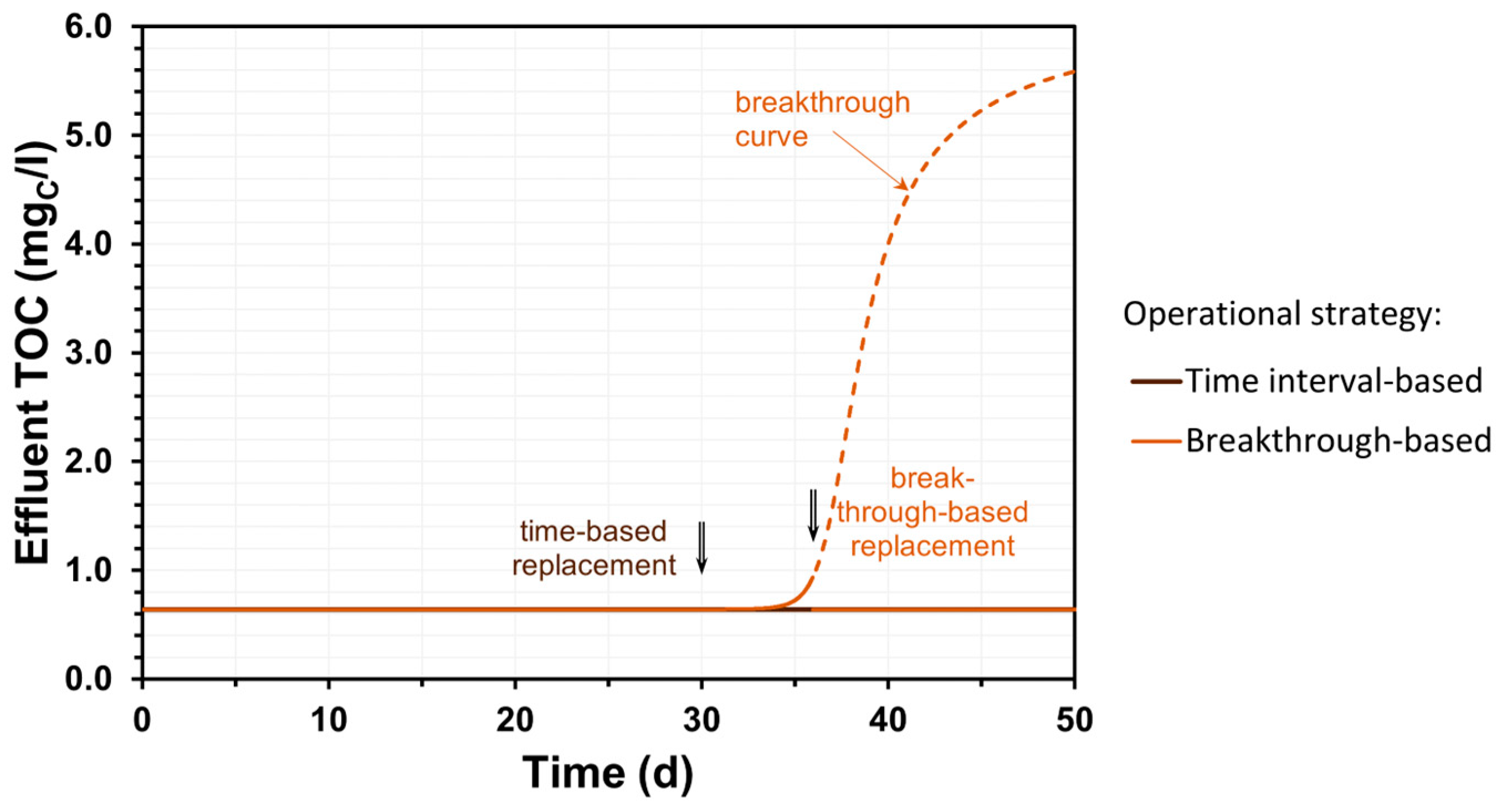
3.3. GAC Operational Strategies
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| α | alpha (wastewater/clean water) correction factor for mass transfer coefficient |
| abub | specific contact area between the gas bubble surface and liquid phase [m2 m−3] |
| asur | specific contact area between the surface gas and liquid phase [m2 m−3] |
| Adiff,sp | area per diffuser [m2] |
| Ar | liquid surface [m2] |
| β | beta (wastewater/clean water) correction factor for the saturation concentration |
| BTC | TOC breakthrough capacity (in concentration unit) [gC m−3] |
| BTCm | TOC adsorption capacity (at breakpoint, in mass fraction unit) [gC gAC−1] |
| Cmid | midpoint concentration of breakthrough curve, with asymmetry correction [gC m−3] |
| Cmid,symm | midpoint concentration of curve, without asymmetry correction [gC m−3] |
| coefflead,h,diff | leading coefficient in a diffuser submergence correction term [m−1] |
| coefflin,h,diff | linear coefficient in a diffuser submergence correction term [m−1] |
| dbub | bubble Sauter mean diameter [m] |
| ddiff | diffuser density [m2 m−2] |
| Di,25 | diffusion coefficient of gas state variable i in water [m2 d−1] |
| divd,diff | divisor value in a diffuser density correction term [m2 m−2] |
| ε | gas hold-up [m3gas m−3] |
| EQC,ad,total | carbon equivalent for all adsorbed components on a GAC bed [g C m−3] |
| expSSOTE | exponent in SSOTE correlation [d m−3gas] |
| F | diffuser fouling factor |
| Fac | replaced activated carbon mass flow [g d−1] |
| fcover | covered fraction of the reactor surface |
| FGi | mass flow of gas phase state variable i [g d−1] |
| fh,sat,eff | effective saturation depth fraction |
| fkL,i | fraction in the liquid side for the mass transfer of gas state variable i |
| FLi | mass flow of liquid phase state variable i [g d−1] |
| fwave | waviness factor |
| Gi | concentration of gas phase state variable i in off-gas, per liquid volume [g m−3] |
| Gi,air,inp | concentration of gas phase state variable i in the air input [%V V−1] |
| Gi,atm | concentration of gas phase state variable i in the atmosphere [%V V−1] |
| Gi,percent | concentration of gas phase state variable i in off-gas, percentage [%V V−1] |
| hdiff | diffuser submergence [m] |
| hdiff,floor | diffuser height from floor [m] |
| Henryi,dt | temperature dependency factor for Henry coefficient of gas i [K] |
| Henryi,SATP | Henry coefficient of gas i, standard (SATP) temperature (25 °C) [mol m−3 Pa−1] |
| hr | reactor depth [m] |
| hsat,eff | effective saturation depth [m] |
| hsea | elevation above sea level [m] |
| iC,i | equivalent mass of soluble organic state variable i per unit mass of carbon [g gC−1] |
| kL,i,bub,st,cw | liquid-side mass transfer coefficient for gas bubbles, standard conditions [m d−1] |
| kL,i,sur,st,cw | liquid-side mass transfer coefficient for liquid surface, standard conditions [m d−1] |
| kLai,bub | volumetric mass transfer coefficient for gas bubbles, field conditions [d−1] |
| kLai,bub,st,cw | volumetric mass transfer coefficient for gas bubbles, standard conditions [d−1] |
| kLai,sur | volumetric mass transfer coefficient for liquid surface, field conditions [d−1] |
| kLai,sur,st,cw | volumetric mass transfer coefficient for liquid surface, standard conditions [d−1] |
| Lair | temperature lapse rate for air pressure calculation [K m−1] |
| Li | concentration of liquid phase state variable i [g m−3] |
| Li,ad | adsorbed soluble organic state variable i mass per bed volume [g m−3] |
| Mac,cycle | mass of activated carbon filled per cycle [g] |
| magnmid,asymm | magnitude of the breakthrough curve midpoint asymmetry correction term |
| MMair | molar mass of air [g mol−1] |
| MMEQ,i | equivalent molar mass of gas phase state variable i [g mol−1] |
| ndiff | number of diffusers |
| ngas,bub | molar quantity of gas bubbles per unit liquid volume [mol m−3] |
| Nrepl | activated carbon bed replacement cycle frequency [d−1] |
| pair | air pressure at field elevation [Pa] |
| pgas | gas phase pressure [Pa] |
| pNTP | pressure at standard (NTP) conditions (101,325 Pa) [Pa] |
| powd,diff | power value in a diffuser density correction term |
| powh,diff | power value in a diffuser submergence correction term |
| powmid,asymm | power of the breakthrough curve midpoint asymmetry correction term |
| ppartial,i,bub | partial pressure of gas state variable i in the gas phase [Pa] |
| ppartial,i,bub,st | partial pressure of gas state variable i in the gas phase, standard conditions [Pa] |
| ppartial,i,sur | partial pressure of gas state variable i in the atmosphere [Pa] |
| ppartial,i,sur,st | partial pressure of gas state variable i in the atmosphere, standard conditions [Pa] |
| pst,h,sat,eff | pressure at standard conditions and effective saturation depth [Pa] |
| pv,T | saturated vapor pressure of water at temperature T [Pa] |
| θ | Arrhenius temperature correction factor for the mass transfer coefficient |
| Q | volumetric flow of wastewater [m3 d−1] |
| Qair,NTP | air flow at standard (NTP) conditions [m3gas d−1] |
| Qair,NTP,sp | air flow per diffuser at standard (NTP) conditions [m3gas d−1] |
| Qgas,transfer,NTP | gas transfer flow at standard (NTP) conditions [m3gas d−1] |
| Qgas,outp,NTP | off-gas flow at standard (NTP) conditions [m3gas d−1] |
| ρac | apparent density of granular activated carbon [gAC m−3] |
| rateFi | mass rate of state variable i [g d−1] |
| ratei | reaction rate for the state variable [g m−3 d−1] |
| RemGAC,i | removal ratio of soluble organic state variable i by granular activated carbon |
| rj | process rate regarding process j (from Gujer matrix) [g m−3 d−1] |
| Si,bub,sat | saturation concentration at the gas bubble interface [g m−3] |
| Si,bub,sat,st,cw | saturation concentration at the gas bubble interface, standard conditions [g m−3] |
| Si,sur,sat | saturation concentration at the atmospheric interface [g m−3] |
| Si,sur,sat,st,cw | saturation concentration at the atmospheric interface, standard conditions [g m−3] |
| slbreak | slope of the breakthrough curve [m3 gC−1] |
| SO2 | dissolved oxygen concentration [gO2 m−3] |
| SOTRbub | standard oxygen transfer rate from bubbles [g d−1] |
| SSOTE | specific standard oxygen transfer efficiency [% m−1] |
| SSOTE0 | intercept in SSOTE correlation [% m−1] |
| SSOTEasym | asymptote in SSOTE correlation [% m−1] |
| T | liquid temperature [°C] |
| Tair,K | field air temperature [K] |
| TK | liquid temperature in an SI unit [K] |
| TNTP,K | temperature at standard (NTP) conditions (20 °C) [K] |
| trepl | duration of activated carbon bed replacement [d] |
| TSATP,K | temperature at standard (SATP) conditions (25 °C) [K] |
| Vac | activated carbon bed volume [m3] |
| Vgas | gas phase volume [m3gas] |
| Vgas,NTP | gas phase volume at standard (NTP) conditions [m3gas] |
| vj,i | stoichiometric coefficient of state variable i in process j |
| Vr | reactive volume [m3] |
Appendix A. Gujer Matrix Development
| Symbol | Process Name |
|---|---|
| 1 | OHO growth on VFAs, O2 |
| 2 | OHO growth on VFAs, NOx |
| 3 | OHO growth on benzene, O2 |
| 4 | OHO growth on benzene, NOx |
| 5 | OHO growth on toluene, O2 |
| 6 | OHO growth on toluene, NOx |
| 7 | OHO growth on ethylbenzene, O2 |
| 8 | OHO growth on ethylbenzene, NOx |
| 9 | OHO growth on xylene, O2 |
| 10 | OHO growth on xylene, NOx |
| 11 | OHO growth on SB, O2 |
| 12 | OHO growth on SB, NOx |
| 13 | SB fermentation with high VFA (OHO growth, anaerobic) |
| 14 | SB fermentation with low VFA (OHO growth, anaerobic) |
| 15 | Benzene fermentation with low VFA (OHO growth, anaerobic) |
| 16 | Toluene fermentation with low VFA (OHO growth, anaerobic) |
| 17 | Ethylbenzene fermentation with low VFA (OHO growth, anaerobic) |
| 18 | Xylene fermentation with low VFA (OHO growth, anaerobic) |
| 19 | OHO decay |
| 20 | NITO growth |
| 21 | NITO decay |
| 22 | AMETO growth |
| 23 | AMETO decay |
| 24 | HMETO growth |
| 25 | HMETO decay |
| 26 | XB hydrolysis |
| 27 | XB anaerobic hydrolysis (fermentation) |
| 28 | SN,B ammonification |
| 29 | NOx assimilative reduction |
| 30 | FeP precipitation |
| 31 | FeP redissolution |
| 32 | AlP precipitation |
| 33 | AlP redissolution |
| 34 | Elimination of surfactants |
| 35 | Methane gas transfer—bubbles |
| 36 | Hydrogen gas transfer—bubbles |
| 37 | Oxygen gas transfer—bubbles |
| 38 | Nitrogen gas transfer—bubbles |
| 39 | Benzene gas transfer—bubbles |
| 40 | Toluene gas transfer—bubbles |
| 41 | Ethylbenzene gas transfer—bubbles |
| 42 | Xylene gas transfer—bubbles |
| 43 | Methane gas transfer—surface |
| 44 | Hydrogen gas transfer—surface |
| 45 | Oxygen gas transfer—surface |
| 46 | Nitrogen gas transfer—surface |
| 47 | Benzene gas transfer—surface |
| 48 | Toluene gas transfer—surface |
| 49 | Ethylbenzene gas transfer—surface |
| 50 | Xylene gas transfer—surface |
| SBENE | STENE | SEBENE | SXENE | SB | XB | SU | XU | XE | XOHO | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | |||||||||
| 2 | 1 | |||||||||
| 3 | −1/YOHO,BTEX,ox | 1 | ||||||||
| 4 | −1/YOHO,BTEX,anox | 1 | ||||||||
| 5 | −1/YOHO,BTEX,ox | 1 | ||||||||
| 6 | −1/YOHO,BTEX,anox | 1 | ||||||||
| 7 | −1/YOHO,BTEX,ox | 1 | ||||||||
| 8 | −1/YOHO,BTEX,anox | 1 | ||||||||
| 9 | −1/YOHO,BTEX,ox | 1 | ||||||||
| 10 | −1/YOHO,BTEX,anox | 1 | ||||||||
| 11 | −1/YOHO,SB,ox | 1 | ||||||||
| 12 | −1/YOHO,SB,anox | 1 | ||||||||
| 13 | −1/YOHO,SB,ana | 1 | ||||||||
| 14 | −1/YOHO,SB,ana | 1 | ||||||||
| 15 | −1/YOHO,BTEX,ana | 1 | ||||||||
| 16 | −1/YOHO,BTEX,ana | 1 | ||||||||
| 17 | −1/YOHO,BTEX,ana | 1 | ||||||||
| 18 | −1/YOHO,BTEX,ana | 1 | ||||||||
| 19 | 1 − fE | fE | −1 | |||||||
| 21 | 1 − fE | fE | ||||||||
| 23 | 1 − fE | fE | ||||||||
| 25 | 1 − fE | fE | ||||||||
| 26 | 1 | −1 | ||||||||
| 27 | 1 − fH2 | −1 | ||||||||
| 29 | −EEQNO3 × XOHO/XBIO,kin | |||||||||
| 39 | 1 | |||||||||
| 40 | 1 | |||||||||
| 41 | 1 | |||||||||
| 42 | 1 | |||||||||
| 47 | 1 | |||||||||
| 48 | 1 | |||||||||
| 49 | 1 | |||||||||
| 50 | 1 |
| SVFA | |
|---|---|
| 1 | −1/YOHO,VFA,ox |
| 2 | −1/YOHO,VFA,anox |
| 13 | (1 − YOHO,SB,ana − YOHO,H2,ana,high)/YOHO,SB,ana |
| 14 | (1 − YOHO,SB,ana − YOHO,H2,ana,low)/YOHO,SB,ana |
| 15 | (1 − YOHO,SB,ana − YOHO,H2,ana,low)/YOHO,SB,ana |
| 16 | (1 − YOHO,SB,ana − YOHO,H2,ana,low)/YOHO,SB,ana |
| 17 | (1 − YOHO,SB,ana − YOHO,H2,ana,low)/YOHO,SB,ana |
| 18 | (1 − YOHO,SB,ana − YOHO,H2,ana,low)/YOHO,SB,ana |
| 22 | −1/YAMETO |
| XNITO | XAMETO | XHMETO | |
|---|---|---|---|
| 20 | 1 | ||
| 21 | −1 | ||
| 22 | 1 | ||
| 23 | −1 | ||
| 24 | 1 | ||
| 25 | −1 | ||
| 29 | −EEQNO3 × XNITO/XBIO,kin | −EEQNO3 × XAMETO/XBIO,kin | −EEQNO3 × XHMETO/XBIO,kin |
| SNHx | SNOx | SN2 | |
|---|---|---|---|
| 1 | −iN,BIO | ||
| 2 | −iN,BIO | −(1 − YOHO,VFA,anox)/(EEQN2,NO3 × YOHO,VFA,anox) | (1 − YOHO,VFA,anox)/(EEQN2,NO3 × YOHO,VFA,anox) |
| 3 | −iN,BIO | ||
| 4 | −iN,BIO | −(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) | (1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) |
| 5 | −iN,BIO | ||
| 6 | −iN,BIO | −(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) | (1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) |
| 7 | −iN,BIO | ||
| 8 | −iN,BIO | −(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) | (1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) |
| 9 | −iN,BIO | ||
| 10 | −iN,BIO | −(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) | (1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) |
| 11 | −iN,BIO | ||
| 12 | −iN,BIO | −(1 − YOHO,SB,anox)/(EEQN2,NO3 × YOHO,SB,anox) | (1 − YOHO,SB,anox)/(EEQN2,NO3 × YOHO,SB,anox) |
| 13 | −iN,BIO | ||
| 14 | −iN,BIO | ||
| 15 | −iN,BIO | ||
| 16 | −iN,BIO | ||
| 17 | −iN,BIO | ||
| 18 | −iN,BIO | ||
| 19 | −fE × (iN,XE − iN,BIO) | ||
| 20 | −1/YNITO − iN,BIO | 1/YNITO | |
| 21 | −fE × (iN,XE − iN,BIO) | ||
| 22 | −iN,BIO | ||
| 23 | −fE × (iN,XE − iN,BIO) | ||
| 24 | −iN,BIO | ||
| 25 | −fE × (iN,XE − iN,BIO) | ||
| 28 | 1 | ||
| 29 | 1 + EEQNO3 × iN,BIO | −1 | |
| 38 | 1 | ||
| 46 | 1 |
| SN,B | XN,B | SPO4 | XP,B | SO2 | SCH4 | SH2 | |
|---|---|---|---|---|---|---|---|
| 1 | −iP,BIO | −(1 − YOHO,VFA,ox)/YOHO,VFA,ox | |||||
| 2 | −iP,BIO | ||||||
| 3 | −iP,BIO | −(1 − YOHO,BTEX,ox)/YOHO,BTEX,ox | |||||
| 4 | −iP,BIO | ||||||
| 5 | −iP,BIO | −(1 − YOHO,BTEX,ox)/YOHO,BTEX,ox | |||||
| 6 | −iP,BIO | ||||||
| 7 | −iP,BIO | −(1 − YOHO,BTEX,ox)/YOHO,BTEX,ox | |||||
| 8 | −iP,BIO | ||||||
| 9 | −iP,BIO | −(1 − YOHO,BTEX,ox)/YOHO,BTEX,ox | |||||
| 10 | −iP,BIO | ||||||
| 11 | −iP,BIO | −(1 − YOHO,SB,ox)/YOHO,SB,ox | |||||
| 12 | −iP,BIO | ||||||
| 13 | −iP,BIO | YOHO,H2,ana,high/YOHO,SB,ana | |||||
| 14 | −iP,BIO | YOHO,H2,ana,low/YOHO,SB,ana | |||||
| 15 | −iP,BIO | YOHO,H2,ana,low/YOHO,BTEX,ana | |||||
| 16 | −iP,BIO | YOHO,H2,ana,low/YOHO,BTEX,ana | |||||
| 17 | −iP,BIO | YOHO,H2,ana,low/YOHO,BTEX,ana | |||||
| 18 | −iP,BIO | YOHO,H2,ana,low/YOHO,BTEX,ana | |||||
| 19 | (1 − fE) × iN,BIO | (1 − fE) × iP,BIO | |||||
| 20 | −iP,BIO | −(EEQNO3 − YNITO)/YNITO | |||||
| 21 | (1 − fE) × iN,BIO | (1 − fE) × iP,BIO | |||||
| 22 | −iP,BIO | (1 − YAMETO)/YAMETO | |||||
| 23 | (1 − fE) × iN,BIO | (1 − fE) × iP,BIO | |||||
| 24 | −iP,BIO | (1 − YHMETO)/YHMETO | −1/YHMETO | ||||
| 25 | (1 − fE) × iN,BIO | (1 − fE) × iP,BIO | |||||
| 26 | XN,B/XB | −XN,B/XB | XP,B/XB | −XP,B/XB | |||
| 27 | XN,B/XB | −XN,B/XB | XP,B/XB | −XP,B/XB | fH2 | ||
| 28 | −1 | ||||||
| 29 | EEQNO3 × iP,BIO | ||||||
| 30 | −fP,Fe | ||||||
| 31 | fP,Fe | ||||||
| 32 | −fP,Al | ||||||
| 33 | fP,Al | ||||||
| 35 | 1 | ||||||
| 36 | 1 | ||||||
| 37 | 1 | ||||||
| 43 | 1 | ||||||
| 44 | 1 | ||||||
| 45 | 1 |
| SALK | XFeOH | XFeP | XAlOH | XAlP | |
|---|---|---|---|---|---|
| 1 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 2 | (−(1 − YOHO,VFA,anox)/(EEQN2,NO3 × YOHO,VFA,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 3 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 4 | (−(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 5 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 6 | (−(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 7 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 8 | (−(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 9 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 10 | (−(1 − YOHO,BTEX,anox)/(EEQN2,NO3 × YOHO,BTEX,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 11 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 12 | (−(1 − YOHO,SB,anox)/(EEQN2,NO3 × YOHO,SB,anox) × CHNO3 − iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 13 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 14 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 15 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 16 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 17 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 18 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 19 | −fE × (iN,XE − iN,BIO) × CHNHx | ||||
| 20 | ((−1/YNITO − iN,BIO) × CHNHx + 1/YNITO × CHNO3 − iP,BIO × CHPO4) | ||||
| 21 | −fE × (iN,XE − iN,BIO) × CHNHx | ||||
| 22 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 23 | −fE × (iN,XE − iN,BIO) × CHNHx | ||||
| 24 | (−iN,BIO × CHNHx − iP,BIO × CHPO4) | ||||
| 25 | −fE × (iN,XE − iN,BIO) × CHNHx | ||||
| 26 | XP,B/XB × CHPO4 | ||||
| 27 | XP,B/XB × CHPO4 | ||||
| 28 | CHNHx | ||||
| 29 | ((1 + EEQNO3 × iN,BIO) × CHNHx − CHNO3 + EEQNO3 × iP,BIO × CHPO4) | ||||
| 30 | −fP,Fe × CHPO4 | −1 | 1 | ||
| 31 | fP,Fe × CHPO4 | 1 | −1 | ||
| 32 | −fP,Al × CHPO4 | −1 | 1 | ||
| 33 | fP,Al × CHPO4 | 1 | −1 |
| SALPHA | GCH4 | GH2 | GO2 | GN2 | GBENE | GTENE | GEBENE | GXENE | |
|---|---|---|---|---|---|---|---|---|---|
| 34 | 1 | ||||||||
| 35 | −1 | ||||||||
| 36 | −1 | ||||||||
| 37 | −1 | ||||||||
| 38 | −1 | ||||||||
| 39 | −1 | ||||||||
| 40 | −1 | ||||||||
| 41 | −1 | ||||||||
| 42 | −1 |
| Rate | |
|---|---|
| 1 | µOHO,T × XOHO × MsatSVFA,KVFA × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 2 | µOHO,T × XOHO × ηOHO,anox × MsatSVFA,KVFA × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 3 | µOHO,BENE,T × XOHO × MsatSBENE,KBENE × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 4 | µOHO,BENE,T × XOHO × ηOHO,anox × MsatSBENE,KBENE × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 5 | µOHO,TENE,T × XOHO × MsatSTENE,KTENE × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 6 | µOHO,TENE,T × XOHO × ηOHO,anox × MsatSTENE,KTENE × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 7 | µOHO,EBENE,T × XOHO × MsatSEBENE,KEBENE × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 8 | µOHO,EBENE,T × XOHO × ηOHO,anox × MsatSEBENE,KEBENE × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 9 | µOHO,XENE,T × XOHO × MsatSXENE,KXENE × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 10 | µOHO,XENE,T × XOHO × ηOHO,anox × MsatSXENE,KXENE × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 11 | µOHO,T × MsatSB,KSB × MinhSVFA,KVFA × XOHO × MsatSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 12 | µOHO,T × ηOHO,anox × MsatSB,KSB × MinhSVFA,KVFA × XOHO × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO |
| 13 | µFERM,OHO,T × XOHO × LogsatSVFA,KVFA,FERM × MsatSB,KSB,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 14 | µFERM,OHO,T × XOHO × LoginhSVFA,KVFA,FERM × MsatSB,KSB,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 15 | µFERM,OHO,BENE,T × XOHO × LoginhSVFA,KVFA,FERM × MsatSBENE,KBENE,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 16 | µFERM,OHO,TENE,T × XOHO × LoginhSVFA,KVFA,FERM × MsatSTENE,KTENE,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 17 | µFERM,OHO,EBENE,T × XOHO × LoginhSVFA,KVFA,FERM × MsatSEBENE,KEBENE,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 18 | µFERM,OHO,XENE,T × XOHO × LoginhSVFA,KVFA,FERM × MsatSXENE,KXENE,ana × MinhSO2,KO2,OHO × MinhSNOx,KNOx,OHO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 19 | bOHO,T × XOHO × (MsatSO2,KO2,OHO + ηb,anox × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO + ηb,ana × MinhSNOx,KNOx,OHO × MinhSO2,KO2,OHO) |
| 20 | µNITO,T × MsatSNHx,KNHx,NITO × XNITO × MsatSO2,KO2,NITO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 21 | bNITO,T × XNITO × (MsatSO2,KO2,NITO + ηb,anox × MsatSNOx,KNOx,NITO × MinhSO2,KO2,NITO + ηb,ana × MinhSNOx,KNOx,NITO × MinhSO2,KO2,NITO + mtox,ana) |
| 22 | µAMETO,T × HsatSVFA,AMETO × XAMETO × MinhSO2,KiO2,AMETO × MinhSNOx,KNOx,AMETO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 23 | bAMETO,T × XAMETO × (MsatSO2,KiO2,AMETO + ηb,anox × MsatSNOx,KNOx,AMETO × MinhSO2,KiO2,AMETO + ηb,ana × MinhSNOx,KNOx,AMETO × MinhSO2,KiO2,AMETO) |
| 24 | µHMETO,T × MsatSH2,KH2,HMETO × XHMETO × MinhSO2,KiO2,HMETO × MinhSNOx,KNOx,HMETO × MsatSNHx,KNHx,BIO × MsatSPO4,KPO4,BIO × MsatSALK,KALK |
| 25 | bHMETO,T × XHMETO × (MsatSO2,KiO2,HMETO + ηb,anox × MsatSNOx,KNOx,HMETO × MinhSO2,KiO2,HMETO + ηb,ana × MinhSNOx,KNOx,HMETO × MinhSO2,KiO2,HMETO) |
| 26 | qHYD,T × XBIO,kin × MRsatXB,XBIO,kin,KHYD × (MsatSO2,KO2,OHO + ηb,anox × MsatSNOx,KNOx,OHO × MinhSO2,KO2,OHO) × MsatSALK,KALK |
| 27 | qHYD,T × XBIO,kin × MRsatXB,XBIO,kin,KHYD × ηb,ana × MinhSNOx,KNOx,OHO × MinhSO2,KO2,OHO × MsatSALK,KALK |
| 28 | qAMMON,T × SN,B × XBIO,kin |
| 29 | qASSIM,T × MsatSNOx,KNOx,ASSIM × MinhSNHx,KiNHx,ASSIM × XBIO,kin |
| 30 | qFeOH,PREC,Me × SPO4 × XFeOH |
| 31 | qFeOH,DISSOL,Me × XFeP × MsatSALK,KALK |
| 32 | qAlOH,PREC,Me × SPO4 × XAlOH |
| 33 | qAlOH,DISSOL,Me × XAlP × MsatSALK,KALK |
| 34 | qALPHA,O2 × XVSS × dampALPHA × (SALPHA,sat — SALPHA) |
| 35 | kLaGCH4,bub × (SGCH4,bub,sat — SCH4) |
| 36 | kLaGH2,bub × (SGH2,bub,sat — SH2) |
| 37 | kLaGO2,bub × (SGO2,bub,sat — SO2) |
| 38 | kLaGN2,bub × (SGN2,bub,sat — SN2) |
| 39 | kLaGBENE,bub × (SGBENE,bub,sat — SBENE) |
| 40 | kLaGTENE,bub × (SGTENE,bub,sat — STENE) |
| 41 | kLaGEBENE,bub × (SGEBENE,bub,sat — SEBENE) |
| 42 | kLaGXENE,bub × (SGXENE,bub,sat — SXENE) |
| 43 | kLaGCH4,sur × (SGCH4,sur,sat — SCH4) |
| 44 | kLaGH2,sur × (SGH2,sur,sat — SH2) |
| 45 | kLaGO2,sur × (SGO2,sur,sat — SO2) |
| 46 | kLaGN2,sur × (SGN2,sur,sat — SN2) |
| 47 | kLaGBENE,sur × (SGBENE,sur,sat — SBENE) |
| 48 | kLaGTENE,sur × (SGTENE,sur,sat — STENE) |
| 49 | kLaGEBENE,sur × (SGEBENE,sur,sat — SEBENE) |
| 50 | kLaGXENE,sur × (SGXENE,sur,sat — SXENE) |
| Symbol | Name | Expression |
|---|---|---|
| Msat(var; k) | Monod saturation | var/(k + var) |
| Minh(var; k) | Monod inhibition | k/(k + var) |
| MRsat(s;x;k) | Monod ratio saturation | (s/x)/(s/x + k) |
| Logsat(var; halfval; slope) | Logistic saturation | 1/(1 + Exp((halfval − var) × slope)) |
| Loginh(var; halfval; slope) | Logistic inhibition | 1/(1 + Exp((var − halfval) × slope)) |
| Hsat(var; halfval; halfinh) | Haldane equation | var/(halfval + var + (var2/halfinh)) |
Appendix B. BTEX Kinetic and Stoichiometric Model Parameters
| Ordinary Heterotrophic Organism Kinetics (OHO) | |||
|---|---|---|---|
| Symbol | Name | Value | Unit |
| µOHO,BENE | Maximum specific growth rate of OHOs on benzene | 0.006 | d−1 |
| µOHO,TENE | Maximum specific growth rate of OHOs on toluene | 0.014 | d−1 |
| µOHO,EBENE | Maximum specific growth rate of OHOs on ethylbenzene | 0.014 | d−1 |
| µOHO,XENE | Maximum specific growth rate of OHOs on xylene | 0.010 | d−1 |
| µFERM,OHO,BENE | Fermentation growth rate of OHOs on benzene | 0.0030 | d−1 |
| µFERM,OHO,TENE | Fermentation growth rate of OHOs on toluene | 0.0042 | d−1 |
| µFERM,OHO,EBENE | Fermentation growth rate of OHOs on ethylbenzene | 0.0035 | d−1 |
| µFERM,OHO,XENE | Fermentation growth rate of OHOs on xylene | 0.0050 | d−1 |
| KBENE | Half-saturation of benzene for OHOs | 6.8 | gCOD m−3 |
| KTENE | Half-saturation of toluene for OHOs | 14.8 | gCOD m−3 |
| KEBENE | Half-saturation of ethylbenzene for OHOs | 3.8 | gCOD m−3 |
| KXENE | Half-saturation of xylene for OHOs | 17.6 | gCOD m−3 |
| KBENE,ana | Half-saturation of benzene in fermentation by OHOs | 238 | gCOD m−3 |
| KTENE,ana | Half-saturation of toluene in fermentation by OHOs | 310 | gCOD m−3 |
| KEBENE,ana | Half-saturation of ethylbenzene in fermentation by OHOs | 67 | gCOD m−3 |
| KXENE,ana | Half-saturation of xylene in fermentation by OHOs | 615 | gCOD m−3 |
| Stoichiometric yields | |||
| Symbol | Name | Value | Unit |
| YOHO,BTEX,ox | Yield of OHOs on BTEX under aerobic conditions | 0.55 | g XOHO g SBTEX−1 |
| YOHO,BTEX,anox | Yield of OHOs on BTEX under anoxic conditions | 0.35 | g XOHO g SBTEX−1 |
| YOHO,BTEX,ana | Yield of OHOs on BTEX under anaerobic conditions | 0.10 | g XOHO g SBTEX−1 |
Appendix C. Gas Transfer, Aeration and BTEX Model Parameters
| Henry Coefficients | |||
|---|---|---|---|
| Symbol | Name | Value | Unit |
| HenryBENE,25 | Henry coefficient for benzene at 25 °C | 1.70 × 10−3 | mol m−3 Pa−1 |
| HenryBENE,dt | Henry’s law temperature dependency factor of benzene | 4150 | K |
| HenryTENE,25 | Henry coefficient for toluene at 25 °C | 1.50 × 10−3 | mol m−3 Pa−1 |
| HenryTENE,dt | Henry’s law temperature dependency factor of toluene | 4150 | K |
| HenryEBENE,25 | Henry coefficient for ethylbenzene at 25 °C | 1.27 × 10−3 | mol m−3 Pa−1 |
| HenryEBENE,dt | Henry’s law temperature dependency factor of ethylbenzene | 5100 | K |
| HenryXENE,25 | Henry coefficient for xylene at 25 °C | 1.56 × 10−3 | mol m−3 Pa−1 |
| HenryXENE,dt | Henry’s law temperature dependency factor of xylene | 4083 | K |
| Diffusion coefficients | |||
| Symbol | Name | Value | Unit |
| DBENE,25 | Diffusion coefficient of benzene in water at 25 °C | 9.13 × 10−5 | m2 d−1 |
| DTENE,25 | Diffusion coefficient of toluene in water at 25 °C | 7.89 × 10−5 | m2 d−1 |
| DEBENE,25 | Diffusion coefficient of ethylbenzene in water at 25 °C | 7.27 × 10−5 | m2 d−1 |
| DXENE,25 | Diffusion coefficient of xylene in water at 25 °C | 7.08 × 10−5 | m2 d−1 |
| Oxygen transfer efficiency correlation parameters | |||
| Symbol | Name | Value | Unit |
| SSOTE0 | Intercept in SSOTE correlation | 7.77 | % m−1 |
| expSSOTE | Exponent (absolute value) in SSOTE correlation | 0.01041 | d m−3gas |
| SSOTEasym | Asymptote in SSOTE correlation | 5.75 | % m−1 |
| divd,diff | Divisor value in a diffuser density correction term | 0.1173 | m2 m−2 |
| powd,diff | Power value in a diffuser density correction term | 0.1329 | |
| coefflead,h,diff | Leading coefficient in a diffuser submergence correction term | 0.011 | m−1 |
| powh,diff | Power value in a diffuser submergence correction term | 1.6031 | |
| coefflin,h,diff | Linear coefficient in a diffuser submergence correction term | −0.0229 | m−1 |
| Specific molecular masses | |||
| Symbol | Name | Value | Unit |
| MMEQ,GBENE | Equivalent molar mass of benzene | 239.97 | gCOD mol−1 |
| MMEQ,GTENE | Equivalent molar mass of toluene | 287.96 | gCOD mol−1 |
| MMEQ,GEBENE | Equivalent molar mass of ethylbenzene | 335.95 | gCOD mol−1 |
| MMEQ,GXENE | Equivalent molar mass of xylene | 335.95 | gCOD mol−1 |
Appendix D. GAC Model Parameters
| State Variable Equivalent Mass Ratios to Carbon | |||
|---|---|---|---|
| Symbol | Name | Value | Unit |
| iC,VFA | COD-to-carbon-mass ratio of VFA | 5.33 | gCOD gC−1 |
| iC,BENE | COD-to-carbon-mass ratio of benzene | 19.98 | gCOD gC−1 |
| iC,TENE | COD-to-carbon-mass ratio of toluene | 23.98 | gCOD gC−1 |
| iC,EBENE | COD-to-carbon-mass ratio of ethylbenzene | 27.97 | gCOD gC−1 |
| iC,XENE | COD-to-carbon-mass ratio of xylene | 27.97 | gCOD gC−1 |
| iC,SB | COD-to-carbon-mass ratio of readily biodegradable substrate | 3.20 | gCOD gC−1 |
| iC,SU | COD-to-carbon-mass ratio of soluble unbiodegradable organics | 2.80 | gCOD gC−1 |
| iC,SN,B | Nitrogen-to-carbon-mass ratio of soluble biodegradable organic N | 1.17 | gN gC−1 |
| Breakthrough curve parameters | |||
| Symbol | Name | Value | Unit |
| fbreak | Breakpoint fraction | 0.05 | |
| slbreak | Breakthrough curve slope | 0.00015 | m3 g−1 |
| powmid,asymm | Power of the midpoint asymmetry correction term | 20.00 | |
| magnmid,asymm | Magnitude of the midpoint asymmetry correction term | 0.50 | |
References
- Scheierling, S.M.; Bartone, C.; Mara, D.D.; Drechsel, P. Improving Wastewater Use in Agriculture: An Emerging Priority; World Bank Policy Research Working Paper Series 5412; The World Bank: Washington, DC, USA, 2010. [Google Scholar] [CrossRef]
- Scheierling, S.M.; Bartone, C.R.; Mara, D.D.; Drechsel, P. Towards an agenda for improving wastewater use in agriculture. Water Int. 2011, 36, 420–440. [Google Scholar] [CrossRef]
- Ofori, S.; Puškáčová, A.; Růžičková, I.; Wanner, J. Treated wastewater reuse for irrigation: Pros and cons. Sci. Total Environ. 2021, 760, 144026. [Google Scholar] [CrossRef] [PubMed]
- Pedrero, F.; Kalavrouziotis, I.; Alarcón, J.J.; Koukoulakis, P.; Asano, T. Use of treated municipal wastewater in irrigated agriculture—Review of some practices in Spain and Greece. Agric. Water Manag. 2010, 97, 1233–1241. [Google Scholar] [CrossRef]
- Jaramillo, M.F.; Restrepo, I. Wastewater Reuse in Agriculture: A Review about Its Limitations and Benefits. Sustainability 2017, 9, 1734. [Google Scholar] [CrossRef]
- Khalil, S.; Kakar, M.K. Agricultural use of untreated urban wastewater in Pakistan. Asian J. Agric. Rural Dev. 2011, 1, 21–26. [Google Scholar]
- Sheikholeslami, Z.; Kebria, D.Y.; Qaderi, F. Nanoparticle for degradation of BTEX in produced water; an experimental procedure. J. Mol. Liq. 2018, 264, 476–482. [Google Scholar] [CrossRef]
- Dehghani, M.; Abbasi, A.; Taherzadeh, Z.; Dehghani, S. Exposure assessment of wastewater treatment plant employees to BTEX: A biological monitoring approach. Sci. Rep. 2022, 12, 21433. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef]
- Dhivakar, V.; Rajan, T. BTEX compounds removal from wastewater by using UV&UV/H2O2 process. Int. J. Recent Eng. Sci. 2018, 5, 22–25. [Google Scholar] [CrossRef]
- Elmobarak, W.F.; Hameed, B.H.; Almomani, F.; Abdullah, A.Z. A Review on the Treatment of Petroleum Refinery Wastewater Using Advanced Oxidation Processes. Catalysts 2021, 11, 782. [Google Scholar] [CrossRef]
- Mello, J.M.M.; Brandao, H.L.; Valerio, A.; de Souza, A.A.U.; de Oliveira, D.; da Silva, A. Biodegradation of BTEX compounds from petrochemical wastewater: Kinetic and toxicity. J. Water Process Eng. 2019, 32, 100914. [Google Scholar] [CrossRef]
- Gusmão, V.R.; Martins, T.H.; Chinalia, F.A.; Sakamoto, I.K.; HenriqueThiemann, O.; Varesche, M.B.A. BTEX and ethanol removal in horizontal-flow anaerobic immobilized biomass reactor, under denitrifying condition. Process Biochem. 2006, 41, 1391–1400. [Google Scholar] [CrossRef]
- Kuyukina, M.S.; Krivoruchko, A.V.; Ivshina, I.B. Advanced Bioreactor Treatments of Hydrocarbon-Containing Wastewater. Appl. Sci. 2020, 10, 831. [Google Scholar] [CrossRef]
- Trusek-Holownia, A.; Noworyta, A. Advanced treatment of wastewater with BTEX. Desalination Water Treat. 2012, 50, 440–445. [Google Scholar] [CrossRef]
- Takáčová, A.; Smolinská, M.; Semerád, M.; Matúš, P. Degradation of BTEX by microalgae Parachlorella kessleri. Pet. Coal 2015, 57, 2. [Google Scholar]
- Anjum, H.; Johari, K.; Gnanasundaram, N.; Ganesapillai, M.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. A review on adsorptive removal of oil pollutants (BTEX) from wastewater using carbon nanotubes. J. Mol. Liq. 2019, 277, 1005–1025. [Google Scholar] [CrossRef]
- El-Naas, M.H.; Acio, J.A.; El Telib, A.E. Aerobic biodegradation of BTEX: Progresses and prospects. J. Environ. Chem. Eng. 2014, 2, 1104–1122. [Google Scholar] [CrossRef]
- Carvajal, A.; Akmirza, I.; Navia, D.; Pérez, R.; Muñoz, R.; Lebrero, R. Anoxic denitrification of BTEX: Biodegradation kinetics and pollutant interactions. J. Environ. Manag. 2018, 214, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Kasi, M.; Wadhawan, T.; McEvoy, J.; Padmanabhan, G.; Khan, E. Effect of Carbon Source during Enrichment on BTEX Degradation by Anaerobic Mixed Bacterial Cultures. Biodegradation 2013, 24, 279–293. [Google Scholar] [CrossRef] [PubMed]
- de Nardi, I.R.; Zaiat, M.; Foresti, E. Kinetics of BTEX degradation in a packed-bed anaerobic reactor. Biodegradation 2007, 18, 83. [Google Scholar] [CrossRef]
- Lee, K.C.; Rittmann, B.E.; Shi, J.; McAvoy, D. Advanced steady-state model for the fate of hydrophobic and volatile compounds in activated sludge. Water Environ. Res. 1998, 70, 1118–1131. [Google Scholar] [CrossRef]
- Pomiès, M.; Wisniewski, C.; Choubert, J.M.; Coquery, M. Modelling of micropollutant removal in biological wastewater treatments: A review. Sci. Total Environ. 2012, 443, 733–748. [Google Scholar] [CrossRef]
- Orhon, D.; Çokgör, E.U. COD fractionation in wastewater characterization—The state of the art. J. Chem. Technol. Biotechnol. 1997, 68, 283–293. [Google Scholar] [CrossRef]
- Patry, G.G.; Takács, I. Settling of flocculent suspensions in secondary clarifiers. Water Res. 1992, 26, 473–479. [Google Scholar] [CrossRef]
- Henze, M.; Gujer, W.; Mino, T.; Matsuo, T.; Wentzel, M.C.; Marais, G.V.R.; Van Loosdrecht, M.C. Activated sludge model no. 2d, ASM2d. Water Sci. Technol. 1999, 39, 165–182. [Google Scholar] [CrossRef]
- Arnell, M.; Ahlström, M.; Wärff, C.; Saagi, R.; Jeppsson, U. Plant-Wide Modelling and Analysis of WWTP Temperature Dynamics for Sustainable Heat Recovery from Wastewater. Water Sci. Technol. 2021, 84, 1023–1036. [Google Scholar] [CrossRef]
- Rieger, L.; Gillot, S.; Langergraber, G.; Ohtsuki, T.; Shaw, A.; Takács, I. Guidelines for Using Activated Sludge Models; IWA Publishing: London, UK, 2012; ISBN 978-1-84339-174-6. [Google Scholar]
- Henze, M.; Grady, L., Jr.; Gujer, W.; Marais, G.; Matsuo, T. Activated Sludge Model No 1; IAWPRC Publishing: London, UK, 1987. [Google Scholar]
- Dynamita. Sumo22 User Manual; Dynamita SARL: Sigale, France, 2022. [Google Scholar]
- Gazsó, Z.; Házi, F.; Kenyeres, I.; Váci, L. Full-Scale Wastewater Treatment Plant Simulation for Real-Time Optimization. Water Pract. Technol. 2017, 12, 848–856. [Google Scholar] [CrossRef]
- Herrmann-Heber, R.; Reinecke, S.F.; Hampel, U. Dynamic Aeration for Improved Oxygen Mass Transfer in the Wastewater Treatment Process. Chem. Eng. J. 2019, 386, 122068. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Design Manual: Fine Pore Aeration Systems; EPA/625/1-89/023; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1989. [Google Scholar]
- Sincero, A.P.; Sincero, G.A. Physical-Chemical Treatment of Water and Wastewater, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; ISBN 978-1-58716-124-7. [Google Scholar]
- Stenstrom, M.K.; Leu, S.-Y.; Jiang, P. Theory to Practice: Oxygen Transfer and the New ASCE Standard. Proc. Water Environ. Fed. 2006, 7, 4838–4852. [Google Scholar] [CrossRef]
- DelSontro, T.; McGinnis, D.; Wehrli, B.; Ostrovsky, I. Size Does Matter: Importance of Large Bubbles and Small-Scale Hot Spots for Methane Transport. Environ. Sci. Technol. 2015, 49, 1268–1276. [Google Scholar] [CrossRef]
- Jensen, M.B.; Kofoed, M.V.W.; Fischer, K.; Voigt, N.V.; Agneessens, L.M.; Batstone, D.J.; Ottosen, L.D.M. Venturi-Type Injection System as a Potential H2 Mass Transfer Technology for Full-Scale in Situ Biomethanation. Appl. Energy 2018, 222, 840–846. [Google Scholar] [CrossRef]
- Roberts, P.V.; Munz, C.; Dändliker, P. Modeling Volatile Organic Solute Removal by Surface and Bubble Aeration. J. Water Pollut. Control Fed. 1984, 56, 157–163. [Google Scholar]
- Khalil, A.; Rosso, D.; DeGroot, C.T. Effects of Flow Velocity and Bubble Size Distribution on Oxygen Mass Transfer in Bubble Column Reactors—A Critical Evaluation of the Computational Fluid Dynamics-Population Balance Model. Water Environ. Res. 2021, 93, 2274–2297. [Google Scholar] [CrossRef] [PubMed]
- Hayduk, W.; Laudie, H. Prediction of Diffusion Coefficients for Nonelectrolytes in Dilute Aqueous Solutions. AIChE J. 1974, 20, 611–615. [Google Scholar] [CrossRef]
- Yaws, C.L. Handbook of Transport Property Data: Viscosity, Thermal Conductivity, and Diffusion Coefficients of Liquids and Gases; Library of Physico-Chemical Property Data; Gulf Publishing Company: Houston, TX, USA, 1995; ISBN 978-0-88415-392-4. [Google Scholar]
- New Jersey Department of Environmental Protection. Chemical Properties for Calculation of Impact to Ground Water Soil Remediation Standards; New Jersey Department of Environmental Protection: Trenton, NJ, USA, 2008. [Google Scholar] [CrossRef]
- Batstone, D.; Flores-Alsina, X. Generalised Physicochemical Model (PCM) for Wastewater Processes; IWA Publishing: London, UK, 2022; ISBN 978-1-78040-982-5. [Google Scholar]
- Bencsik, D.; Takács, I.; Rosso, D. Dynamic Alpha Factors: Prediction in Time and Evolution along Reactors. Water Res. 2022, 216, 118339. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency. Summary Report: Fine Pore (Fine Bubble) Aeration Systems; EPA/625/8-85/010; U.S. Environmental Protection Agency: Cincinnati, OH, USA, 1989. [Google Scholar]
- Jiang, L.-M.; Chen, L.; Zhou, Z.; Sun, D.; Li, Y.; Zhang, M.; Liu, Y.; Du, S.; Chen, G.; Yao, J. Fouling Characterization and Aeration Performance Recovery of Fine-Pore Diffusers Operated for 10 Years in a Full-Scale Wastewater Treatment Plant. Bioresour. Technol. 2020, 307, 123197. [Google Scholar] [CrossRef]
- Bencsik, D.; Wadhawan, T.; Takács, I.; Bott, C.; Rosso, D. Improved Aeration Modelling Using Innovative Concepts for Prediction of Key Factors in Oxygen Transfer. In Proceedings of the Innovations in Process Engineering 2021, Virtual, 9–10 and 15–16 June 2022; Water Environment Federation: Alexandria, VA, USA, 2021. [Google Scholar]
- Morgan, P.F.; Bewtra, J.K. Air diffuser efficiencies. J. Water Pollut. Control Fed. 1960, 32, 1047–1059. [Google Scholar]
- Eckenfelder, W.W. Factors affecting the aeration efficiency of sewage and industrial wastes. Sew. Ind. Wastes 1959, 31, 60–70. [Google Scholar]
- Wagner, M.R.; Pöpel, H.J. Oxygen transfer and aeration efficiency—Influence of diffuser submergence, diffuser density, and blower type. Water Sci. Technol. 1998, 38, 1–6. [Google Scholar] [CrossRef]
- Pöpel, H.J.; Wagner, M.R. Modelling of Oxygen Transfer in Deep Diffused-Aeration Tanks and Comparison with Full-Scale Plant Data. Water Sci. Technol. 1994, 30, 71–80. [Google Scholar] [CrossRef]
- Rosso, D.; Iranpour, R.; Stenstrom, M.K. Fifteen Years of Offgas Transfer Efficiency Measurements on Fine-Pore Aerators: Key Role of Sludge Age and Normalized Air Flux. Water Environ. Res. 2005, 77, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Plósz, B.G.; Jobbágy, A.; Grady, C.P.L. Factors Influencing Deterioration of Denitrification by Oxygen Entering an Anoxic Reactor through the Surface. Water Res. 2003, 37, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef]
- Mackay, D.; Shiu, W.Y. A Critical Review of Henry’s Law Constants for Chemicals of Environmental Interest. J. Phys. Chem. Ref. Data 1981, 10, 1175–1199. [Google Scholar] [CrossRef]
- Staudinger, J.; Roberts, P.V. A Critical Review of Henry’s Law Constants for Environmental Applications. Crit. Rev. Environ. Sci. Technol. 1996, 26, 205–297. [Google Scholar] [CrossRef]
- Staudinger, J.; Roberts, P.V. A Critical Compilation of Henry’s Law Constant Temperature Dependence Relations for Organic Compounds in Dilute Aqueous Solutions. Chemosphere 2001, 44, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Water Environment Federation. Design of Municipal Wastewater Treatment Plants MOP 8, 5th ed.; McGraw-Hill Education: Alexandria, VA, USA, 2009; ISBN 978-0-07-166358-8. [Google Scholar]
- Cecen, F.; Aktas, Ö. Activated Carbon for Water and Wastewater Treatment: Integration of Adsorption and Biological Treatment; WILEY-VCH: Weinheim, Germany, 2011; ISBN 978-3-527-63945-8. [Google Scholar]
- Benedek, P.; Major, V.; Takács, I. Mathematical Model Suggested for a Carbon-Activated Sludge System. Water Res. 1985, 19, 407–413. [Google Scholar] [CrossRef]
- Sun, Y.-X.; Yang, Z.; Ye, T.; Shi, N.; Tian, Y. Evaluation of the Treatment of Reverse Osmosis Concentrates from Municipal Wastewater Reclamation by Coagulation and Granular Activated Carbon Adsorption. Environ. Sci. Pollut. Res. 2016, 23, 13543–13553. [Google Scholar] [CrossRef] [PubMed]
- Vahala, R.; Rintala, J.; Järvinen, A. Removal of TOC by Two-Step GAC Filtration in Cold Humic Waters. In Proceedings of the NOM Workshop, Poitiers, France, 18 September 1996. [Google Scholar]
- Alex, J.; Benedetti, L.; Copp, J.; Gernaey, K.; Jeppsson, U.; Nopens, I.; Pons, M.; Rieger, L.; Rosen, C.; Steyer, J.-P. Benchmark Simulation Model No. 1 (BSM1); Report by the IWA Taskgroup on Benchmarking of Control Strategies for WWTPs; Lund University: Lund, Sweden, 2008. [Google Scholar]
- Mrowiec, B. Effect of BTX on Biological Treatment of Sewage. Environ. Prot. Eng. 2009, 35, 197–206. [Google Scholar]
- Smith, R.C.; Elger, S.O.; Mleziva, S. Implementation of solids retention time (SRT) control in wastewater treatment. Xylem Anal. 2015, 20, 1–6. [Google Scholar]
- Benstoem, F.; Nahrstedt, A.; Boehler, M.; Knopp, G.; Montag, D.; Siegrist, H.; Pinnekamp, J. Performance of granular activated carbon to remove micropollutants from municipal wastewater—A meta-analysis of pilot-and large-scale studies. Chemosphere 2017, 185, 105–118. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.; Wang, Y.; Liu, S.; Liu, S.; Yang, Y.; Jiang, H.; Zhang, Y.; Qi, L.; Wang, H. Adsorption characteristics of organics in the effluent of ultra-short SRT wastewater treatment by single-walled, multi-walled, and graphitized multi-walled carbon nanotubes. Sci. Rep. 2018, 8, 17245. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.S.; Romão, L.P.C.; Araújo, B.R.; Lucas, S.C.O.; Maciel, S.T.A.; Wisniewski Jr, A.; Alexandre, M.D.R. Environmental strategies to remove volatile aromatic fractions (BTEX) from petroleum industry wastewater using biomass. Bioresour. Technol. 2012, 105, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Torfs, E.; Nicolaï, N.; Daneshgar, S.; Copp, J.B.; Haimi, H.; Ikumi, D.; Johnson, B.; Plosz, B.B.; Snowling, S.; Townley, L.R.; et al. The Transition of WRRF Models to Digital Twin Applications. Water Sci. Technol. 2022, 85, 2840–2853. [Google Scholar] [CrossRef]
- Zietzschmann, F.; Stützer, C.; Jekel, M. Granular activated carbon adsorption of organic micro-pollutants in drinking water and treated wastewater–aligning breakthrough curves and capacities. Water Res. 2016, 92, 180–187. [Google Scholar] [CrossRef]


| Parameter | Value | Unit |
|---|---|---|
| Influent properties | ||
| Flow | 18,446 | m3 d−1 |
| COD | 360 | gCOD m−3 |
| Filtered COD | 144 | gCOD m−3 |
| TOC | 114 | gC m−3 |
| TKN | 47 | gN m−3 |
| NH4-N | 30 | gN m−3 |
| Tank dimensions | ||
| Anoxic 1 zone volume | 1000 | m3 |
| Anoxic 2 zone volume | 1000 | m3 |
| Aerobic 1 zone volume | 1333 | m3 |
| Aerobic 2 zone volume | 1333 | m3 |
| Aerobic 3 zone volume | 1333 | m3 |
| Clarifier surface area | 1500 | m2 |
| Clarifier depth | 4 | m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bencsik, D.; Wadhawan, T.; Házi, F.; Karches, T. Plant-Wide Models for Optimizing the Operation and Maintenance of BTEX-Contaminated Wastewater Treatment and Reuse. Environments 2024, 11, 88. https://doi.org/10.3390/environments11050088
Bencsik D, Wadhawan T, Házi F, Karches T. Plant-Wide Models for Optimizing the Operation and Maintenance of BTEX-Contaminated Wastewater Treatment and Reuse. Environments. 2024; 11(5):88. https://doi.org/10.3390/environments11050088
Chicago/Turabian StyleBencsik, Dániel, Tanush Wadhawan, Ferenc Házi, and Tamás Karches. 2024. "Plant-Wide Models for Optimizing the Operation and Maintenance of BTEX-Contaminated Wastewater Treatment and Reuse" Environments 11, no. 5: 88. https://doi.org/10.3390/environments11050088







