Properties Analysis and Preparation of Biochar–Graphene Composites Under a One-Step Dip Coating Method in Water Treatment
Abstract
:1. Introduction
2. Methods
2.1. Experimental Materials and Equipment
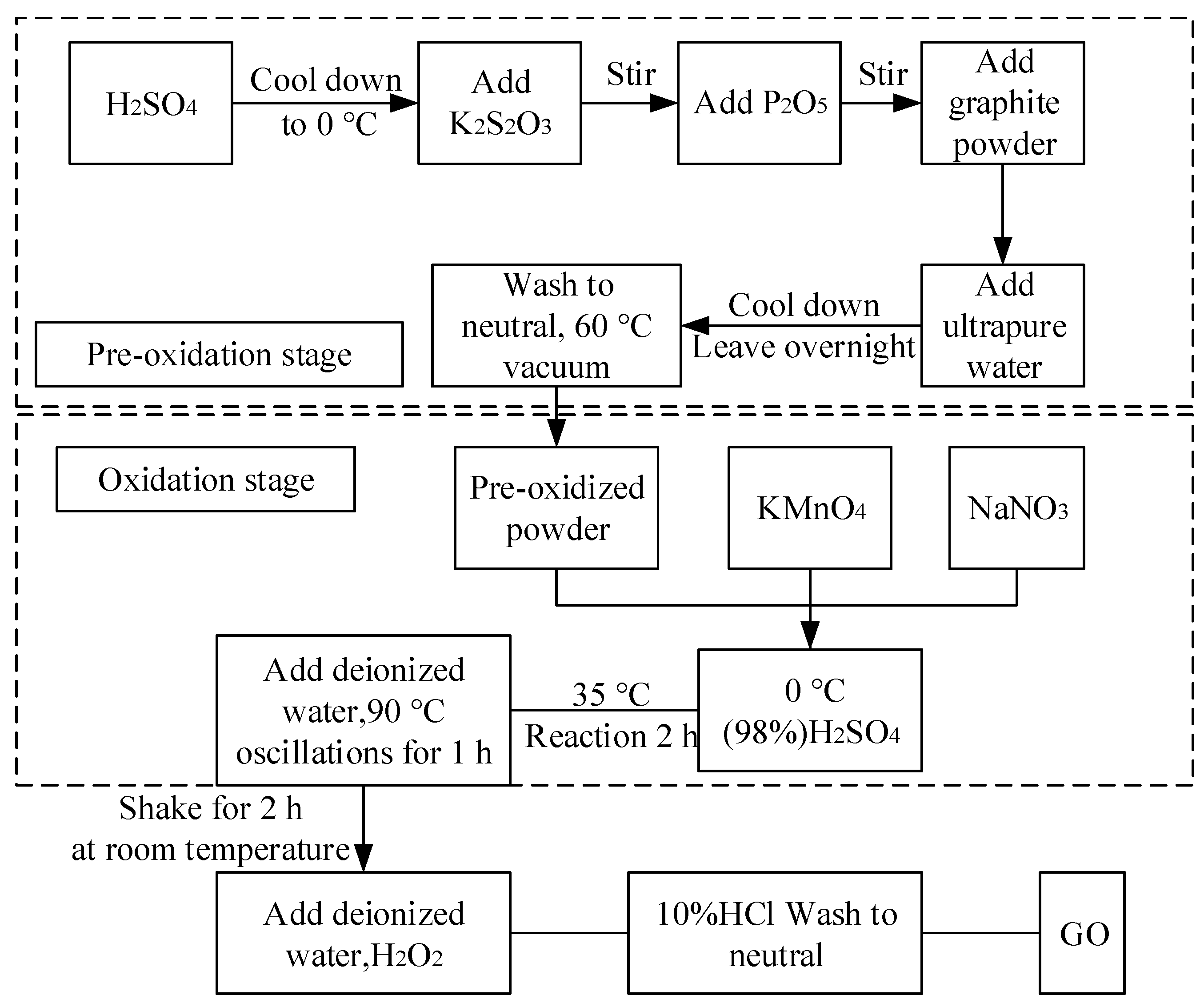
2.2. Synthesis of the GO
2.3. Preparation of BG Composites Based on One-Step Dip Coating Method
2.3.1. Preparation of Biochar
2.3.2. Preparation of BG Composites
2.4. Characterization of Biochar and BG Composites
2.5. Adsorption Kinetics and Isothermal Model
3. Results
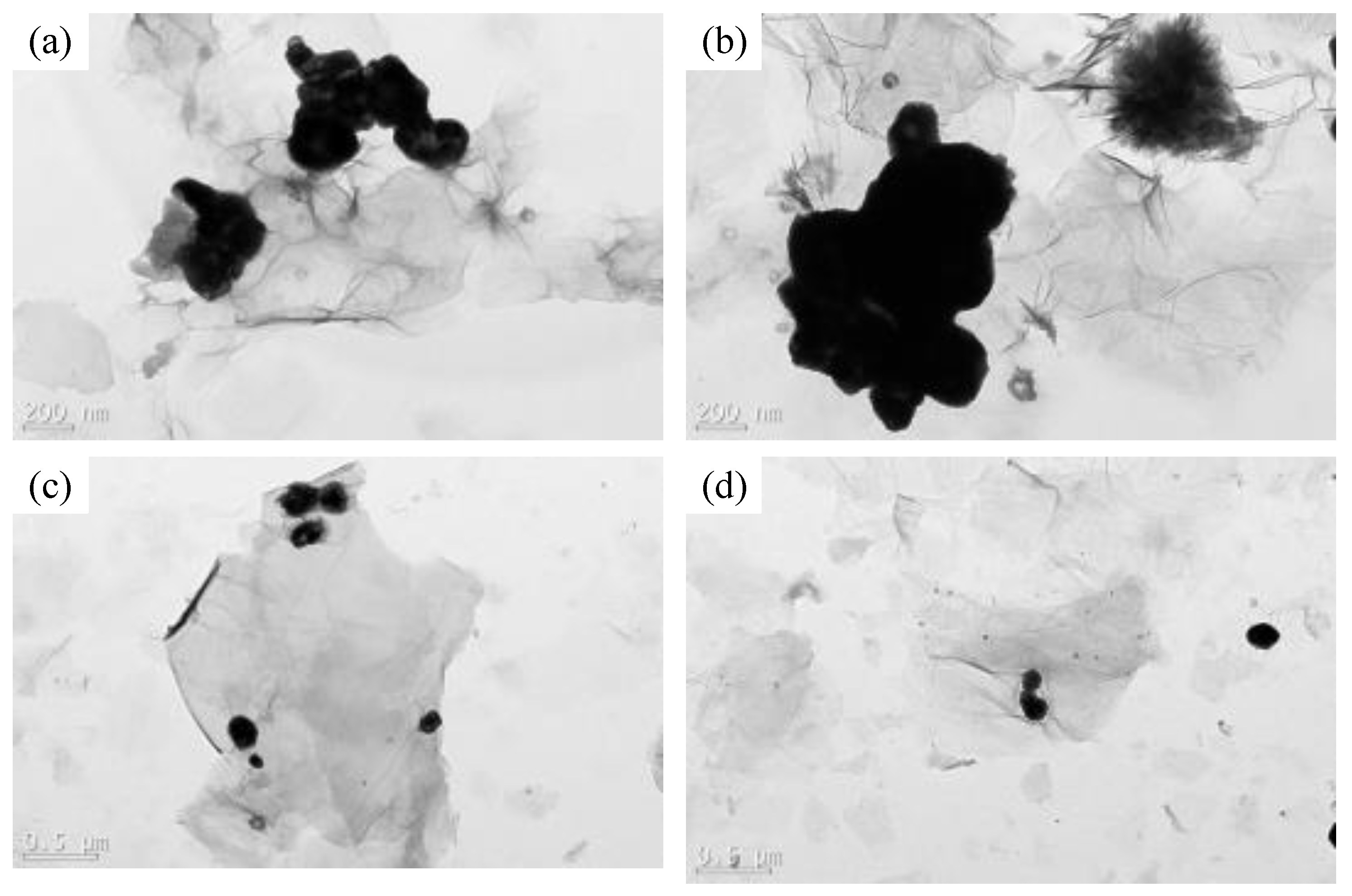
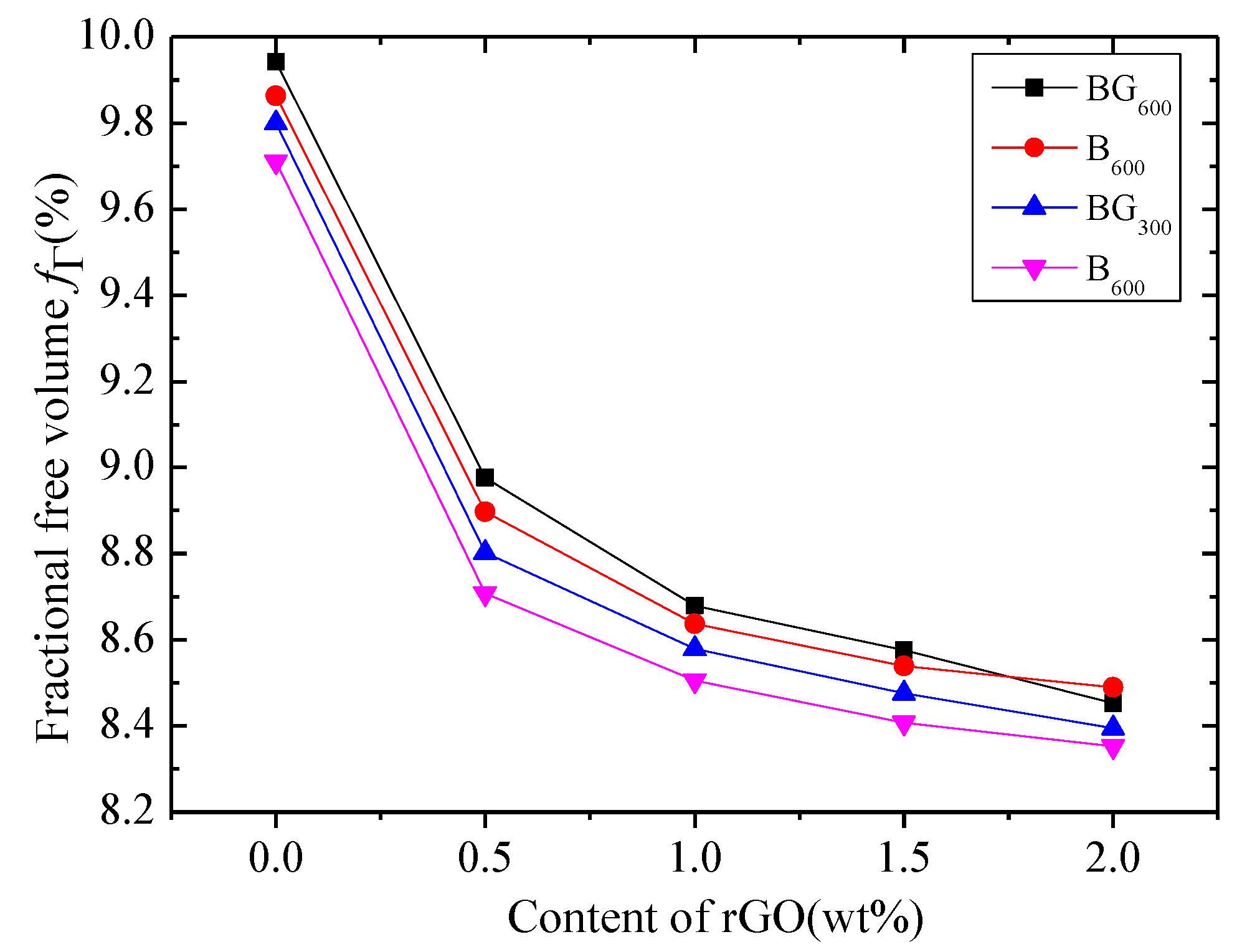
3.1. Structural Characterization of Biochar and BG Composites
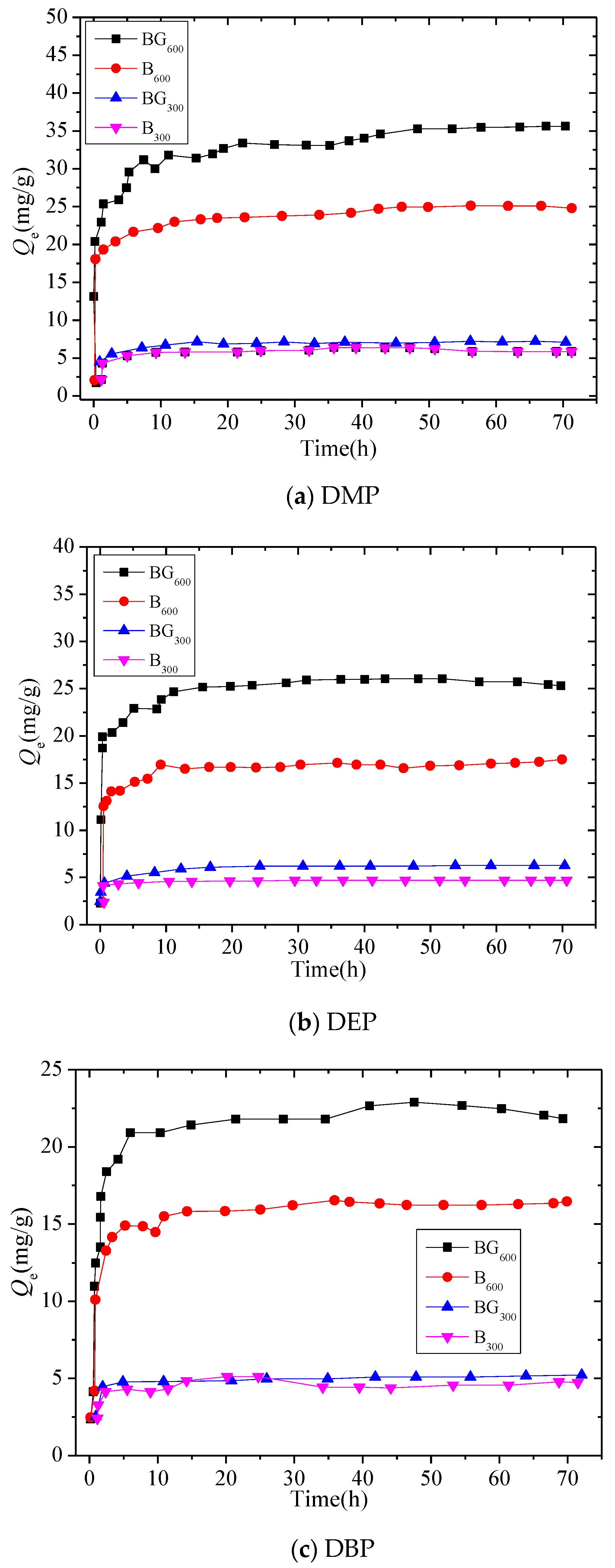
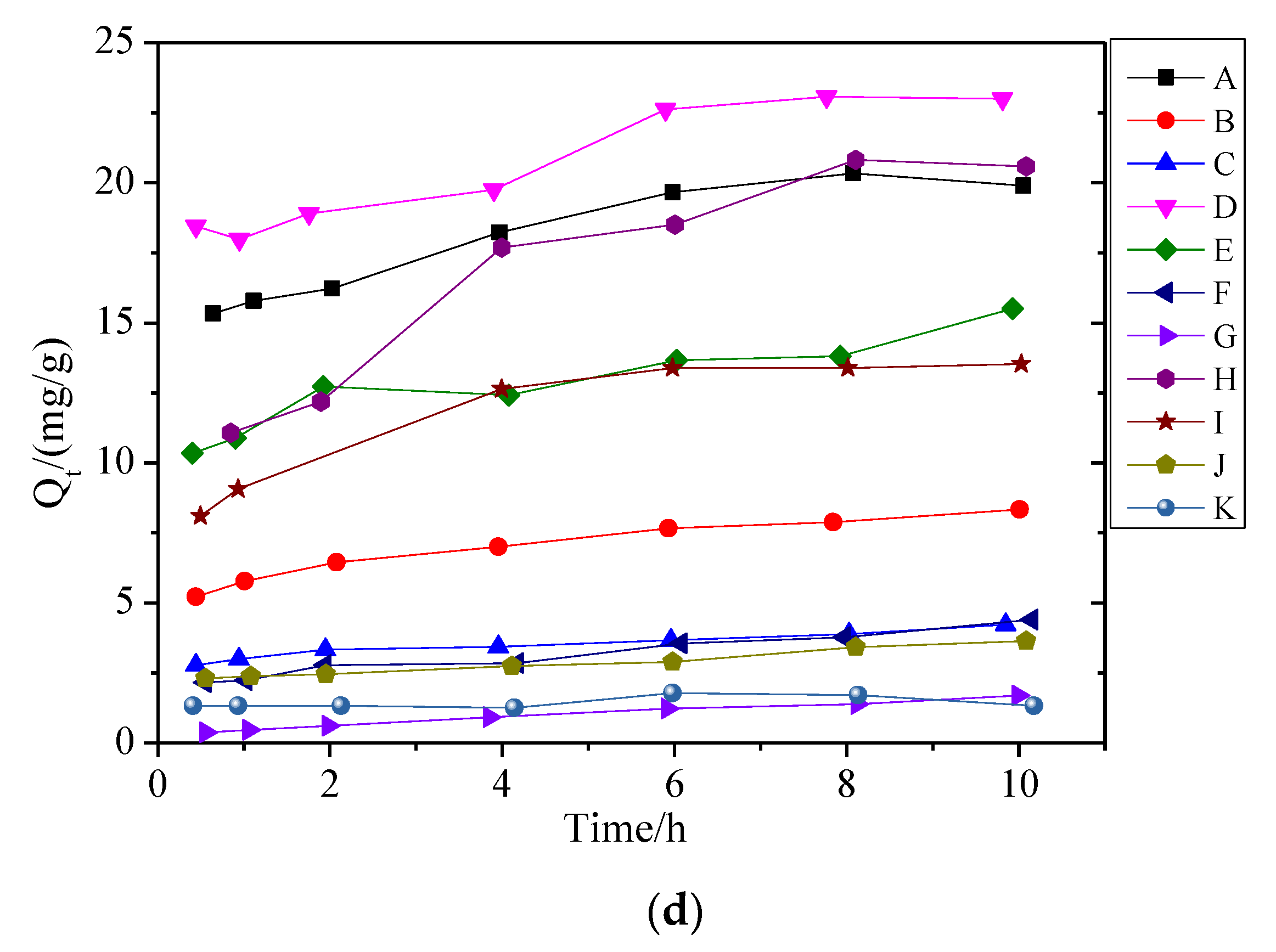
3.2. Adsorption Kinetics of Biochar and BG Composites
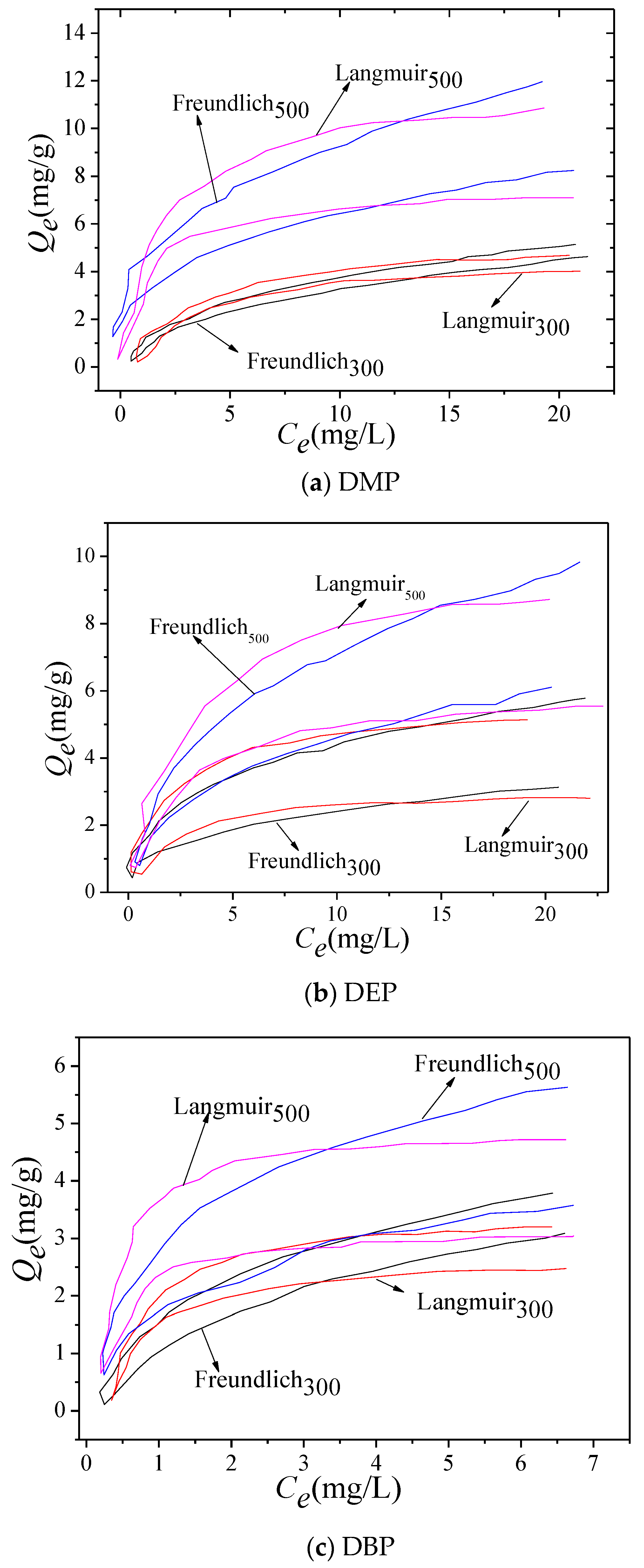
3.3. Adsorption Isotherms of PAEs on BG Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Abdul, G.; Zhu, X.; Chen, B. Structural characteristics of biochar-graphene nanosheet composites and their adsorption performance for phthalic acid esters. Chem. Eng. J. 2017, 319, 9–20. [Google Scholar] [CrossRef]
- Huang, Q.; Song, S.; Chen, Z.; Hu, B.W.; Chen, J.R.; Wang, X.K. Biochar-based materials and their applications in removal of organic contaminants from wastewater: State-of-the-art review. Biochar 2019, 1, 45–73. [Google Scholar] [CrossRef] [Green Version]
- Nupearachchi, C.N.; Mahatantila, K.; Vithanage, M. Application of graphene for decontamination of water; Implications for sorptive removal. Groundw. Sustain. Dev. 2017, 5, 206–215. [Google Scholar] [CrossRef]
- Zhang, C.M.; Fu, X.L.; Zhang, X.X.; Li, J.Z. The Effects of Metal Complexes of Nano-Graphene Oxide to Thermal Decomposition of FOX-7. Nanomaterials 2020, 10, 144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.Q.; Zhang, P.; Liang, B.; Liu, Y.X.; Xu, T.; Wang, L.F.; Cao, B.; Pan, K. Graphene oxide as an effective barrier on a porous nanofibrous membrane for water treatment. ACS Appl. Mater. Interfaces 2016, 8, 6211–6218. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.W.; Wu, T.F.; Shi, J.; Teng, K.Y.; Wang, W.; Ma, M.J.; Li, J.; Qian, X.M.; Li, C.Y.; Fan, J.T. Photocatalytic antifouling PVDF ultrafiltration membranes based on synergy of graphene oxide and TiO2 for water treatment. J. Membr. Sci. 2016, 520, 281–293. [Google Scholar] [CrossRef]
- Wang, H.; Mi, X.; Li, Y.; Zhan, S. 3D graphene-based macrostructures for water treatment. Adv. Mater. 2020, 32, 1806843. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, S.; Quan, X.; Hu, Y.; Zhao, H. Integration of microfiltration and visible-light-driven photocatalysis on g-C3N4 nanosheet/reduced graphene oxide membrane for enhanced water treatment. Appl. Catal. B 2016, 194, 134–140. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Ma, Z.; Wang, X.; Gao, C. Multilayered graphene oxide membranes for water treatment: A review. Carbon 2018, 139, 964–981. [Google Scholar] [CrossRef]
- Karkooti, A.; Yazdi, A.Z.; Chen, P.; McGregor, M.; Nazemifard, N.; Sadrzadeh, M. Development of advanced nanocomposite membranes using graphene nanoribbons and nanosheets for water treatment. J. Membr. Sci. 2018, 560, 97–107. [Google Scholar] [CrossRef]
- Ye, S.; Liu, Y.; Feng, J. Low-density, mechanical compressible, water-induced self-recoverable graphene aerogels for water treatment. ACS Appl. Mater. Interfaces 2017, 9, 22456–22464. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Ghorai, U.K.; Samanta, M.; Santra, A.; Das, G.; Chattopadhyay, K.K. Graphene wrapped Copper Phthalocyanine nanotube: Enhanced photocatalytic activity for industrial waste water treatment. Appl. Surf. Sci. 2017, 418, 156–162. [Google Scholar] [CrossRef]
- Bai, T.; Lv, L.; Du, W.; Fang, W.P.; Wang, Y.S. Improving the Tribological and Anticorrosion Performance of Waterborne Polyurethane Coating by the Synergistic Effect between Modified Graphene Oxide and Polytetrafluoroethylene. Nanomaterials 2020, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Fan, X.X.; Zhang, H.G.; Quan, X. A multifunctional graphene-based nanofiltration membrane under photo-assistance for enhanced water treatment based on layer-by-layer sieving. Appl. Catal. B 2018, 224, 204–213. [Google Scholar] [CrossRef]
- Meng, L.; Hu, Q.; Shi, C.; Huang, C. Roles of Graphene Additives in Optimizing the Microstructure and Properties of Ni–Cr–Graphene Coatings. Coatings 2020, 10, 104. [Google Scholar] [CrossRef] [Green Version]
- Arshad, A.; Iqbal, J.; Siddiq, M.; Ali, M.U.; Shabbir, H.; Nazeer, B.; Saleem, M.S. Solar light triggered catalytic performance of graphene-CuO nanocomposite for waste water treatment. Ceram. Int. 2017, 43, 10654–10660. [Google Scholar] [CrossRef]
- Huang, Y.; Ruan, G.H.; Ruan, Y.J.; Zhang, W.J.; Li, X.X.; Du, F.Y.; Hu, C.J.; Li, J.P. Hyper crosslinked porous polymers hybridized with graphene oxide for water treatment: Dye adsorption and degradation. RSC Adv. 2018, 8, 13417–13422. [Google Scholar] [CrossRef] [Green Version]
- Thakur, A.K.; Singh, S.P.; Kleinberg, M.N.; Gupta, A.; Aenusch, C.J. Laser-Induced Graphene–PVA Composites as Robust Electrically Conductive Water Treatment Membranes. ACS Appl. Mater. Interfaces 2019, 11, 10914–10921. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Tian, X.; Yang, C.; Zhou, Z. Facile synthesis of Fe3O4 nanoparticles decorated on 3D graphene aerogels as broad-spectrum sorbents for water treatment. Appl. Surf. Sci. 2016, 369, 11–18. [Google Scholar] [CrossRef]
- Abd-Elhamid, A.I.; Aly, H.F.; Soliman, H.A.M.; Shanshory, A.A.E.I. Graphene oxide: Follow the oxidation mechanism and its application in water treatment. J. Mol. Liq. 2018, 265, 226–237. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H. Engineering sub-nanometer channels in two-dimensional materials for membrane gas separation. Membranes 2018, 8, 100. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Sun, J.; Che, Y.; Lv, F.; Liu, F. Antibacterial properties of chitosan chloride-graphene oxide composites modified quartz sand filter media in water treatment. Int. J. Biol. Macromol. 2019, 121, 760–773. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Liu, G.; Han, K.; Ye, H.; Wei, S.; Zhou, Y. One-step facile synthesis of graphene oxide/TiO2 composite as efficient photocatalytic membrane for water treatment: Crossflow filtration operation and membrane fouling analysis. Chem. Eng. Process. Process Intensif. 2017, 120, 20–26. [Google Scholar] [CrossRef]
- Nakagawa, K.; Araya, S.; Kunimatsu, M.; Yoshioka, T.; Shintani, T.; Kamio, E. Matsuyama. Fabrication of Stacked Graphene Oxide Nanosheet Membranes Using Triethanolamine as a Crosslinker and Mild Reducing Agent for Water Treatment. Membranes 2018, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Han, K.; Zhou, Y.; Ye, H.Q.; Zhang, X.; Hu, J.B.; Li, X.J. Facile synthesis of highly dispersed Ag doped graphene oxide/titanate nanotubes as a visible light photocatalytic membrane for water treatment. ACS Sustain. Chem. Eng. 2018, 6, 6256–6263. [Google Scholar] [CrossRef]
- Arshadi Rastabi, S.; Sarraf Mamoory, R.; Blomquist, N.; Phadatare, M.; Olin, H. Synthesis of a NiMoO4/3D-rgo Nanocomposite via Starch Medium Precipitation Method for Supercapacitor Performance. Batteries 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Suh, D.W.; Hong, S.P.; Yoon, J. A surface-modified EDTA-reduced graphene oxide membrane for nanofiltration and anti-biofouling prepared by plasma post-treatment. Environ. Sci. 2019, 6, 2292–2298. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Nakhjiri, M.T.; Shakeri, E.; Khankeshipour, N.; Ghorbani, F. Carboxymethylcellulose-quaternary graphene oxide nanocomposite polymer hydrogel as a biodegradable draw agent for osmotic water treatment process. Cellulose 2019, 26, 1841–1853. [Google Scholar] [CrossRef]
- Wang, Z. Efficient adsorption of dibutyl phthalate from a-queous solution by activated carbon developed from phoenix leaves. Int. J. Environ. Sci. Technol. 2015, 12, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Moazzen, M.; Khaneghah, A.M.; Sharialifar, N.; Ahmadloo, M.; ES, I.; Baghani, N.A.; Yousefinejsd, S.; Alimohammadi, M.; Azari, A.; Dobaradaran, S.; et al. Multi-walled carbon nanotubes modified with iron oxide and silver nanoparticles (MWCNT-Fe3O4/Ag) as a novel adsorbent for determining PAEs in carbonaled soft drinks using magnetic SPE-GC/MS method. Arab. J. Chem. 2019, 12, 476488. [Google Scholar] [CrossRef]
- Chen, M.; Gong, D.F. 9 Discrimination of breast tumors in ultrasonic images using an ensemble classifier based on TensorFlow framework with feature selection. J. Invest. Med. 2019, 67 (Suppl. 1), A3. [Google Scholar]


| The Name of the Reagent | Chemical Equation | Purity | Manufacturer |
|---|---|---|---|
| Graphite powder | C | Analytical pure | Qingdao Tianyuan Da Graphite Co. LTD |
| Potassium permanganate | KMnO4 | Analytical pure | Hubei Wanye Pharmaceutical Co. LTD |
| Phosphorus pentoxide | P2O5 | Analytical pure | Shanghai Alighting Biochemical Technology Co. LTD |
| Potassium dichromate | Cr2K2O7 | Analytical pure | Shanghai Alighting Biochemical Technology Co. LTD |
| Sodium nitrate | NaNO3 | Analytical pure | Hebei Xuxing New Energy Technology Co. LTD |
| Concentrated sulfuric acid | H2SO4 | Guaranteed reagent | Zhengzhou Longda Chemical Co. LTD |
| Hydrogen peroxide | H2O2 | Analytical pure | Sino Pharma Chemical Reagent Co. LTD |
| Potassium persulfate | K2S2O8 | Analytical pure | Jinan Zhendong Chemical Co. LTD |
| Sodium chloride | NaCl | Analytical pure | Zhengzhou Chunqiu Chemical Co. LTD |
| Nitric acid | HNO3 | Guaranteed reagent | Sino Pharma Chemical Reagent Co. LTD |
| Sodium hydroxide | NaOH | Analytical pure | Nanjing Chemical Reagent Co. LTD |
| Concentrated hydrochloric acid | HCl | Guaranteed reagent | Sino Pharma Chemical Reagent Co. LTD |
| Diphenyl carbonyl dihydrazine | C13H14N4O | Analytical pure | Nanjing Chemical Reagent Co. LTD |
| Acetone | CH3COCH3 | Guaranteed reagent | Sino Pharma Chemical Reagent Co. LTD |
| Dimethyl phthalate | C10H10O4 | Analytical pure | Shanghai Alighting Biochemical Technology Co., LTD |
| Diethyl phthalate | C12H14O4 | Analytical pure | Shanghai Alighting Biochemical Technology Co., LTD |
| Dibutyl phthalate | C16H22O4 | Analytical pure | Shanghai Alighting Biochemical Technology Co., LTD |
| Names of Samples | BET-N2 * | BET-CO2 * | SA (N2-CO2) | ||
|---|---|---|---|---|---|
| SA/(m2g−1) | PV(m2g−1) | SA(m2g−1) | PV(m2g−1) | ||
| B300 | 8.40 | 0.014 | 117.70 | 0.055 | 109 |
| BG300 | 11.01 | 0.023 | 121.94 | 0.057 | 110 |
| B600 | 221.3 | 0.037 | 367.71 | 0.183 | 165 |
| BG600 | 251.82 | 0.044 | 455.98 | 0.182 | 139 |
| Phthalate | Names of Samples | Pseudo First Order Dynamic Model | Pseudo Second Order Dynamic Model | Internal Diffusion Model | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Qe/(mg/g) | k1(h−1) | radj2 | Qe/(mg/g) | k1(h−1) | radj2 | Qe/(mg/g) | k1(h−1) | radj2 | ||
| DMP | B300 | 3.88 | 1.92 | 0.843 | 4.05 | 0.783 | 0.939 | 2.81 | 0.123 | 0.934 |
| BG300 | 4.09 | 1.65 | 0.794 | 4.26 | 0.542 | 0.901 | 2.81 | 0.126 | 0.976 | |
| B600 | 20.15 | 1.71 | 0.821 | 20.09 | 0.127 | 0.948 | 14.76 | 0.607 | 0.876 | |
| BG600 | 28.12 | 1.28 | 0.805 | 19.32 | 0.063 | 0.927 | 19.35 | 1.09 | 0.854 | |
| DEP | B300 | 2.46 | 3.49 | 0.943 | 2.37 | 3.376 | 0.982 | 2.19 | 1.036 | 0.813 |
| BG300 | 3.51 | 1.08 | 0.836 | 3.26 | 0.447 | 0.945 | 2.27 | 0.145 | 0.901 | |
| B600 | 13.93 | 2.01 | 0.851 | 15.11 | 0.225 | 0.943 | 11.16 | 0.446 | 0.919 | |
| BG600 | 21.74 | 2.21 | 0.787 | 21.87 | 0.142 | 0.899 | 16.89 | 0.571 | 0.883 | |
| DBP | B300 | 2.41 | 2.32 | 0.837 | 2.69 | 1.38 | 0.917 | 2.07 | 0.049 | 0.712 |
| BG300 | 3.02 | 2.04 | 0.865 | 3.07 | 1.06 | 0.962 | 2.31 | 0.105 | 0.917 | |
| B600 | 14.27 | 1.91 | 0.901 | 13.65 | 0.142 | 0.916 | 10.08 | 0.467 | 0.645 | |
| BG600 | 19.85 | 0.52 | 0.876 | 20.04 | 0.051 | 0.963 | 10.67 | 1.21 | 0.876 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Z.; Wang, W.; Gao, H.; Liang, D. Properties Analysis and Preparation of Biochar–Graphene Composites Under a One-Step Dip Coating Method in Water Treatment. Appl. Sci. 2020, 10, 3689. https://doi.org/10.3390/app10113689
Yu Z, Wang W, Gao H, Liang D. Properties Analysis and Preparation of Biochar–Graphene Composites Under a One-Step Dip Coating Method in Water Treatment. Applied Sciences. 2020; 10(11):3689. https://doi.org/10.3390/app10113689
Chicago/Turabian StyleYu, Ze, Wenxuan Wang, He Gao, and Daxin Liang. 2020. "Properties Analysis and Preparation of Biochar–Graphene Composites Under a One-Step Dip Coating Method in Water Treatment" Applied Sciences 10, no. 11: 3689. https://doi.org/10.3390/app10113689




