Time-Domain Nuclear Magnetic Resonance Determination of Wettability Alteration: Analysis for Low-Salinity Water
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Sample Preparation
2.2.2. NMR T Relaxation and Diffusion Coefficient Measurements
2.2.3. Contact Angle and Interfacial Tension
3. Results
3.1. Interfacial Tension and Contact Angle
3.2. Glass Beads-Fluid Interaction
3.2.1. Oil-Surface Interaction
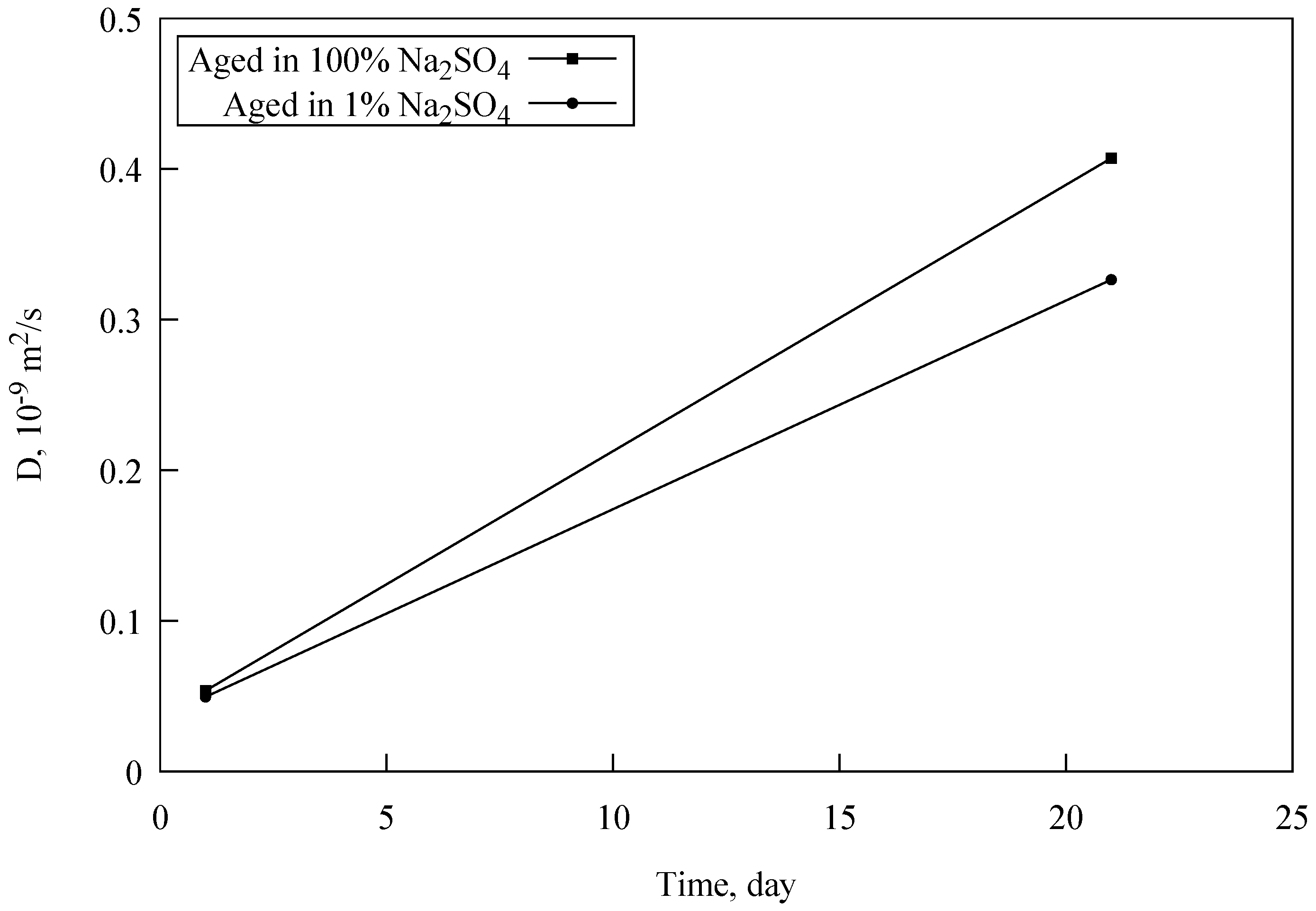
3.2.2. Brine-Surface Interaction
3.3. Rock-Fluid Interaction
Partial Saturation
4. Discussion
5. Conclusions
- Time domain NMR analysis of simple systems can be used to determine the effect of a specific fluid on a surface, and therefore its contribution to wettability.
- Time domain NMR can be used to estimate pore-size distribution quantitatively in small plugs or large-diameter rock samples for any lithology using a simple and quick experiment. This can be useful to correlate results from other experimental techniques.
- The NMR T is an effective tool to detect wettability alteration. It can be useful to map the conditions where changes in wettability occur, for example, temperature, time, brines or chemical products designed for this purpose. At this point of the research, qualitative conclusions can be drawn.
- Wettability alteration induced as a the result of brine salinity modification has been consistently detected with TD-NMR measurements, that is, T and D distribution shifts.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EOR | Enhanced oil recovery |
| RF | Recovery factor |
| TD-NMR | Time-domain Nuclear Magnetic Resonance |
| IFT | Interfacial tension |
| GB | Glass beads |
| TGB | Treated glass beads |
References
- Craig, F.F. The Reservoir Engineering Aspects of Waterflooding; Doherty Memorial Fund of AIME: New York, NY, USA, 1971; Volume 3. [Google Scholar]
- Hirasaki, G.J. Wettability: Fundamentals and surface forces. SPE Form. Eval. 1991, 6, 217–226. [Google Scholar] [CrossRef]
- Al-Muthana, A.; Hursan, G.G.; Ma, S.M.; Valori, A.; Nicot, B.; Singer, P.M. Wettability as a function of pore size by NMR. In Proceedings of the SCA Conference Paper, Aberdeen, UK, 27–30 August 2012; Volume 31. [Google Scholar]
- Wang, J.; Xiao, L.; Liao, G.; Zhang, Y.; Guo, L.; Arns, C.H.; Sun, Z. Theoretical investigation of heterogeneous wettability in porous media using NMR. Sci. Rep. 2018, 8, 13450. [Google Scholar] [CrossRef]
- Buckley, J.; Takamura, K.; Morrow, N. Influence of electrical surface charges on the wetting properties of crude oils. SPE Reserv. Eng. 1989, 4, 332–340. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, Q.; Saeedi, A. Role of ion exchange, surface complexation, and albite dissolution in low salinity water flooding in sandstone. J. Petroleum Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Wagner, O.; Leach, R. Improving oil displacement efficiency by wettability adjustment. Trans. AIME 1959, 216, 65–72. [Google Scholar] [CrossRef]
- Jadhunandan, P.P. Effects of Brine Composition, Crude Oil, and Aging Conditions on Wettability and Oil Recovery. Ph.D. Thesis, Department of Petroleum Engineering, New Mexico Institute of Mining & Technology, Socorro, NM, USA, 1990. [Google Scholar]
- Jadhunandan, P.; Morrow, N. Spontaneous imbibition of water by crude oil/brine/rock systems. In Situ 1991, 15. [Google Scholar]
- Jadhunandan, P.; Morrow, N.R. Effect of wettability on waterflood recovery for crude-oil/brine/rock systems. SPE Reserv. Eng. 1995, 10, 40–46. [Google Scholar] [CrossRef]
- Morrow, N.R. Wettability and its effect on oil recovery. J. Pet. Technol. 1990, 42, 1–476. [Google Scholar] [CrossRef]
- Brady, P.V.; Krumhansl, J.L. A surface complexation model of oil–brine–sandstone interfaces at 100 C: Low salinity waterflooding. J. Pet. Sci. Eng. 2012, 81, 171–176. [Google Scholar] [CrossRef]
- Graue, A.; Viksund, B.G.; Eilertsen, T.; Moe, R. Systematic wettability alteration by aging sandstone and carbonate rock in crude oil. J. Pet. Sci. Eng. 1999, 24, 85–97. [Google Scholar] [CrossRef]
- Al-Mahrooqi, S.; Grattoni, C.; Muggeridge, A.; Jing, X. Wettability alteration during aging: The application of NMR to monitor fluid redistribution. In Proceedings of the International Symposium of the Society of Core Analysts, Toronto, ON, Canada, 21–25 August 2005; pp. 21–25. [Google Scholar]
- Awolayo, A.; Sarma, H.; AlSumaiti, A. An experimental investigation into the impact of sulfate ions in smart water to improve oil recovery in carbonate reservoirs. Transp. Porous Media 2016, 111, 649–668. [Google Scholar] [CrossRef]
- Hognesen, E.J.; Strand, S.; Austad, T. Waterflooding of preferential oil-wet carbonates: Oil recovery related to reservoir temperature and brine composition. In Proceedings of the SPE Europec/EAGE Annual Conference, Madrid, Spain, 13–16 June 2005. Society of Petroleum Engineers. [Google Scholar]
- Mohamed, M.I.; Alvarado, V. Smart Water Flooding in Berea Sandstone at Low Temperature: Is Wettability Alteration the Sole Mechanism at Play? In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. Society of Petroleum Engineers. [Google Scholar]
- Zahid, A.; Shapiro, A.; Stenby, E.H.; Yan, W. Managing injected water composition to improve oil recovery: A case study of North Sea chalk reservoirs. Energy Fuels 2012, 26, 3407–3415. [Google Scholar] [CrossRef] [Green Version]
- Bidhendi, M.M.; Garcia-Olvera, G.; Morin, B.; Oakey, J.S.; Alvarado, V. Interfacial Viscoelasticity of Crude Oil/Brine: An Alternative Enhanced-Oil-Recovery Mechanism in Smart Waterflooding. SPE J. 2018. [Google Scholar] [CrossRef]
- Tabrizy, V.A.; Hamouda, A.; Denoyel, R. Influence of magnesium and sulfate ions on wettability alteration of calcite, quartz, and kaolinite: Surface energy analysis. Energy Fuels 2011, 25, 1667–1680. [Google Scholar] [CrossRef] [Green Version]
- Anderson, W.G. Wettability literature survey—Part 1: Rock/oil/brine interactions and the effects of core handling on wettability. J. Pet. Technol. 1986, 38, 1–125. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability literature survey—Part 3: The effects of wettability on the electrical properties of porous media. J. Pet. Technol. 1986, 38, 1–371. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability literature survey—Part 4: Effects of wettability on capillary pressure. J. Pet. Technol. 1987, 39, 1–283. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability literature survey part 5: The effects of wettability on relative permeability. J. Pet. Technol. 1987, 39, 1–453. [Google Scholar] [CrossRef]
- Amott, E. Observations Relating to the Wettability of Porous Rock; Society of Petroleum Engineers: Richardson, TX, USA, 1959. [Google Scholar]
- Morrow, N.R.; Mason, G. Recovery of oil by spontaneous imbibition. Curr. Opin. Colloid Interface Sci. 2001, 6, 321–337. [Google Scholar] [CrossRef]
- Treiber, L.; Owens, W. A laboratory evaluation of the wettability of fifty oil-producing reservoirs. Soc. Pet. Eng. J. 1972, 12, 531–540. [Google Scholar] [CrossRef]
- Hirasaki, G.J.; Huang, C.C.; Zhang, G.Q. Interpretation of wettability in sandstones wit NMR analysis. Petrophysics 2000, 41. [Google Scholar]
- Guan, H.; Brougham, D.; Sorbie, K.; Packer, K. Wettability effects in a sandstone reservoir and outcrop cores from NMR relaxation time distributions. J. Pet. Sci. Eng. 2002, 34, 35–54. [Google Scholar] [CrossRef]
- Fleury, M.; Deflandre, F. Quantitative evaluation of porous media wettability using NMR relaxometry. Magn. Reson. Imaging 2003, 21, 385–387. [Google Scholar] [CrossRef]
- Flaum, M.; Chen, J.; Hirasaki, G. NMR Diffusion Editing for D? T2 Maps: Application to Recognition of Wettability Change. Petrophysics 2005, 46. [Google Scholar]
- Looyestijn, W.J.; Hofman, J. Wettability-index determination by nuclear magnetic resonance. SPE Reserv. Eval. Eng. 2006, 9, 146–153. [Google Scholar] [CrossRef]
- Chen, J.; Hirasaki, G.; Flaum, M. NMR wettability indices: Effect of OBM on wettability and NMR responses. J. Pet. Sci. Eng. 2006, 52, 161–171. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, L.; Liao, G.; Zhang, Y.; Cui, Y.; Sun, Z.; Dong, Y.; Hu, L. NMR characterizing mixed wettability under intermediate-wet condition. Magn. Reson. Imaging 2018. [Google Scholar] [CrossRef]
- Howard, J. Quantitative estimates of porous media wettability from proton NMR measurements. Magn. Reson. Imaging 1998, 16, 529–533. [Google Scholar] [CrossRef]
- Hsu, W.F.; Li, X.; Flumerfelt, R. Wettability of porous media by NMR relaxation methods. In Proceedings of the SPE Annual Technical Conference and Exhibition, Washington, DC, USA, 4–7 October 1992. Society of Petroleum Engineers. [Google Scholar]
- Brown, R.J.; Fatt, I. Measurements of fractional wettability of oil fields’ rocks by the nuclear magnetic relaxation method. In Proceedings of the Fall Meeting of the Petroleum Branch of AIME, Los Angeles, CA, USA, 14–17 October 1956. Society of Petroleum Engineers. [Google Scholar]
- Brady, P.V.; Thyne, G. Functional wettability in carbonate reservoirs. Energy Fuels 2016, 30, 9217–9225. [Google Scholar] [CrossRef]
- Chang, D.; Vinegar, H.J.; Morriss, C.; Straley, C. Effective porosity, producible fluid and permeability in carbonates from NMR logging. In Proceedings of the SPWLA 35th Annual Logging Symposium. Society of Petrophysicists and Well-Log Analysts, Tulsa, OK, USA, 19–22 June 1994. [Google Scholar]
- Coates, G.R.; Xiao, L.; Prammer, M.G. NMR Logging: Principles and Applications; Haliburton Energy Services: Houston, TX, USA, 1999; Volume 344. [Google Scholar]
- Wang, H.; Alvarado, V. Ionic strength-dependent pre-asymptoic diffusion coefficient distribution in porous media-Determination through the pulsed field gradient technique. J. Nat. Gas Sci. Eng. 2018, 49, 250–259. [Google Scholar] [CrossRef]
- Buntkowsky, G.; Breitzke, H.; Adamczyk, A.; Roelofs, F.; Emmler, T.; Gedat, E.; Grünberg, B.; Xu, Y.; Limbach, H.H.; Shenderovich, I.; et al. Structural and dynamical properties of guest molecules confined in mesoporous silica materials revealed by NMR. Phys. Chem. Chem. Phys. 2007, 9, 4843–4853. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Dore, J.C.; Webber, J.B.W.; Khushalani, D.; Jähnert, S.; Findenegg, G.; Hansen, T. Neutron diffraction and NMR relaxation studies of structural variation and phase transformations for water/ice in SBA-15 silica: I. The over-filled case. J. Phys. Condens. Matter 2006, 18, 10009. [Google Scholar] [CrossRef]
- Vogel, M. NMR studies on simple liquids in confinement. Eur. Phys. J. Spec. Top. 2010, 189, 47–64. [Google Scholar] [CrossRef]
- Carr, H.; Purcell, E. Effects of Diffusion on Free Precession in Nuclear Magnetic Resonance Experiments. Phys. Rev. 1954, 94, 630–638. [Google Scholar] [CrossRef]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci. Instrum. 1958, 29, 688–691. [Google Scholar] [CrossRef] [Green Version]
- Hahn, E. Spin echoes. Phys. Rev. 1950, 80, 580–594. [Google Scholar] [CrossRef]
- Blümich, B. NMR Imaging of Materials; Oxford Science Publications: New York, NY, USA, 2004. [Google Scholar]
- Callaghan, P. Principles of Nuclear Magnetic Resonance Microscopy; Oxford Science Publications: New York, NY, USA, 1993. [Google Scholar]
- Stilbs, P. Fourier transform pulsed-gradient spin-echo studies of molecular diffusion. Prog. Nucl. Magn. Reson. Spectrosc. 1987, 19, 1–45. [Google Scholar] [CrossRef]
- Hamouda, A.A.; Rezaei Gomari, K.A. Influence of temperature on wettability alteration of carbonate reservoirs. In Proceedings of the SPE/DOE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 22–26 April 2006. Society of Petroleum Engineers. [Google Scholar]
- Sohal, M.A.; Thyne, G.; Søgaard, E.G. Effect of the temperature on wettability and optimum wetting conditions for maximum oil recovery in a carbonate reservoir system. Energy Fuels 2017, 31, 3557–3566. [Google Scholar] [CrossRef]
- Wang, Y.l.; Ma, L.; Bai, B.j.; Jiang, G.c.; Jin, J.f.; Wang, Z.b. Wettability alteration of sandstone by chemical treatments. J. Chem. 2013, 2013. [Google Scholar] [CrossRef] [Green Version]
- Saulick, Y.; Lourenço, S.; Baudet, B. Effect of particle size on the measurement of the apparent contact angle in sand of varying wettability under air-dried conditions. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 9, p. 09003. [Google Scholar]
- Alotaibi, M.B.; Nasralla, R.A.; Nasr-El-Din, H.A. Wettability studies using low-salinity water in sandstone reservoirs. SPE Reserv. Eval. Eng. 2011, 14, 713–725. [Google Scholar] [CrossRef]
- Taborda, E.; Franco, C.; Lopera, S.; Alvarado, V.; Cortes, F. Effect of Nanoparticles/Nanofluids on the Rheology of Heavy Crude Oil and Its Mobility on Porous Media at Reservoir Condition. Fuel 2016, 184, 222–232. [Google Scholar] [CrossRef]















| Salinity | Condition | Contact Angle |
|---|---|---|
| 1% | Untreated | |
| Treated | ||
| 100% | Untreated | |
| Treated |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medina-Rodriguez, B.X.; Reilly, T.; Wang, H.; Smith, E.R.; Garcia-Olvera, G.; Alvarado, V.; Aryana, S. Time-Domain Nuclear Magnetic Resonance Determination of Wettability Alteration: Analysis for Low-Salinity Water. Appl. Sci. 2020, 10, 1017. https://doi.org/10.3390/app10031017
Medina-Rodriguez BX, Reilly T, Wang H, Smith ER, Garcia-Olvera G, Alvarado V, Aryana S. Time-Domain Nuclear Magnetic Resonance Determination of Wettability Alteration: Analysis for Low-Salinity Water. Applied Sciences. 2020; 10(3):1017. https://doi.org/10.3390/app10031017
Chicago/Turabian StyleMedina-Rodriguez, Bryan X., Teresa Reilly, Heng Wang, Erik Robert Smith, Griselda Garcia-Olvera, Vladimir Alvarado, and Saman Aryana. 2020. "Time-Domain Nuclear Magnetic Resonance Determination of Wettability Alteration: Analysis for Low-Salinity Water" Applied Sciences 10, no. 3: 1017. https://doi.org/10.3390/app10031017






