NMR CAPIBarA: Proof of Principle of a Low-Field Unilateral Magnetic Resonance System for Monitoring of the Placenta during Pregnancy
Abstract
:Featured Application
Abstract
1. Introduction
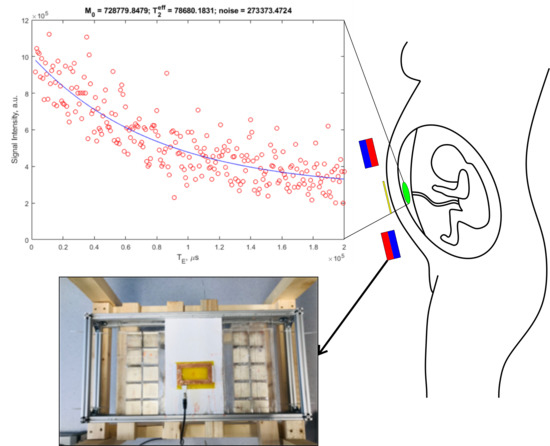
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glover, L. Why Does an MRI Cost So Darn Much? 2014. Available online: http://money.com/money/2995166/why-does-mri-cost-so-much/ (accessed on 26 November 2019).
- Dean, R. Expecting? How much does an ultrasound cost? 2014. Available online: https://blog.bernardbenefits.com/bid/196963/expecting-how-much-does-an-ultrasound-cost (accessed on 26 November 2019).
- Gowland, P.A.; Freeman, A.; Issa, B.; Boulby, P.; Duncan, K.R.; Baker, P.N.; Bowtell, R.W.; Johnson, I.R.; Worthington, B.S. In vivo relaxation time measurements in the human placenta using echo planar imaging at 0.5 T. Magn. Reson. Imaging 1998, 16, 241–247. [Google Scholar] [CrossRef]
- Wright, C.; Morris, D.M.; Baker, P.N.; Crocker, I.P.; Gowland, P.A.; Parker, G.J.; Sibley, C.P. Magnetic resonance imaging relaxation time measurements of the placenta at 1.5 T. Placenta 2011, 32, 1010–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kameyama, N.K.; Kido, A.; Himoto, Y.; Moribata, Y.; Minamiguchi, S.; Konishi, I.; Togashi, K. What is the most suitable MR signal index for quantitative evaluation of placental function using Half-Fourier acquisition single-shot turbo spin-echo compared with T2-relaxation time? Acta Radiol. 2017, 59, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Siding, M.; Peters, D.A.; Frokjaer, J.B.; Christiansen, O.B.; Petersen, A.; Uldbjerg, N.; Sorensen, A. Placental magnetic resonance imaging T2 * measurements in normal pregnancies and in those complicated by fetal growth restriction. Ultrasound Obstet. Gynecol. 2016, 6, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Kleinberg, R.L.; Jackson, J.A. An Introduction to the History of NMR Well Logging. Concepts Magn. Reson. 2001, 13, 340–342. [Google Scholar] [CrossRef]
- Eidmann, G.; Savelsberg, R.; Blumle, P.; Blumich, B. The NMR MoUSE, a mobile universal surface explorer. J. Magn. Reson. A 1996, 122, 104–109. [Google Scholar] [CrossRef]
- Blumich, B.; Blumler, P.; Eidmann, G.; Guthausen, A.; Haken, R.; Schmitz, U.; Saito, K.; Zimmer, G. The NMR-mouse: Construction, excitation, and applications. Magn. Reson. Imaging 1998, 16, 479–484. [Google Scholar] [CrossRef]
- NMR-MOUSE. Available online: magritek.com/products/nmr-mouse/ (accessed on 16 October 2019).
- Van Landeghem, M.; Danieli, E.; Perlo, J.; Blümich, B.; Casanova, F. Low-gradient single-sided NMR sensor for one-shot profiling of human skin. J. Magn. Reson. 2012, 215, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Rössler, E.; Mattea, C.; Stapf, S. Feasibility of high-resolution one-dimensional relaxation imaging at low magnetic field using a singlesided NMR scanner applied to articular cartilage. J. Magn. Reson. 2015, 251, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Brizi, L.; Barbieri, M.; Baruffaldi, F.; Bortolotti, V.; Fersini, F.; Liu, H.; d’Eurydice, M.N.; Obruchkov, S.; Zong, F.; Galvosas, P.; et al. Bone volume–to–total volume ratio measured in trabecular bone by single-sided NMR devices. Magn. Reson. Med. 2018, 79, 501–510. [Google Scholar] [CrossRef] [PubMed]
- Tourell, M.C.; Ali, T.S.; Hugo, H.J.; Pyke, C.; Yang, S.; Lloyd, T.; Thompson, E.W.; Momot, K.I. T1-based sensing of mammographic density using single-sided portable NMR. Magn. Reson. Med. 2018, 80, 1243–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ali, T.S.; Tourell, M.C.; Hugo, H.J.; Pyke, C.; Yang, S.; Lloyd, T.; Thompson, E.W.; Momot, K.I. Transverse relaxation-based assessment of mammographic density and breast tissue composition by single-sided portable NMRe. Magn. Reson. Med. 2019, 82, 1199–1213. [Google Scholar] [CrossRef] [PubMed]
- Dabaghyan, M.; Muradyan, I.; Hrovat, A.; Butler, J.; Frederick, E.; Zhou, F.; Kyriazis, A.; Hardin, C.; Patz, S.; Hrovat, M. A portable single-sided magnet system for remote NMR measurements of pulmonary function. NMR Biomed. 2014, 27, 1479–1489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meiboom, S.; Gill, D. Modified spin-echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Henoumont, C.C.; Laurent, S.; Vander Elst, L. How to perform accurate and reliable measurements of longitudinal and transverse relaxation times of MRI contrast media in aqueous solutions. Contrast Media Mol. Imaging 2009, 4, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Zia, S. Placental Location and Pregnancy Outcome. J. Turk. Ger. Gynecol. Assoc. 2013, 14, 190–193. [Google Scholar] [CrossRef] [PubMed]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morris, R.H.; Almazrouei, N.K.; Trabi, C.L.; Newton, M.I. NMR CAPIBarA: Proof of Principle of a Low-Field Unilateral Magnetic Resonance System for Monitoring of the Placenta during Pregnancy. Appl. Sci. 2020, 10, 162. https://doi.org/10.3390/app10010162
Morris RH, Almazrouei NK, Trabi CL, Newton MI. NMR CAPIBarA: Proof of Principle of a Low-Field Unilateral Magnetic Resonance System for Monitoring of the Placenta during Pregnancy. Applied Sciences. 2020; 10(1):162. https://doi.org/10.3390/app10010162
Chicago/Turabian StyleMorris, Robert H., Najlaa K. Almazrouei, Christophe L. Trabi, and Michael I. Newton. 2020. "NMR CAPIBarA: Proof of Principle of a Low-Field Unilateral Magnetic Resonance System for Monitoring of the Placenta during Pregnancy" Applied Sciences 10, no. 1: 162. https://doi.org/10.3390/app10010162