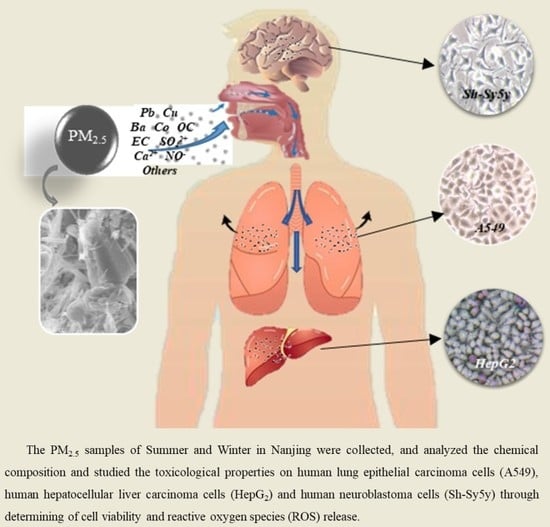
Chemical Characterization of Two Seasonal PM2.5 Samples in Nanjing and Its Toxicological Properties in Three Human Cell Lines
Abstract
:1. Introduction
2. Materials and Methods
2.1. PM2.5 Collection
2.2. PM2.5 Chemical Analysis
2.3. Cytotoxicity
2.3.1. PM2.5 Preparation and Cell Culture
2.3.2. Cell Viability
2.3.3. ROS Assay
2.4. Data Analysis
3. Results
3.1. PM2.5 Mass Concentrations and Chemical Characteristics
3.1.1. Heavy Metals
3.1.2. Water-Soluble Ions
3.1.3. OC and EC
3.2. PM2.5 Cytotoxicity
3.2.1. Cell Viability
3.2.2. ROS Induction
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kan, H.; Chen, R.; Tong, S. Ambient air pollution, climate change, and population health in China. Environ. Int. 2012, 42, 10–19. [Google Scholar] [CrossRef]
- Niu, X.; Ho, S.S.H.; Ho, K.F.; Huang, Y.; Sun, J.; Wang, Q.; Zhou, Y.; Zhao, Z.; Cao, J. Atmospheric levels and cytotoxicity of polycyclic aromatic hydrocarbons and oxygenated-PAHs in PM2.5 in the Beijing-Tianjin-Hebei region. Environ. Pollut. 2017, 231, 1075–1084. [Google Scholar] [CrossRef]
- Delfino, R.J.; Sioutas, C.; Malik, S. Potential role of ultrafine particles in associations between airborne particle mass and cardiovascular health. Environ. Health Perspect. 2005, 113, 934–946. [Google Scholar] [CrossRef]
- Morgan, T.E.; Davis, D.A.; Iwata, N.; Tanner, J.A.; Snyder, D.; Ning, Z.; Kam, W.; Hsu, Y.-T.; Winkler, J.W.; Chen, J.-C.; et al. Glutamatergic Neurons in Rodent Models Respond to Nanoscale Particulate Urban Air Pollutants in Vivo and in Vitro. Environ. Health Perspect. 2011, 119, 1003–1009. [Google Scholar] [CrossRef]
- Cohen, A.J.; Anderson, H.R.; Ostro, B.; Pandey, K.D.; Krzyzanowski, M.; Kunzli, N.; Gutschmidt, K.; Pope, A.; Romieu, I.; Samet, J.M.; et al. The global burden of disease due to outdoor air pollution. J. Toxicol. Environ. Health Part A Curr. Issues 2005, 68, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Vos, T.; Flaxman, A.D. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010 (vol 380, pg 2224, 2012). Lancet 2013, 381, 1276. [Google Scholar]
- Spurny, K.R. Chemical mixtures in atmospheric aerosols and their correlation to lung diseases and lung cancer occurence in the general population. Toxicol. Lett. 1996, 88, 271–277. [Google Scholar] [CrossRef]
- Sarnat, J.A.; Marmur, A.; Klein, M.; Kim, E.; Russell, A.G.; Sarnat, S.E.; Mulholland, J.A.; Hopke, P.K.; Tolbert, P.E. Fine particle sources and cardiorespiratory morbidity: An application of chemical mass balance and factor analytical source-apportionment methods. Environ. Health Perspect. 2008, 116, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Dergham, M.; Lepers, C.; Verdin, A.; Cazier, F.; Billet, S.; Courcot, D.; Shirali, P.; Garcon, G. Temporal-spatial variations of the physicochemical characteristics of air pollution Particulate Matter (PM2.5–0.3) and toxicological effects in human bronchial epithelial cells (SEAS-2B). Environ. Res. 2015, 137, 256–267. [Google Scholar] [CrossRef]
- Gao, P.; Lei, T.; Jia, L.; Song, Y.; Lin, N.; Du, Y.; Feng, Y.; Zhang, Z.; Cui, F. Exposure and health risk assessment of PM2.5-bound trace metals during winter in university campus in Northeast China. Sci. Total Environ. 2017, 576, 628–636. [Google Scholar] [CrossRef]
- Satsangi, P.G.; Yadav, S.; Pipal, A.S.; Kumbhar, N. Characteristics of trace metals in fine (PM2.5) and inhalable (PM10) particles and its health risk assessment along with in-silico approach in indoor environment of India. Atmos. Environ. 2014, 92, 384–393. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Ding, Z.; Wang, T.; Lian, H.; Sun, Y.; Wu, J. Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmos. Environ. 2012, 57, 146–152. [Google Scholar] [CrossRef]
- Jeng, H.A. Chemical composition of ambient particulate matter and redox activity. Environ. Monit. Assess. 2010, 169, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, J.; Soong, R.; Wu, B.; Chan, A.W.H. Relationship between chemical composition and oxidative potential of secondary organic aerosol from polycyclic aromatic hydrocarbons. Atmos. Chem. Phys. 2018, 18, 3987–4003. [Google Scholar] [CrossRef]
- Weber, S.; Uzu, G.; Calas, A.; Chevrier, F.; Besombes, J.-L.; Charron, A.; Salameh, D.; Jezek, I.; Mocnik, G.; Jaffrezo, J.-L. An apportionment method for the oxidative potential of atmospheric particulate matter sources: Application to a one-year study in Chamonix, France. Atmos. Chem. Phys. 2018, 18, 9617–9629. [Google Scholar] [CrossRef]
- Huang, M.; Kang, Y.; Wang, W.; Chan, C.Y.; Wang, X.; Wong, M.H. Potential cytotoxicity of water-soluble fraction of dust and particulate matters and relation to metal(loid)s based on three human cell lines. Chemosphere 2015, 135, 61–66. [Google Scholar] [CrossRef]
- US Environmental Protection Agency, Office of Health and Environmental Assessment, Exposure Assessment Group. Exposure Factors Handbook; Final Report; US Environmental Protection Agency: Washington, DC, USA, 1997.
- Calcabrini, A.; Meschini, S.; Marra, M.; Falzano, L.; Colone, M.; De Berardis, B.; Paoletti, L.; Arancia, G.; Fiorentini, C. Fine environmental particulate engenders alterations in human lung epithelial A549 cells. Environ. Res. 2004, 95, 82–91. [Google Scholar] [CrossRef]
- Adamson, I.Y.; Prieditis, H.; Vincent, R. Pulmonary toxicity of an atmospheric particulate sample is due to the soluble fraction. Toxicol. Appl. Pharmacol. 1999, 157, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Knasmuller, S.; Mersch-Sundermann, V.; Kevekordes, S.; Darroudi, F.; Huber, W.W.; Hoelzl, C.; Bichler, J.; Majer, B.J. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology 2004, 198, 315–328. [Google Scholar] [CrossRef]
- Allen, J.L.; Oberdorster, G.; Morris-Schaffer, K.; Wong, C.; Klocke, C.; Sobolewski, M.; Conrad, K.; Mayer-Proschel, M.; Cory-Slechta, D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: Parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology 2017, 59, 140–154. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Maciel, A.; Reynoso-Robles, R.; Torres-Jardon, R.; Mukherjee, P.S.; Calderon-Garciduenas, L. Combustion-Derived Nanoparticles in Key Brain Target Cells and Organelles in Young Urbanites: Culprit Hidden in Plain Sight in Alzheimer’s Disease Development. J. Alzheimers Dis. 2017, 59, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Ritz, B.; Lee, P.-C.; Hansen, J.; Lassen, C.F.; Ketzel, M.; Sorensen, M.; Raaschou-Nielsen, O. Traffic-Related Air Pollution and Parkinson’s Disease in Denmark: A Case-Control Study. Environ. Health Perspect. 2016, 124, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.-H.; Kim, J.-A.; Hong, S.-I.; Jung, Y.-H.; Kim, H.-C.; Lee, S.-Y.; Jang, C.-G. Loganin protects against hydrogen peroxide-induced apoptosis by inhibiting phosphorylation of JNK, p38, and ERK 1/2 MAPKs in SH-SY5Y cells. Neurochem. Int. 2011, 58, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Perrone, M.G.; Gualtieri, M.; Ferrero, L.; Lo Porto, C.; Udisti, R.; Bolzacchini, E.; Camatini, M. Seasonal variations in chemical composition and in vitro biological effects of fine PM from Milan. Chemosphere 2010, 78, 1368–1377. [Google Scholar] [CrossRef]
- Kang, Y.; Cheung, K.C.; Wong, M.H. Polycyclic aromatic hydrocarbons (PAHs) in different indoor dusts and their potential cytotoxicity based on two human cell lines. Environ. Int. 2010, 36, 542–547. [Google Scholar] [CrossRef]
- Zou, Y.; Jin, C.; Su, Y.; Li, J.; Zhu, B. Water soluble and insoluble components of urban PM2.5 and their cytotoxic effects on epithelial cells (A549) in vitro. Environ. Pollut. 2016, 212, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.L.; Lu, X.; Wu, M.L. Pollution characters and source apportionment of heavy metals in PM2.1 in Nanjing. China. Chin. J. Environ. Eng. 2014, 8, 652–658. [Google Scholar]
- Yang, W.; Yin, Y.; Wei, Y.; Chen, K. Characteristics and sources of metal elements in PM2.5 during hazy days in Nanjing. China. China Environ. Sci. 2010, 30, 12–17. [Google Scholar]
- Qi, L.; Zhang, Y.; Ma, Y.; Chen, M.; Ge, X.; Ma, Y.; Zheng, J.; Wang, Z.; Li, S. Source identification of trace elements in the atmosphere during the second Asian Youth Games in Nanjing, China: Influence of control measures on air quality. Atmos. Pollut. Res. 2016, 7, 547–556. [Google Scholar] [CrossRef]
- Zhou, S.; Yuan, Q.; Li, W.; Lu, Y.; Zhang, Y.; Wang, W. Trace metals in atmospheric fine particles in one industrial urban city: Spatial variations, sources, and health implications. J. Environ. Sci. 2014, 26, 205–213. [Google Scholar] [CrossRef]
- Yang, L.; Cheng, S.; Wang, X.; Nie, W.; Xu, P.; Gao, X.; Yuan, C.; Wang, W. Source identification and health impact of PM2.5 in a heavily polluted urban atmosphere in China. Atmos. Environ. 2013, 75, 265–269. [Google Scholar] [CrossRef]
- Nie, D.; Wu, Y.; Chen, M.; Liu, H.; Zhang, K.; Ge, P.; Yuan, Y.; Ge, X. Bioaccessibility and health risk of trace elements in fine particulate matter in different simulated body fluids. Atmos. Environ. 2018, 186, 1–8. [Google Scholar] [CrossRef]
- MohseniBandpi, A.; Eslami, A.; Shahsavani, A.; Khodagholi, F.; Alinejad, A. Physicochemical characterization of ambient PM2.5 in Tehran air and its potential cytotoxicity in human lung epithelial cells (A549). Sci. Total Environ. 2017, 593–594, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Li, L.; Chen, Y.; Chen, H.; Wu, D.; Wang, J.; Xie, X.; Ge, S.; Ye, Z.; Xu, J.; et al. Aerosol characteristics and sources in Yangzhou, China resolved by offline aerosol mass spectrometry and other techniques. Environ. Pollut. 2017, 225, 74–85. [Google Scholar] [CrossRef]
- Senaratne, I.; Shooter, D. Elemental composition in source identification of brown haze in Auckland, New Zealand. Atmos. Environ. 2004, 38, 3049–3059. [Google Scholar] [CrossRef]
- Taiwo, A.M.; Harrison, R.M.; Shi, Z. A review of receptor modelling of industrially emitted particulate matter. Atmos. Environ. 2014, 97, 109–120. [Google Scholar] [CrossRef]
- Chen, P.L.; Wang, T.J.; Hu, X.; Xie, M. Chemical Mass Balance Source Apportionment of Size-Fractionated Particulate Matter in Nanjing, China. Aerosol Air Qual. Res. 2015, 15, 1855–1867. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, Y.; Sun, Y.; Tian, S.; Cheng, M. Size-resolved aerosol trace elements at a rural mountainous site in Northern China: Importance of regional transport. Sci. Total Environ. 2013, 461, 761–771. [Google Scholar] [CrossRef]
- Tian, H.Z.; Wang, Y.; Xue, Z.G.; Cheng, K.; Qu, Y.P.; Chai, F.H.; Hao, J.M. Trend and characteristics of atmospheric emissions of Hg, As, and Se from coal combustion in China, 1980–2007. Atmos. Chem. Phys. 2010, 10, 11905–11919. [Google Scholar] [CrossRef] [Green Version]
- Bukowiecki, N.; Lienemann, P.; Hill, M.; Figi, R.; Richard, A.; Furger, M.; Rickers, K.; Falkenberg, G.; Zhao, Y.; Cliff, S.S.; et al. Real-World Emission Factors for Antimony and Other Brake Wear Related Trace Elements: Size-Segregated Values for Light and Heavy Duty Vehicles. Environ. Sci. Technol. 2009, 43, 8072–8078. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chen, H.; Yang, X.; Chen, J.; Allen, J.O. Source apportionment of lead-containing aerosol particles in Shanghai using single particle mass spectrometry. Chemosphere 2009, 74, 501–507. [Google Scholar] [CrossRef]
- Karnae, S.; John, K. Source apportionment of fine particulate matter measured in an industrialized coastal urban area of South Texas. Atmos. Environ. 2011, 45, 3769–3776. [Google Scholar] [CrossRef]
- Yang, S.; Song, X.; Zhen, M.; Wang, X.; Huang, C. Characteristic features of the water-soluble inorganic ions in PM2.5 in the process of a severe haze episode in Zhengzhou. China. J. Saf. Environ. 2016, 6, 348–353. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, G.; Yu, R.; Hu, Q.; Liu, X. Characteristics and sources apportionment of water-soluble ions in PM2.5 of Xiamen City, China. China. China Environ. Sci. 2016, 36, 1947–1954. [Google Scholar]
- Gao, H.; Wei, J.; Wang, Y. Seasonal Variation and Source Analysis of Water-soluble Inorganic Salts in PM2.5 in the Southern Suburbs of Beijing. China. Environ. Sci. 2018, 39, 1987–1993. [Google Scholar] [CrossRef]
- Li, W.; Bai, Z.; Shi, J.; Liu, A. Pollution Characteristics and Sources of Fine Particulate Matter in Ambient Air in Tianjin City. China. Res. Environ. Sci. 2010, 23, 394–400. [Google Scholar] [CrossRef]
- Arimoto, R.; Duce, R.A.; Savoie, D.L.; Prospero, J.M.; Talbot, R.; Cullen, J.D.; Tomza, U.; Lewis, N.F.; Ray, B.J. Relationships among aerosol constitutes from Asia and the North Pacific during PEM-West A. J. Geophys. Res. Atmos. 1996, 101, 2011–2023. [Google Scholar] [CrossRef]
- Wang, L.; Ma, Y.; Zheng, J.; Wang, Z.; Zhou, Y.-Y. Characterization Apportionment of Carbonaceous Aerosol and Water-soluble Ions in PM2.5 at Suburban Nanjing. China. Sci. Technol. Eng. 2015, 15, 91–99. [Google Scholar]
- Huang, X.H.H.; Bian, Q.J.; Louie, P.K.K.; Yu, J.Z. Contributions of vehicular carbonaceous aerosols to PM2.5 in a roadside environment in Hong Kong. Atmos. Chem. Phys. 2014, 14, 9279–9293. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, Z.; Luo, L.; Lou, S.; Ma, Y.; Yan, N. Investigation of fungal spore characteristics in PM2.5 through organic tracers in Shanghai, China. Atmos. Pollut. Res. 2018, 9, 894–900. [Google Scholar] [CrossRef]
- Shi, M.; Wu, H.; Zhang, S.; Li, H.; Liu, H. Weekly cycle of magnetic characteristics of the daily PM2.5 and PM2.5–10 in Beijing, China. Atmos. Environ. 2014, 98, 357–367. [Google Scholar] [CrossRef]
- Yang, H.; Yu, J.Z.; Ho, S.S.H.; Xu, J.H.; Wu, W.S.; Wan, C.H.; Wang, X.D.; Wang, X.R.; Wang, L.S. The chemical composition of inorganic and carbonaceous materials in PM2.5 in Nanjing, China. Atmos. Environ. 2005, 39, 3735–3749. [Google Scholar] [CrossRef]
- Chen, K.; Yin, Y.; Wei, Y. Characteristics of aerosols in PM2.5 in Nanjing. China. China Environ. Sci. 2010, 30, 1015–1020. [Google Scholar]
- Li, B.; Zhang, J.; Zhao, Y.; Yuan, S.; Zhao, Q.; Shen, G.; Wu, H. Seasonal variation of urban carbonaceous aerosols in a typical city Nanjing in Yangtze River Delta, China. Atmos. Environ. 2015, 106, 223–231. [Google Scholar] [CrossRef]
- Chen, Y.J.; Sheng, G.Y.; Bi, X.H.; Feng, Y.L.; Mai, B.X.; Fu, J.M. Emission factors for carbonaceous particles and polycyclic aromatic hydrocarbons from residential coal combustion in China. Environ. Sci. Technol. 2005, 39, 1861–1867. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R. Measurement of emissions from air pollution sources. 5.C1-C32 organic compounds from gasoline-powered motor vehicles. Environ. Sci. Technol. 2002, 35, 1716–1728. [Google Scholar] [CrossRef]
- Castro, L.M.; Pio, C.A.; Harrison, R.M.; Smith, D.J.T. Carbonaceous aerosol in urban and rural European atmospheres: Estimation of secondary organic carbon concentrations. Atmos. Environ. 1999, 33, 2771–2781. [Google Scholar] [CrossRef]
- He, Q.; Guo, W.; Zhang, G.; Yan, Y.; Chen, L. Characteristics and Seasonal Variations of Carbonaceous Species in PM2.5 in Taiyuan, China. Atmosphere 2015, 6, 850–862. [Google Scholar] [CrossRef]
- Borgie, M.; Dagher, Z.; Ledoux, F.; Verdin, A.; Cazier, F.; Martin, P.; Hachimi, A.; Shirali, P.; Greige-Gerges, H.; Courcot, D. Comparison between ultrafine and fine particulate matter collected in Lebanon: Chemical characterization, in vitro cytotoxic effects and metabolizing enzymes gene expression in human bronchial epithelial cells. Environ. Pollut. 2015, 205, 250–260. [Google Scholar] [CrossRef] [PubMed]
- de Kok, T.M.C.M.; Driece, H.A.L.; Hogervorst, J.G.F.; Briede, J.J. Toxicological assessment of ambient and traffic-related particulate matter: A review of recent studies. Mutat. Res. Rev. Mutat. Res. 2006, 613, 103–122. [Google Scholar] [CrossRef]
- Liu, Q.; Baumgartner, J.; Zhang, Y.; Schauer, J.J. Source apportionment of Beijing air pollution during a severe winter haze event and associated pro-inflammatory responses in lung epithelial cells. Atmos. Environ. 2016, 126, 28–35. [Google Scholar] [CrossRef]
- Bressi, M.; Sciare, J.; Ghersi, V.; Bonnaire, N.; Nicolas, J.B.; Petit, J.E.; Moukhtar, S.; Rosso, A.; Mihalopoulos, N.; Feron, A. A one-year comprehensive chemical characterisation of fine aerosol (PM2.5) at urban, suburban and rural background sites in the region of Paris (France). Atmos. Chem. Phys. 2013, 13, 7825–7844. [Google Scholar] [CrossRef]
- Cao, J.J.; Zhu, C.S.; Tie, X.X.; Geng, F.H.; Xu, H.M.; Ho, S.S.H.; Wang, G.H.; Han, Y.M.; Ho, K.F. Characteristics and sources of carbonaceous aerosols from Shanghai, China. Atmos. Chem. Phys. 2013, 13, 803–817. [Google Scholar] [CrossRef]
- Könczöl, M.; Ebeling, S.; Goldenberg, E.; Treude, F.; Gminski, R.; Gieré, R.; Grobéty, B.; Rothen-Rutishauser, B.; Merfort, I.; Mersch-Sundermann, V. Cytotoxicity and genotoxicity of size-fractionated iron oxide (magnetite) in A549 human lung epithelial cells: Role of ROS, JNK, and NF-κB. J. Chem. Res. Toxicol. 2011, 24, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Moller, P.; Jacobsen, N.R.; Folkmann, J.K.; Danielsen, P.H.; Mikkelsen, L.; Hemmingsen, J.G.; Vesterdal, L.K.; Forchhammer, L.; Wallin, H.; Loft, S. Role of oxidative damage in toxicity of particulates. Free Radic. Res. 2010, 44, 1–46. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Zhang, F.; Rui, W.; Long, F.; Wang, L.; Feng, Z.; Chen, D.; Ding, W. PM2.5-induced oxidative stress triggers autophagy in human lung epithelial A549 cells. Toxicol. In Vitro 2013, 27, 1762–1770. [Google Scholar] [CrossRef]
- Bai, R.; Guan, L.; Zhang, W.; Xu, J.; Rui, W.; Zhang, F.; Ding, W. Comparative study of the effects of PM1-induced oxidative stress on autophagy and surfactant protein B and C expressions in lung alveolar type II epithelial MLE-12 cells. Biochim. Biophys. Acta-Gen. Subj. 2016, 1860, 2782–2792. [Google Scholar] [CrossRef]
- Gehling, W.; Dellinger, B. Environmentally Persistent Free Radicals and Their Lifetimes in PM2.5. Environ. Sci. Technol. 2013, 47, 8172–8178. [Google Scholar] [CrossRef]
- Gehling, W.; Khachatryan, L.; Dellinger, B. Hydroxyl Radical Generation from Environmentally Persistent Free Radicals (EPFRs) in PM2.5. Environ. Sci. Technol. 2014, 48, 4266–4272. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Polidori, A.; Schauer, J.J.; Shafer, M.M.; Cassee, F.R.; Sioutas, C. Physicochemical and Toxicological Profiles of Particulate Matter in Los Angeles during the October 2007 Southern California Wildfires. Environ. Sci. Technol. 2009, 43, 954–960. [Google Scholar] [CrossRef]
- Shafer, M.M.; Perkins, D.A.; Antkiewicz, D.S.; Stone, E.A.; Quraishi, T.A.; Schauer, J.J. Reactive oxygen species activity and chemical speciation of size-fractionated atmospheric particulate matter from Lahore, Pakistan: An important role for transition metals. J. Environ. Monit. 2010, 12, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Daher, N.; Ruprecht, A.; Invernizzi, G.; De Marco, C.; Miller-Schulze, J.; Heo, J.B.; Shafer, M.M.; Shelton, B.R.; Schauer, J.J.; Sioutas, C. Characterization, sources and redox activity of fine and coarse particulate matter in Milan, Italy. Atmos. Environ. 2012, 49, 130–141. [Google Scholar] [CrossRef]
- Saffari, A.; Daher, N.; Shafer, M.M.; Schauer, J.J.; Sioutas, C. Seasonal and spatial variation in reactive oxygen species activity of quasi-ultrafine particles (PM0.25) in the Los Angeles metropolitan area and its association with chemical composition. Atmos. Environ. 2013, 79, 566–575. [Google Scholar] [CrossRef]






| Elements | Summer (n = 33) | Winter (n = 32) | Mass Fraction Ratio Summer/Winter | ||
|---|---|---|---|---|---|
| Concentration (ng/m3) | Mass Fraction (μg/g) | Concentration (ng/m3) | Mass Fraction (μg/g) | ||
| Al | 588.85 ± 284.99 | 12,704.97 ± 6747.99 | 755.05 ± 738.39 | 12,559.83 ± 11,775.82 | 1.01 |
| As | 3.37 ± 2.31 | 65.95 ± 38.61 | 3.05 ± 1.90 | 51.18 ± 27.05 | 1.29 |
| Ba | 20.92 ± 38.82 | 512.71 ± 103.58 | 11.72 ± 4.99 | 198.11 ± 78.77 | 2.59 |
| Cd | 1.58 ± 1.27 | 31.24 ± 26.83 | 1.17 ± 0.71 | 19.18 ± 11.01 | 1.63 |
| Co | 0.73 ± 0.99 | 17.64 ± 3.63 | 0.47 ± 0.17 | 8.02 ± 3.54 | 2.20 |
| Cr | 23.38 ± 7.50 | 524.42 ± 244.98 | 34.70 ± 20.37 | 609.05 ± 434.21 | 0.86 |
| Cu | 35.99 ± 19.86 | 802.44 ± 507.76 | 36.23 ± 29.95 | 602.33 ± 391.26 | 1.33 |
| Mn | 26.98 ± 11.65 | 552.71 ± 183.13 | 29.88 ± 14.90 | 493.90 ± 214.84 | 1.12 |
| Ni | 11.49 ± 3.56 | 252.24 ± 98.97 | 7.86 ± 4.99 | 139.49 ± 100.74 | 1.81 |
| Pb | 86.16 ± 65.88 | 1742.57 ± 501.45 | 48.92 ± 22.24 | 799.80 ± 302.54 | 2.18 |
| Sr | 4.12 ± 1.83 | 86.49 ± 30.45 | 5.51 ± 2.27 | 93.38 ± 40.03 | 0.93 |
| V | 4.64 ± 3.28 | 90.02 ± 57.89 | 2.95 ± 1.67 | 50.17 ± 29.71 | 1.79 |
| Zn | 678.16 ± 325.86 | 14,979.87 ± 10,218.68 | 724.70 ± 438.32 | 12,784.79 ± 8830.23 | 1.17 |
| Al | As | Ba | Cd | Co | Cr | Cu | Mn | Ni | Pb | Sr | V | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | 1 | ||||||||||||
| As | 0.145 | 1 | |||||||||||
| Ba | −0.034 | 0.014 | 1 | ||||||||||
| Cd | 0.119 | 0.366 ** | −0.024 | 1 | |||||||||
| Co | −0.005 | −0.033 | 0.013 | 0.562 ** | 1 | ||||||||
| Cr | 0.383 ** | 0.152 | −0.008 | 0.133 | 0.020 | 1 | |||||||
| Cu | 0.191 | 0.167 | −0.035 | 0.388 ** | 0.069 | 0.311 * | 1 | ||||||
| Mn | 0.210 | 0.419 ** | 0.024 | 0.257 * | 0.097 | 0.341 ** | 0.187 | 1 | |||||
| Ni | 0.320 ** | 0.151 | 0.204 | 0.405 ** | 0.189 | 0.157 | 0.576 ** | 0.154 | 1 | ||||
| Pb | 0.063 | 0.115 | −0.001 | 0.380 ** | 0.127 | 0.098 | 0.452 ** | 0.394 ** | 0.338 ** | 1 | |||
| Sr | 0.489 ** | 0.470 ** | 0.052 | 0.279 * | 0.123 | 0.488 ** | 0.348 ** | 0.640 ** | 0.254 * | 0.207 | 1 | ||
| V | −0.040 | 0.016 | 0.012 | 0.471 ** | 0.303 * | 0.048 | 0.263 * | 0.509 ** | 0.403 ** | 0.568 ** | 0.140 | 1 | |
| Zn | 0.152 | −0.092 | −0.065 | 0.068 | −0.119 | 0.202 | 0.141 | 0.150 | 0.077 | 0.083 | 0.072 | 0.212 | 1 |
| A549 Cells | HepG2 Cells | Sh-Sy5y Cells | ||||
|---|---|---|---|---|---|---|
| LC20 | LC50 | LC20 | LC50 | LC20 | LC50 | |
| Summer | 96 (92–99) (n = 30) | 274 (250–291) (n = 30) | 66 (51–80) (n = 27) | 205 (173–274) (n = 27) | 98 (93–102) (n = 22) | 279 (249–310) (n = 22) |
| Winter | 130 (127–133) (n = 29) | 323 (268–379) (n = 29) | 84 (64–103) (n = 30) | 268 (221–315) (n = 30) | 127 (62–167) (n = 26) | 312 (311–415) (n = 26) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Nie, D.; Chen, M.; Wu, Y.; Ge, X.; Hu, J.; Ge, P.; Li, W.; Huang, B.; Yuan, Y.; et al. Chemical Characterization of Two Seasonal PM2.5 Samples in Nanjing and Its Toxicological Properties in Three Human Cell Lines. Environments 2019, 6, 42. https://doi.org/10.3390/environments6040042
Zhang K, Nie D, Chen M, Wu Y, Ge X, Hu J, Ge P, Li W, Huang B, Yuan Y, et al. Chemical Characterization of Two Seasonal PM2.5 Samples in Nanjing and Its Toxicological Properties in Three Human Cell Lines. Environments. 2019; 6(4):42. https://doi.org/10.3390/environments6040042
Chicago/Turabian StyleZhang, Kai, Dongyang Nie, Mindong Chen, Yun Wu, Xinlei Ge, Jianlin Hu, Pengxiang Ge, Wenjing Li, Bingbo Huang, Yue Yuan, and et al. 2019. "Chemical Characterization of Two Seasonal PM2.5 Samples in Nanjing and Its Toxicological Properties in Three Human Cell Lines" Environments 6, no. 4: 42. https://doi.org/10.3390/environments6040042