Minimizing Chromium Leaching from Low-Alloy Electric Arc Furnace (EAF) Slag by Adjusting the Basicity and Cooling Rate to Control Brownmillerite Formation
Abstract
:1. Introduction
2. Methods and Material
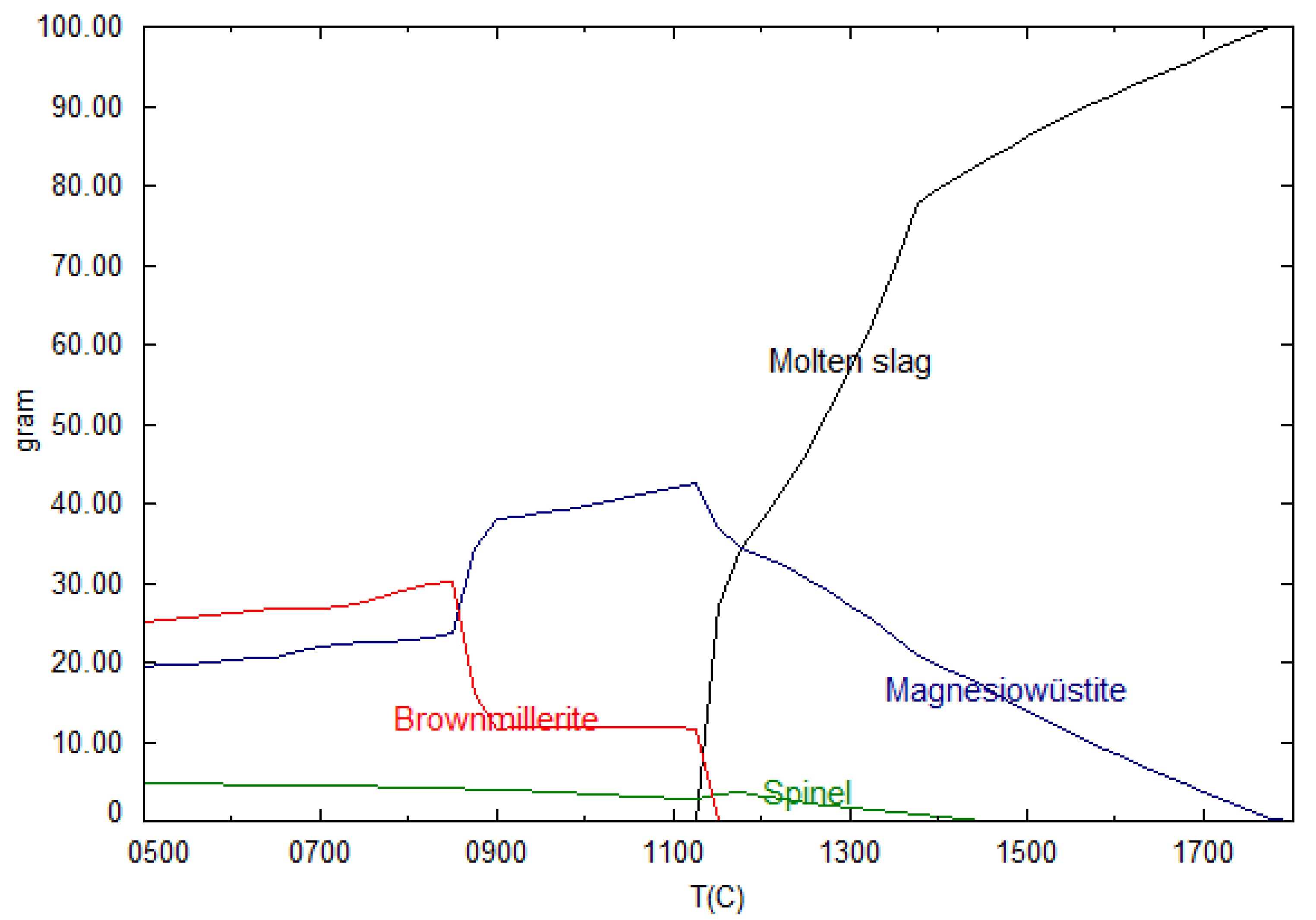
2.1. Thermodynamic Calculations
2.2. Experimental Overview
2.3. EN 12457-2 Leaching Test
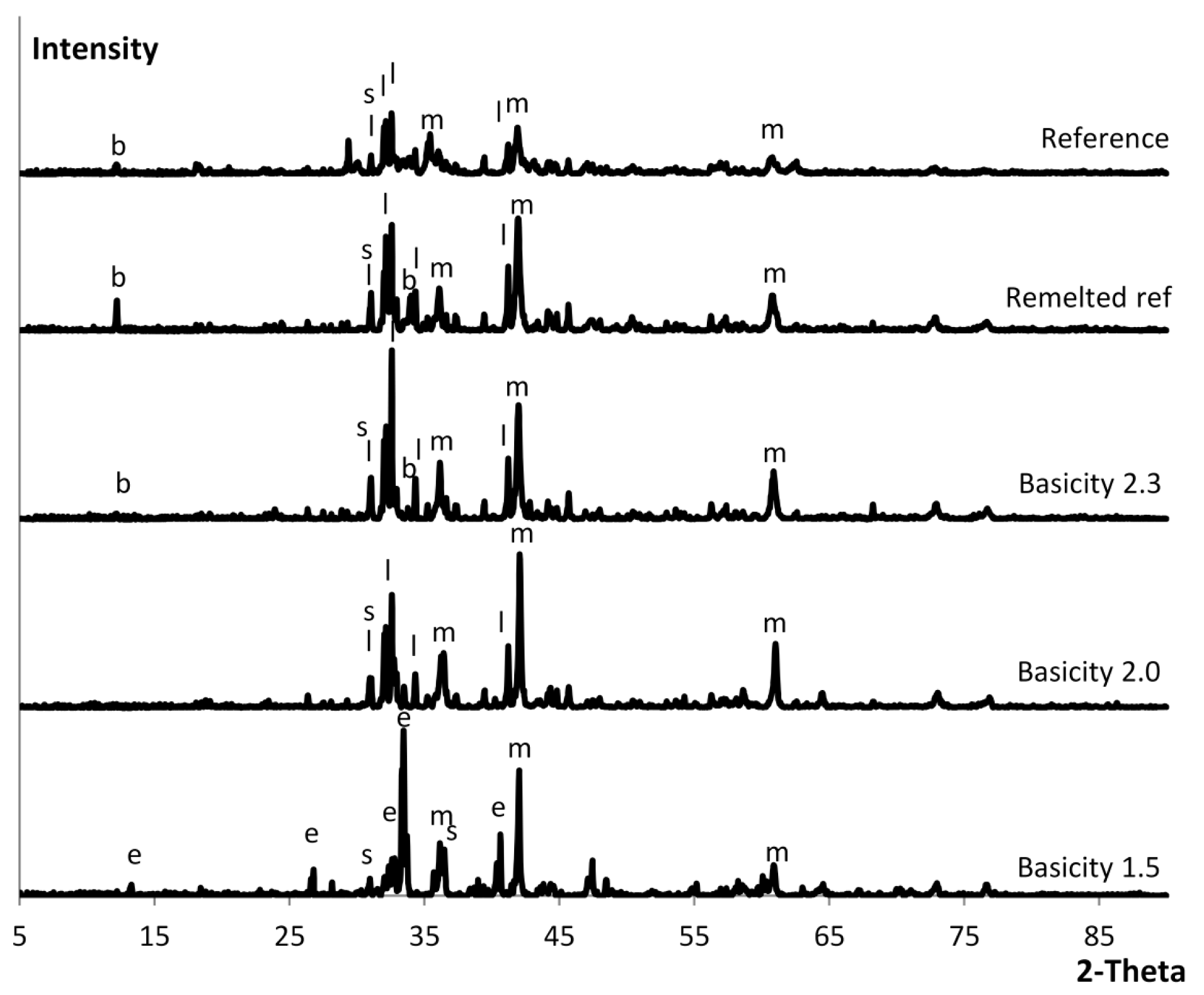
2.4. Mineralogical Characterization
2.5. Laboratory-Scale Experiments
2.5.1. Controlling Basicity
2.5.2. Controlling Cooling
3. Results and Discussion
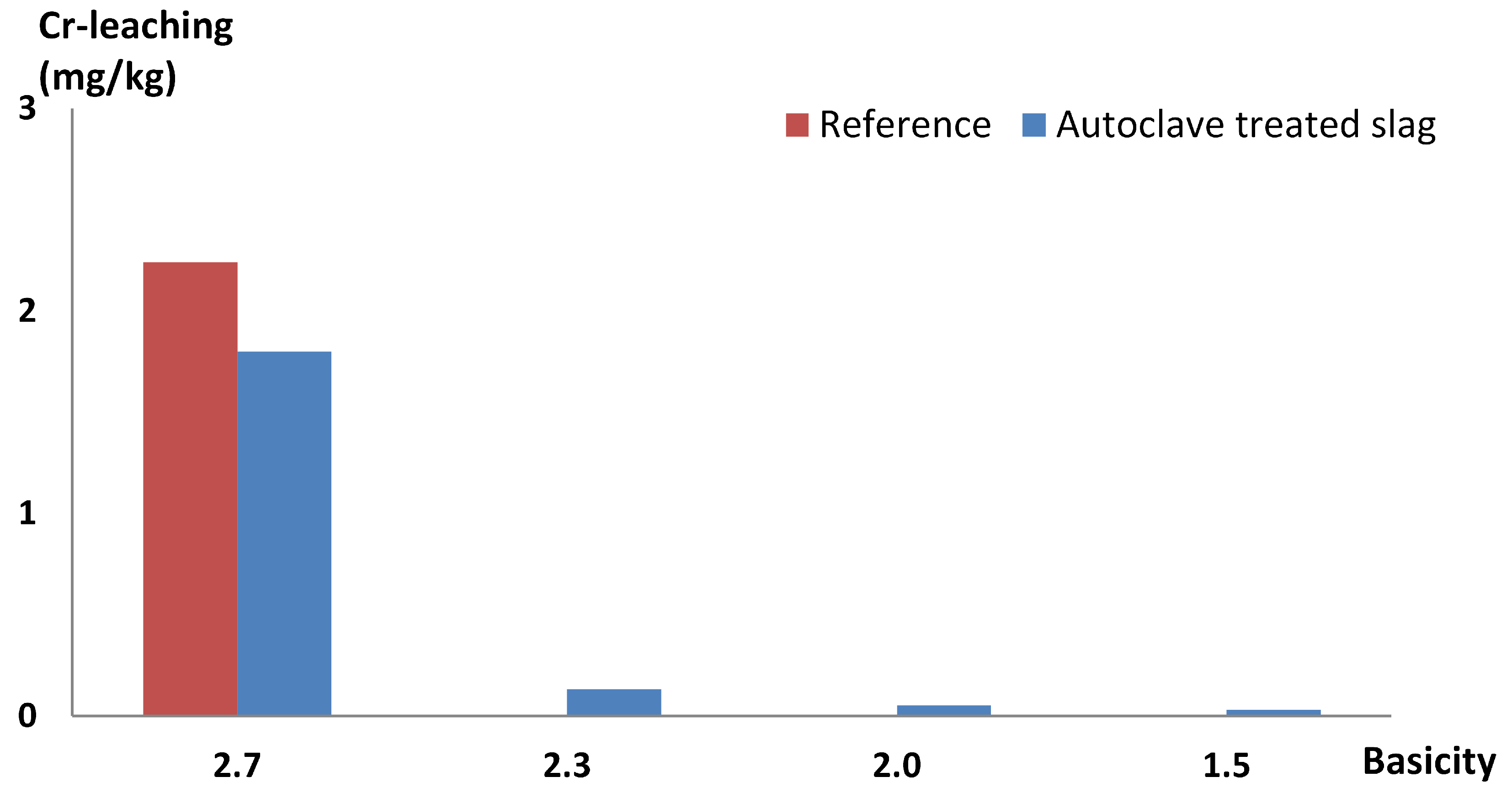
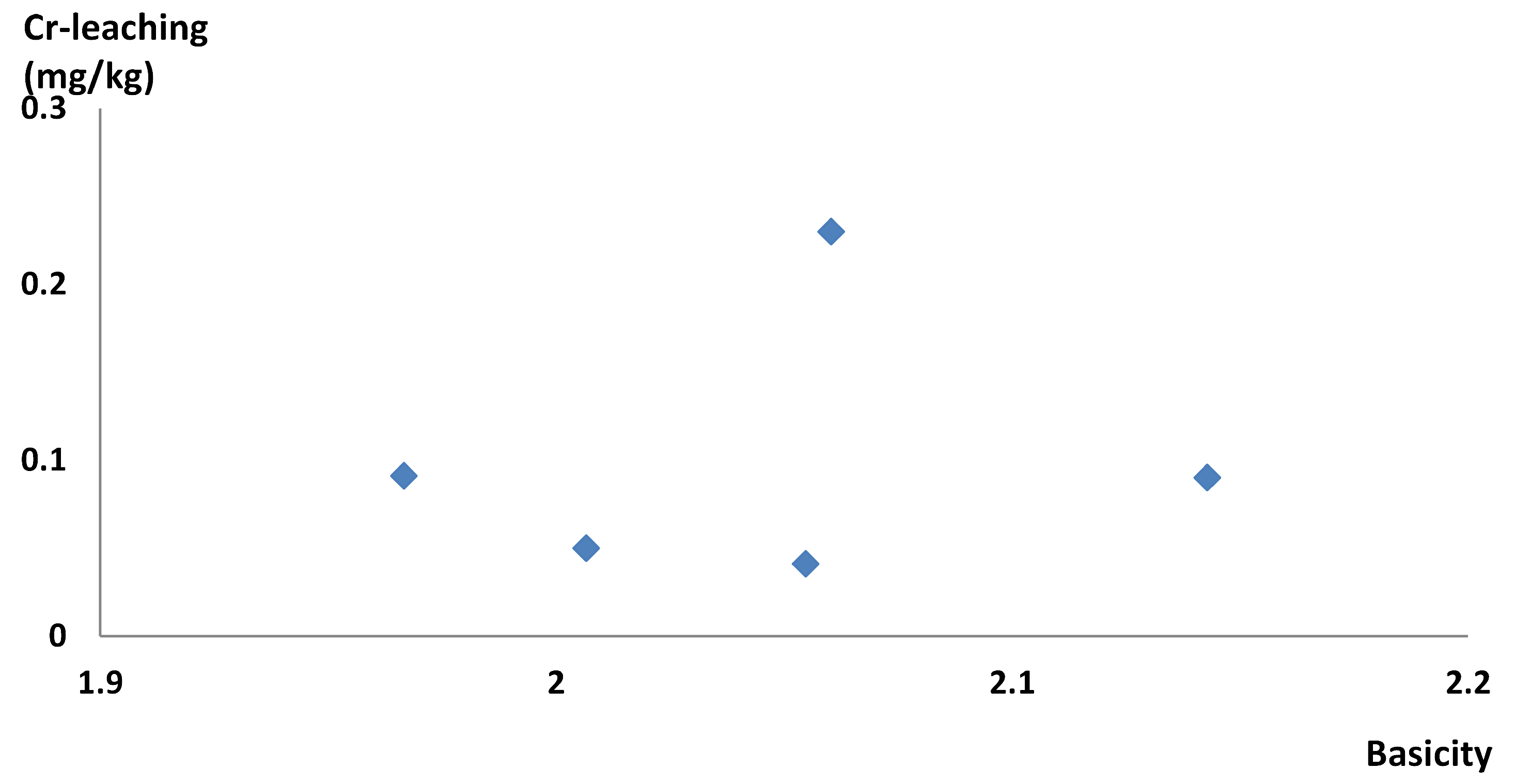
3.1. Controlling Basicity
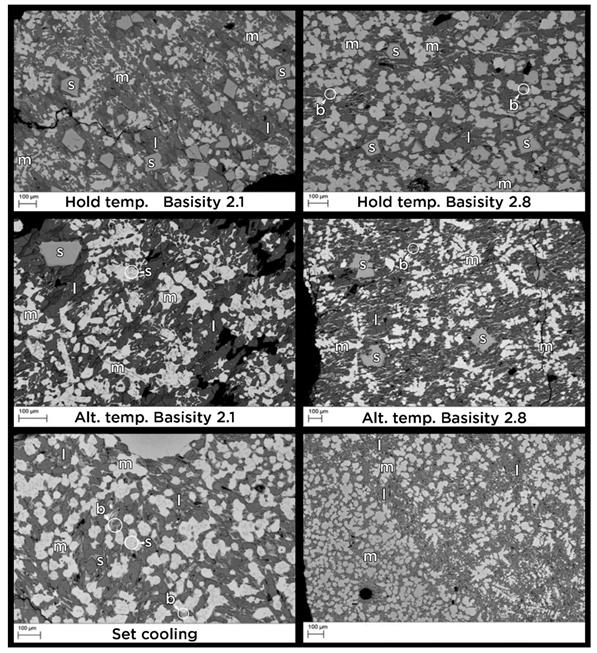
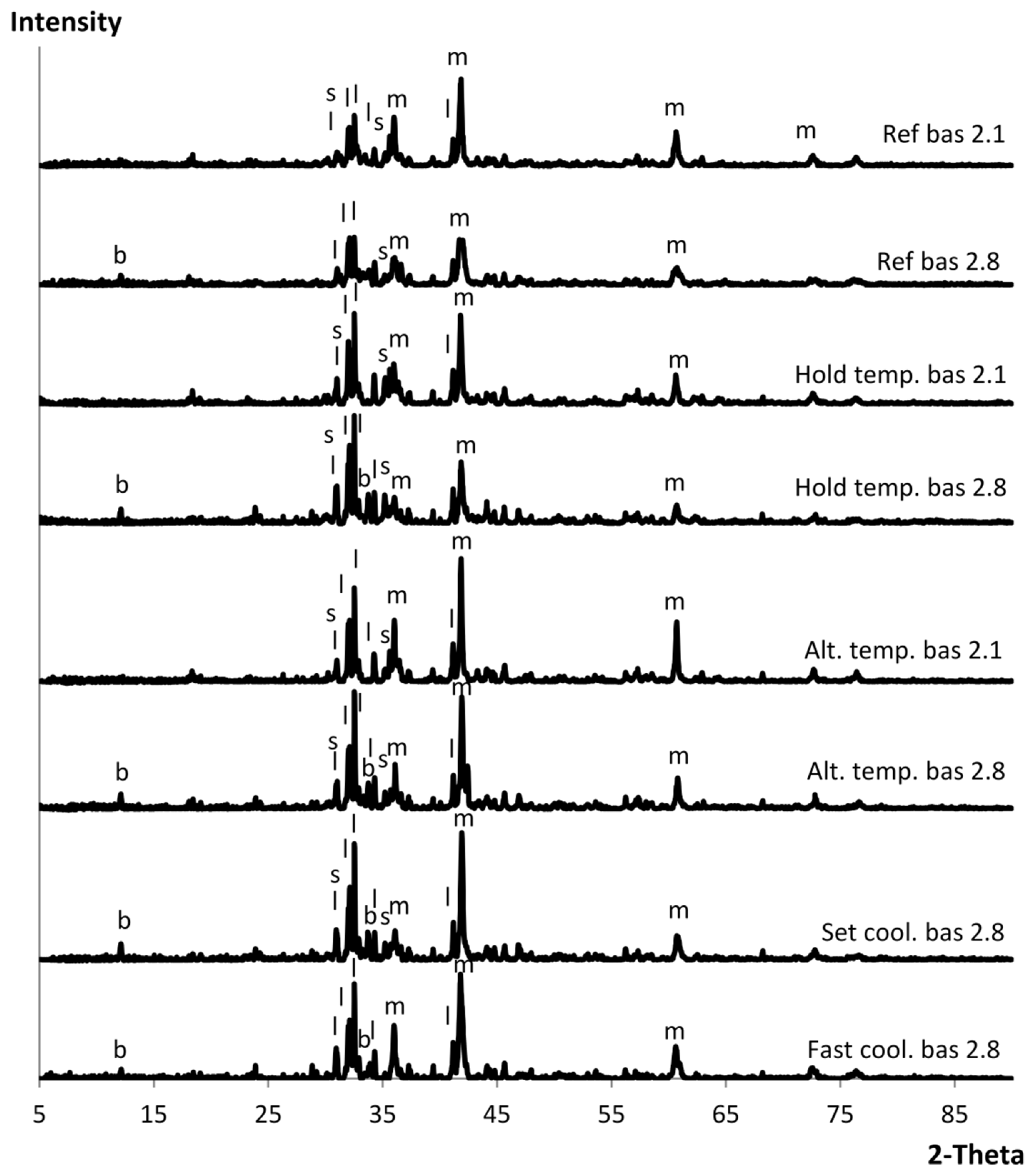
3.2. Controlling Cooling
4. Full-Scale Production Verifications
4.1. Controlling Basicity in Full-Scale Experiments
4.2. Controlling Cooling in Full-Scale Experiments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Produktion Jernkontoret. Available online: http://www.jernkontoret.se/sv/stalindustrin/branschfakta-och-statistik/produktion/ (accessed on 31 January 2018).
- Pålsson, K.; Stemne, J.; Johansson, L.; Blixt, E. Stålindustrin Gör Mer än Stål—Handbok för Restprodukter; Jernkontoret: Stockholm, Sweden, 2012. [Google Scholar]
- Motz, H.; Geiseler, J. Products of steel slags an opportunity to save natural resources. Waste Manag. 2001, 21, 285–293. [Google Scholar] [CrossRef]
- Juckes, L.M. The volume stability of modern steelmaking slags. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 2003, 112, 177–197. [Google Scholar] [CrossRef]
- Durinck, D.; Arnout, S.; Mertens, G.; Boydens, E.; Jones, P.T.; Elsen, J.; Blanpain, B.; Wollants, P. Borate distribution in stabilized stainless-steel slag. J. Am. Ceram. Soc. 2008, 91, 548–554. [Google Scholar] [CrossRef]
- Mudersbach, D.; Kühn, M.; Geisler, J.; Koch, K. Chrome immobilisation in EAF-slags from high-alloy steelmaking: tests at FEhS institute and development of an operational slag treatment process. In Proceedings of the 1st International Slag Valorisation Symposium, Leuven, Belgium, 6–7 April 2009. [Google Scholar]
- Pillay, K.; Von Blottnitz, H.; Petersen, J. Ageing of chromium(III)-bearing slag and its relation to the atmospheric oxidation of solid chromium(III)-oxide in the presence of calcium oxide. Chemosphere 2003, 52, 1771–1779. [Google Scholar] [CrossRef]
- Fakta och Nyckeltal. 2019. Available online: https://www.jernkontoret.se/sv/stalindustrin/branschfakta-och-statistik/fakta-och-nyckeltal/ (accessed on 8 October 2019).
- Pålsson, K.; Stemne, J.; Ruist, G.; Blixt, E. Stålindustrin Gör Mer än Stål—Handbok för Restprodukter 2018; Jernkontoret: Stockholm, Sweden, 2018. [Google Scholar]
- Jansson, Å. Minimal Återverkan på Ljusbågsugnsslagg på Miljön; Bergsskolan i Filipstad: Filipstad, Sweden, 2000. [Google Scholar]
- Nilsson, N. Inverkan av MgO på Ljusbågsugnsslaggens Lakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2002. [Google Scholar]
- Lindström, B. Inverkan av Processparametrar på Ljusbågsugsslaggens Kromlakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2004. [Google Scholar]
- Lindström, B. Arbetet med att Förbättra Ljusbågugnsslaggens Kromlakningsegenskaper vid Ovakos Anläggning i Hofors; Ovako Steel: Hofors, Sweden, 2006. [Google Scholar]
- Fällman, A. Leaching of chromium and barium from steel slag in laboratory and field tests—A solubility controlled process? Waste Manag. 2000, 20, 149–154. [Google Scholar] [CrossRef]
- Aldrian, A.; Raith, J.G.; Höllen, D.; Pomberger, R. Influence of chromium containing spinels in an electric arc furnace slag on the leaching behaviour. J. Solid Waste Technol. Manag. 2015, 41, 357–365. [Google Scholar] [CrossRef]
- Strandkvist, I.; Björkman, B.; Engström, F. Synthesis and dissolution of slag minerals—A study of β-dicalcium silicate, pseudowollastonite and monticellite. Can. Metall. Q. 2015, 54, 446–454. [Google Scholar] [CrossRef]
- Mombelli, D.; Mapelli, C.; Barella, S.; Di Cecca, C.; Le Saout, G.; Garcia-Diaz, E. The effect of microstructure on the leaching behaviour of electric arc furnace (EAF) carbon steel slag. Process Saf. Environ. Prot. 2016, 102, 810–821. [Google Scholar] [CrossRef] [Green Version]
- Strandkvist, I. Inverkan av Järnoxid på Ljusbågsugnsslaggens Kromlakningsegenskaper; Luleå University of Technology: Luleå, Sweden, 2010. [Google Scholar]
- Roininen, J.; Vaara, N.; Ylimaunu, J. Quality Control for Stainless Steel Slag Products. In Proceedings of the 4th European Slag Conference, Oulu, Finland, 20–21 June 2005; pp. 199–210. [Google Scholar]
- Geiseler, J.; Schlösser, R.; Scheel, R.; Koch, K.; Janke, D. Untersuchungen zum Hydratationsverhalten von synthetischen Magnesiowüstiten. Steel Res. 1987, 58, 210–214. [Google Scholar] [CrossRef]
- Strandkvist, I.; Sandström, Å.; Engström, F. Effect of FeO/MgO Ratio on Dissolution and Leaching of Magnesiowüstite. Steel Res. Int. 2017, 88, 1600322. [Google Scholar] [CrossRef]
- Qian, G.R.; Sun, D.D.; Tay, J.H.; Lai, Z.Y. Hydrothermal reaction and autoclave stability of Mg bearing RO phase in steel slag. Br. Ceram. Trans. 2002, 101, 159–164. [Google Scholar] [CrossRef]
- Schlösser, R.; Steffes, B. Commission of the European Communities. In Untersuchungen an Stahlwerksschlacken, Insbesondere im Hinblick auf Ihre Verwendung im Strassenbau; Kommission der Europäischen Gemeinschaften: Luxemburg, 1985. [Google Scholar]
- Mombelli, D.; Mapelli, C.; Di Cecca, C.; Barella, S.; Gruttadauria, A. Electric arc furnace slag: Study on leaching mechanisms and stabilization treatments. [Scorie da forno elettrico ad arco: Studio sui meccanismi di rilascio e trattamenti di stabilizzazione]. Metall. Ital. 2016, 108, 5–17. [Google Scholar]
- Taylor, H.F.W. Cement Chemistry, 2nd ed.; Thomas Telford Ltd.: London, UK, 1997. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.-H.; Kang, Y.-B.; Melancon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad Comput. Coupling Phase Diagr. Thermochem. 2016, 54, 35–53. [Google Scholar] [CrossRef] [Green Version]
- Mombelli, D.; Mapelli, C.; Barella, S.; Gruttadauria, A.; Le Saout, G.; Garcia-Diaz, E. The efficiency of quartz addition on electric arc furnace (EAF) carbon steel slag stability. J. Hazard. Mater. 2014, 279, 586–596. [Google Scholar] [CrossRef]
- Kilau, H.W.; Shah, I.D. Preventing Chromium Leaching from Waste Slag Exposed to Simulated Acid Precipitation: A Laboratory Study; US Department of the Interior, Bureau of Mines: Washington, DC, USA, 1984.
- Neuhold, S.; Van Zomeren, A.; Dijkstra, J.J.; Van der Sloot, H.A.; Drissen, P.; Algermissen, D.; Mudersbach, D.; Schüler, S.; Griessacher, T.; Raith, J.G.; et al. Investigation of possible leaching control mechanisms for chromium and vanadium in electric arc furnace (EAF) slags using combined experimental and modeling approaches. Minerals 2019, 9, 525. [Google Scholar] [CrossRef] [Green Version]
- Engström, F.; Adolfsson, D.; Samuelsson, C.; Sandström, Å.; Björkman, B. A study of the solubility of pure slag minerals. Miner. Eng. 2013, 41, 46–52. [Google Scholar] [CrossRef]
- Strandkvist, I.; Björkman, B.; Engström, F.; Pålsson, K. Chromium leaching from low-alloy EAF slag—Influence of ageing and FeO content. In Proceedings of the 14th ISIJ-VDEh Seminar, the 8th Japan-Nordic Countries Joint Symposium on Science and Technology of Process Metallurgy, ISIJ-VDeh-Jernkontoret Joint Symposium, Osaka, Japan, 15–16 April 2013; p. 57. [Google Scholar]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of steel slag under different cooling conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef] [PubMed]
- Piatak, N.M.; Parsons, M.B.; Seal, R.R., II. Characteristics and environmental aspects of slag: A review. Appl. Geochem. 2015, 57, 236–266. [Google Scholar] [CrossRef]




| Basicity Experiments | Cooling Experiments | |
|---|---|---|
| Lab scale | Basicity 2.7 remelted | Hold temp. 700 °C |
| Basicity 2.3 by SiO2-addition | Alternating temp. 800–1000 °C | |
| Basicity 2.0 by SiO2-addition | Set cooling rate, 4 °C/min | |
| Basicity 1.5 by SiO2-addition | Fast cooling rate, 25 °C within 2 h | |
| Full scale | Basicity 2.2 by SiO2-sand addition | Air-cooled |
| Water-cooled |
| CaO | MgO | SiO2 | Al2O3 | FeO | Fe2O3 | MnO | Cr2O3 |
|---|---|---|---|---|---|---|---|
| 31.0 | 9.12 | 11.4 | 6.4 | 14.5 | 15.8 | 5.5 | 3.1 |
| Basicity | CaO | MgO | SiO2 | Al2O3 | MnO | Cr2O3 | FeO | Fe2O3 | |
|---|---|---|---|---|---|---|---|---|---|
| Slag 1 | 2.1 | 28.5 | 8.14 | 13.3 | 5.62 | 7.05 | 4.05 | 20.6 | 8.10 |
| Slag 2 | 2.8 | 31.0 | 9.43 | 11.1 | 8.89 | 5.96 | 2.92 | 18.2 | 8.21 |
| CaO | MgO | SiO2 | Al2O3 | FeO | MnO | Cr2O3 | |
|---|---|---|---|---|---|---|---|
| Basicity 2.3 | 31.5 | 10.5 | 13.5 | 6.4 | 26.2 | 5.1 | 2.8 |
| Basicity 2.0 | 30.6 | 11.0 | 15.3 | 6.4 | 25.2 | 5.1 | 2.7 |
| Basicity 1.5 | 28.3 | 11.7 | 18.8 | 5.8 | 24.1 | 4.6 | 2.4 |
| Sample | Larnite | Magnesio- | Brownmillerite | Spinel | Merwinite |
|---|---|---|---|---|---|
| Ca2SiO4 | Wüstite MeO | Ca2(Al,Fe)2O5 | AB2O4 | Ca3MgSi2O8 | |
| Reference | XRD, SEM | XRD, SEM | XRD, SEM | XRD, SEM | |
| Remelted | XRD, SEM | XRD, SEM | XRD, SEM | SEM | |
| Basicity 2.3 | XRD, SEM | XRD, SEM | XRD, SEM | SEM | |
| Basicity 2.0 | XRD | XRD | XRD | ||
| Basicity 1.5 | XRD, SEM | XRD, SEM | XRD, SEM |
| Sample | Basicity | Larnite | Magneiso- | Brownmillerite | Spinel |
|---|---|---|---|---|---|
| Ca2SiO4 | Wûstite MeO | Ca2(Al,Fe)2O5 | AB2O4 | ||
| Reference | 2.1 | XRD | XRD | XRD | |
| 2.8 | XRD | XRD | XRD | XRD | |
| Hold. Temp. | 2.1 | XRD, SEM | XRD, SEM | XRD, SEM | |
| 2.8 | XRD, SEM | XRD, SEM | XRD, SEM | XRD, SEM | |
| Alter. Temp. | 2.1 | XRD, SEM | XRD, SEM | XRD, SEM | |
| 2.8 | XRD, SEM | XRD, SEM | XRD, SEM | XRD, SEM | |
| Set Cool. | 2.8 | XRD, SEM | XRD, SEM | XRD, SEM | XRD, SEM |
| Fast Cool. | 2.8 | XRD, SEM | XRD, SEM | XRD, SEM | SEM |
| CaO | MgO | SiO2 | Al2O3 | FeO | Na2O | K2O | |
|---|---|---|---|---|---|---|---|
| Sand 1 | 1.6 | 1.4 | 71.2 | 13.9 | 3.46 | 2.6 | 4.1 |
| Sand 2 | 1.76 | 0.87 | 73.5 | 12.7 | 2.33 | 3.05 | 3.73 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strandkvist, I.; Pålsson, K.; Andersson, A.; Olofsson, J.; Lennartsson, A.; Samuelsson, C.; Engström, F. Minimizing Chromium Leaching from Low-Alloy Electric Arc Furnace (EAF) Slag by Adjusting the Basicity and Cooling Rate to Control Brownmillerite Formation. Appl. Sci. 2020, 10, 35. https://doi.org/10.3390/app10010035
Strandkvist I, Pålsson K, Andersson A, Olofsson J, Lennartsson A, Samuelsson C, Engström F. Minimizing Chromium Leaching from Low-Alloy Electric Arc Furnace (EAF) Slag by Adjusting the Basicity and Cooling Rate to Control Brownmillerite Formation. Applied Sciences. 2020; 10(1):35. https://doi.org/10.3390/app10010035
Chicago/Turabian StyleStrandkvist, Ida, Kjell Pålsson, Anton Andersson, Jenny Olofsson, Andreas Lennartsson, Caisa Samuelsson, and Fredrik Engström. 2020. "Minimizing Chromium Leaching from Low-Alloy Electric Arc Furnace (EAF) Slag by Adjusting the Basicity and Cooling Rate to Control Brownmillerite Formation" Applied Sciences 10, no. 1: 35. https://doi.org/10.3390/app10010035





