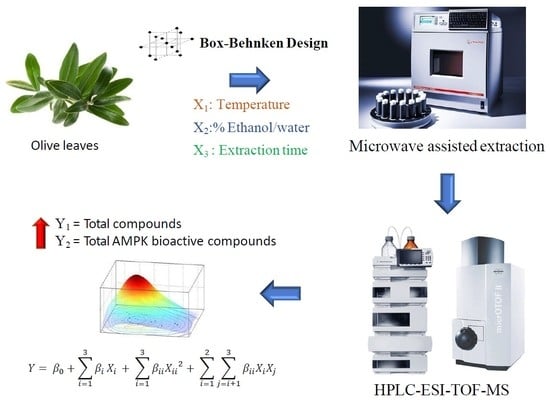
A Box-Behnken Design for Optimal Green Extraction of Compounds from Olive Leaves That Potentially Activate the AMPK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Samples
2.3. Extraction of Phenolic Compounds from Olive Leaves by Microwave-Assisted Extraction (MAE)
2.4. Characterization of MAE Olive Leaf Extract by High-Performance Liquid Chromatography Coupled to Electrospray Ionization Time-of-Flight Mass Spectrometry (HPLC-ESI-TOF-MS)
2.5. Response Surface Methodology for Evaluation of MAE Parameters on Concentration of Compounds in Olive Leaves
3. Results and Discussion
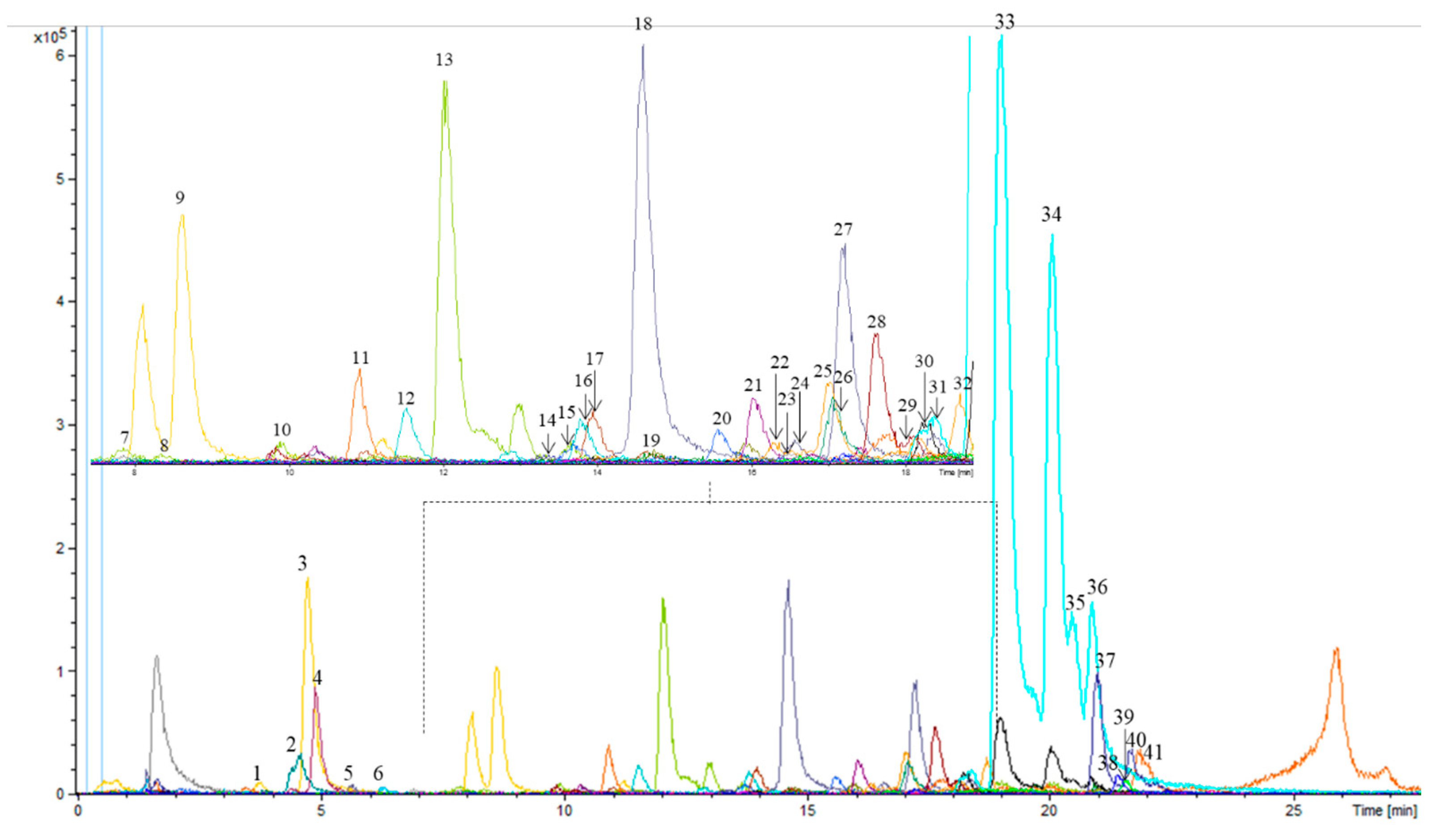
3.1. Determination of Phenolic and Other Compounds in Olive Leaf Samples by HPLC-ESI-TOF-MS
3.2. Optimization of MAE Extraction Conditions
3.2.1. Optimization of Extraction Conditions for the Content of Total Compounds
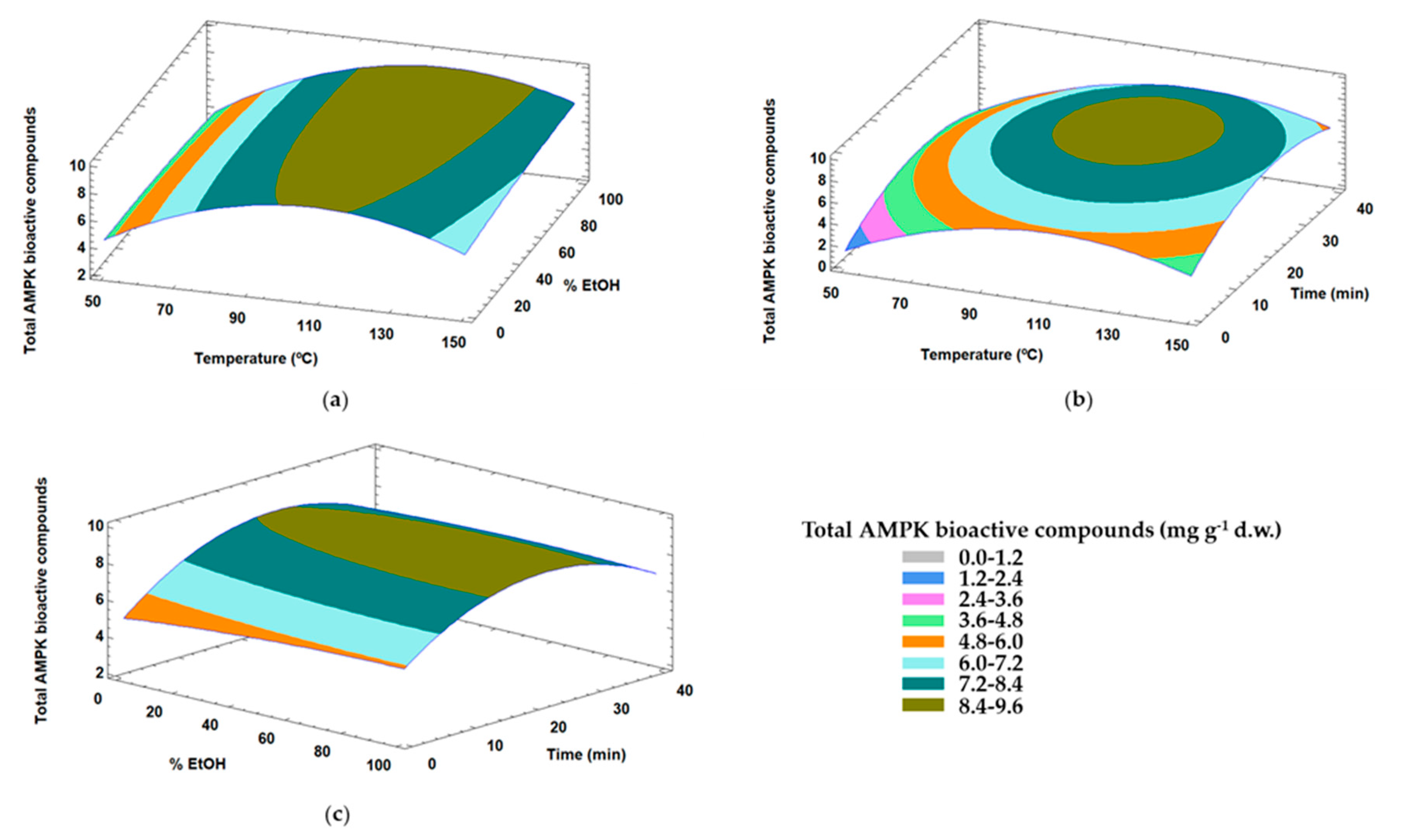
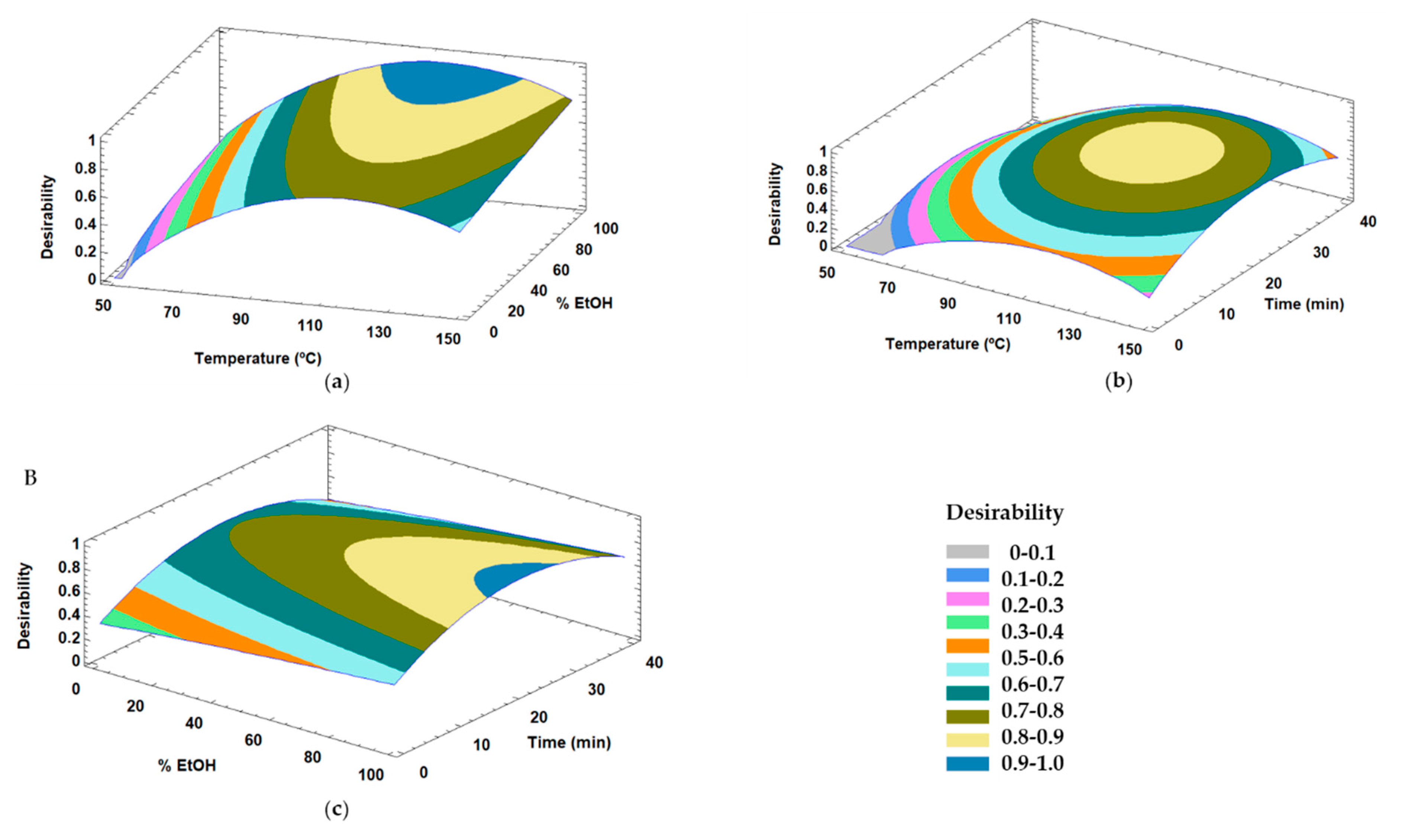
3.2.2. Optimization of Extraction Conditions for the Content of Compounds from Olive Leaves with Capacity to Modulate AMPK
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic compounds and antioxidant activity of olive leaf extracts. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Charisiadis, P.; Margianni, E.; Lamari, F.N.; Gerothanassis, I.P.; Tzakos, A.G. Olive Leaf Extracts Are a Natural Source of Advanced Glycation End Product Inhibitors. J. Med. Food 2013, 16, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omar, S.H. Cardioprotective and neuroprotective roles of oleuropein in olive. Saudi Pharm. J. 2010, 18, 111–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalatbary, A.R.; Zarrinjoei, G. Anti-Inflammatory effect of oleuropein in experimental rat spinal cord trauma. Iran. Red Crescent Med. J. 2012, 14, 229–234. [Google Scholar]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid. Based Complement. Altern. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stalikas, C.D. Extraction, separation, and detection methods for phenolic acids and flavonoids. J. Sep. Sci. 2007, 30, 3268–3295. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Biorefin. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Şahin, S.; Samli, R.; Tan, A.S.B.; Barba, F.J.; Hemat, F.C.; Cravotto, G.; Lorenzo, J.M. Solvent-free microwave-assisted extraction of polyphenols from olive tree leaves: Antioxidant and antimicrobial properties. Molecules 2017, 22, 1056. [Google Scholar]
- Chan, C.; Yusoff, R.; Ngoh, G.; Kung, F.W. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Carciochi, R.A.; D’Alessandro, L.G.; Vauchel, P.; Rodriguez, M.M.; Nolasco, S.M.; Dimitrov, K. Valorization of agrifood by-products by extracting valuable bioactive compounds using green processes. In Ingredients Extraction by Physicochemical Methods in Food; Elsevier: London, UK, 2017; pp. 191–228. ISBN 9780128115213. [Google Scholar]
- Li, Y.; Fabiano-tixier, A.S.; Vian, M.A.; Chemat, F. Solvent-free microwave extraction of bioactive compounds provides a tool for green analytical chemistry. Trends Anal. Chem. 2013, 47, 1–11. [Google Scholar] [CrossRef]
- Veggi, P.C.; Martinez, J.; Meireles, M.A.A. Fundamentals of Microwave Extraction. In Microwave-Assisted Extraction for Bioactive Compounds: Theory and Practice; Chemat, F., Cravotto, G., Eds.; Springer: New York, NY, USA, 2013; pp. 15–52. [Google Scholar]
- De Silvaa, E.O.; Borges, L.L.; da Conceição, E.C.; Bara, M.T.F. Box—Behnken experimental design for extraction of artemisinin from Artemisia annua and validation of the assay method. Rev. Bras. Farmacogn. 2017, 27, 519–524. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H.H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [Green Version]
- Menendez, J.A.; Joven, J.; Aragonès, G.; Barrajón-catalán, E.; Beltrán-debón, R.; Linares, I.B.; Camps, J.; Corominas-faja, B.; Cufí, S.; Fernández-arroyo, S.; et al. Xenohormetic and anti-aging activity of secoiridoid polyphenols present in extra virgin olive oil. Cell Cycle 2013, 12, 555–578. [Google Scholar] [CrossRef] [Green Version]
- Barrajón-Catalán, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Molecular promiscuity of plant polyphenols in the management of age-related diseases: Far beyond their antioxidant properties. Adv. Exp. Med. Biol. 2014, 824, 141–159. [Google Scholar]
- Fryer, L.G.; Carling, D. AMP-activated protein kinase and the metabolic syndrome. Biochem. Soc. Trans. 2005, 33, 362–366. [Google Scholar] [CrossRef] [Green Version]
- Hardie, D.G.; Hawley, S.A.; Scott, J.W. AMP-activated protein kinase—Development of the energy sensor concept. J. Physiol. 2006, 574, 7–15. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Ceddia, R.B. Remodeling glucose and lipid metabolism through AMPK activation: Relevance for treating obesity and Type 2 diabetes. Clin. Lipidol. 2009, 4, 465–477. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, C.; Olivares-Vicente, M.; Rodríguez-Pérez, C.; Herranz-López, M.; Lozano-Sanchez, J.; Segura-Carretero, A.; Fernández-Gutiérrez, A.; Encinar, J.A.; Micol, V. AMPK modulatory activity of olive—Tree leaves phenolic compounds: Bioassay-guided isolation on adipocyte model and in silico approach. PLoS ONE 2017, 12, 1–22. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Rodrigues, G.D.M. Pressurized Liquid Extraction of Oil from Soybean Seeds. Can. J. Chem. Eng. 2017, 95, 2383–2389. [Google Scholar]
- Talhaoui, N.; Goómez-Caravaca, A.M.; Roldaán, C.; Leoón, L.; la Rosa, R.D.; Fernaóndez-Gutieérrez, A.; Segura-Carretero, A. Chemometric analysis for the evaluation of phenolic patterns in olive leaves from six cultivars at different growth stages. J. Agric. Food Chem. 2015, 63, 1722–1729. [Google Scholar] [CrossRef] [PubMed]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De, R.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Determination of phenolic compounds of ‘ Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT Food Sci. Technol. 2014, 58, 28–34. [Google Scholar] [CrossRef]
- Fu, S.; Arráez-Roman, D.; Segura-Carretero, A.; Menéndez, J.A.; Menéndez-Gutiérrez, M.P.; Micol, V.; Fernández-Gutiérrez, A. Qualitative screening of phenolic compounds in olive leaf extracts by hyphenated liquid chromatography and preliminary evaluation of cytotoxic activity against human breast cancer cells. Anal. Bioanal. Chem. 2010, 397, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Taamalli, A.; Arráez-Román, D.; Ibañez, E.; Zarrouk, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Optimization of microwave-assisted extraction for the characterization of olive leaf phenolic compounds by using HPLC-ESI-TOF-MS/IT-MS2. J. Agric. Food Chem. 2012, 60, 791–798. [Google Scholar] [CrossRef] [PubMed]
- Herrero, M.; Temirzoda, T.N.; Segura-Carretero, A.; Quirantes, R.; Plaza, M.; Ibañez, E. New possibilities for the valorization of olive oil by-products. J. Chromatogr. A 2011, 1218, 7511–7520. [Google Scholar] [CrossRef] [Green Version]
- Yateem, H.; Afaneh, I.; Al-Rimawi, F. Optimum conditions for oleuropein extraction from olive leaves. Int. J. Appl. Sci. Technol. 2014, 4, 153–157. [Google Scholar]
- Alupului, A.; Cãlinescu, I.; Lavric, V. Microwave extraction of active principles from medicinal plants. Univ. Politeh. Din Bucuresti Sci. Bull. 2012, 74, 129–142. [Google Scholar]
- Osorio-Tobón, J.F. Recent advances and comparisons of conventional and alternative extraction techniques of phenolic compounds. J. Food Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Hemalatha, S.; Mandal, V.; Mohan, Y. Microwave assisted extraction—An innovative and promising extraction tool for medicinal plant research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Khoddami, A.; Wilkes, M.A.; Roberts, T.H. Techniques for analysis of plant phenolic compounds. Molecules 2013, 18, 2328–2375. [Google Scholar] [CrossRef]
- Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M.; Rafiee, Z.; Jafari, S.M.; Alami, M.; Khomeiri, M. Microwave-assisted extraction of phenolic compounds from olive leaves; a comparison with maceration. J. Anim. Plant Sci. 2011, 21, 738–745. [Google Scholar]
- Japón-Luján, R.; Luque-Rodríguez, J.M.; Luque De Castro, M.D. Multivariate optimisation of the microwave-assisted extraction of oleuropein and related biophenols from olive leaves. Anal. Bioanal. Chem. 2006, 385, 753–759. [Google Scholar] [CrossRef]
- Chanioti, S.; Liadakis, G.; Tzia, C. Solid liquid extraction. In Food Engineering Handbook: Food Process Engineering (Contemporary Food Engineering); Varzakas, T., Tzia, C., Eds.; CRC Press: Boca Raton, FL, USA, 2014; p. 275. [Google Scholar]
- Mustapa, A.N.; Martin, A.; Gallego, J.R.; Mato, R.B.; Cocero, M.J. Microwave-assisted extraction of polyphenols from Clinacanthus nutans Lindau medicinal plant: Energy perspective and kinetics modeling. Chem. Eng. Process. Process Intensif. 2015, 97, 66–74. [Google Scholar] [CrossRef]
- Alañón, M.E.; Ivanović, M.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. Choline chloride derivative-based deep eutectic liquids as novel green alternative solvents for extraction of phenolic compounds from olive leaf. Arab. J. Chem. 2020, 13, 1685–1701. [Google Scholar] [CrossRef]
- Do, Q.D.; Angkawijaya, A.E.; Tran-Nguyen, P.L.; Huynh, L.H.; Soetaredjo, F.E.; Ismadji, S.; Ju, Y.H. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J. Food Drug Anal. 2014, 22, 296–302. [Google Scholar] [CrossRef] [Green Version]
- Radojković, M.; Moreira, M.M.; Soares, C.; Fátima Barroso, M.; Cvetanović, A.; Švarc-Gajić, J.; Morais, S.; Delerue-Matos, C. Microwave-assisted extraction of phenolic compounds from Morus nigra leaves: Optimization and characterization of the antioxidant activity and phenolic composition. J. Chem. Technol. Biotechnol. 2018, 93, 1684–1693. [Google Scholar] [CrossRef] [Green Version]
- Routray, W.; Orsat, V. Microwave-assisted extraction of flavonoids: A review. Food Bioprocess Technol. 2012, 5, 409–424. [Google Scholar] [CrossRef]
- Kim, J.S.; Kang, O.J.; Gweon, O.C. Comparison of phenolic acids and flavonoids in black garlic at different thermal processing steps. J. Funct. Foods 2013, 5, 80–86. [Google Scholar] [CrossRef]
- Akçay, H.; Anagün, A.S. Multi response optimization application on a manufacturing factory. Math. Comput. Appl. 2013, 18, 531–538. [Google Scholar] [CrossRef]

| MAE | X1 | X2 | X3 | Total Compounds (mg g−1 d.w.) | Total AMPK Bioactive Compounds (mg g−1 d.w.) |
|---|---|---|---|---|---|
| 1 | 50 | 100 | 22.5 | 35.72 ± 0.17 b | 3.70 ± 0.10 b,c |
| 2 | 50 | 50 | 40 | 21.85 ± 0.16 a | 2.81 ± 0.05 a |
| 3 | 100 | 50 | 22.5 | 57.37 ± 0.07 f | 8.64 ± 0.08 g,h |
| 4 | 150 | 100 | 22.5 | 74.24 ± 0.50 i | 7.93 ± 0.21 g |
| 5 | 100 | 100 | 5 | 67.18 ± 0.60 h | 6.99 ± 0.16 f |
| 6 | 150 | 0 | 22.5 | 51.36 ± 0.37 d | 6.56 ± 0.21 f |
| 7 | 100 | 100 | 40 | 63.55 ± 0.90 g | 7.05 ± 0.24 f |
| 8 | 50 | 0 | 22.5 | 21.84 ± 0.37 a | 4.06 ± 0.35 c,d |
| 9 | 50 | 50 | 5 | 21.02 ± 0.02 a | 2.87 ± 0.10 a,b |
| 10 | 100 | 0 | 40 | 39.19 ± 0.09 c | 6.97 ± 0.13 f |
| 11 | 100 | 50 | 22.5 | 58.25 ± 0.37 f | 9.04 ± 0.53 h |
| 12 | 100 | 0 | 5 | 35.73 ± 0.07 b | 6.29 ± 0.06 f |
| 13 | 150 | 50 | 40 | 38.81 ± 0.57 c | 5.25 ± 0.21 e |
| 14 | 100 | 50 | 22.5 | 62.49 ± 0.45 g | 9.39 ± 0.02 h |
| 15 | 150 | 50 | 5 | 53.47 ± 0.31 e | 4.80 ± 0.002 d,e |
| Total Compounds | |||||
|---|---|---|---|---|---|
| Source | SS | DF | MS | F-Value | p-Value |
| X1 | 1723.74 | 1 | 1723.74 | 230.05 | 0.0043 * |
| X2 | 1071.32 | 1 | 1071.32 | 142.98 | 0.0069 * |
| X3 | 24.51 | 1 | 24.51 | 3.27 | 0.2122 |
| X11 | 898.99 | 1 | 898.99 | 119.98 | 0.0082 * |
| X12 | 20.26 | 1 | 20.26 | 2.70 | 0.2418 |
| X13 | 59.98 | 1 | 59.98 | 8.01 | 0.1055 |
| X22 | 15.05 | 1 | 15.05 | 2.01 | 0.2921 |
| X23 | 12.60 | 1 | 12.60 | 1.68 | 0.3242 |
| X33 | 367.86 | 1 | 367.86 | 49.09 | 0.0198 * |
| Lack of fit | 112.07 | 3 | 37.36 | 4.99 | 0.1716 |
| Pure error | 14.99 | 2 | 7.49 | ||
| Total (corr.) | 4280.1 | 14 | |||
| R2 | 97.03 | ||||
| Adj R2 | 91.69 | ||||
| Peak | AMPK Bioactive Compounds |
|---|---|
| 9 | Secologanoside isomer b |
| 10 | Elenolic acid glucoside isomer c |
| 14 | Demethyloleuropein |
| 15 | Hydroxyoleuropein/hydroxyoleuroside isomer a |
| 16 | Rutin |
| 17 | Luteolin rutinoside |
| 18 | luteolin glucoside isomer a |
| 19 | Verbascoside |
| 22 | Oleuropein glucoside isomer a |
| 23 | Diosmetin rhamnoside glucoside (diosmin) |
| 24 | luteolin glucoside isomer b |
| 35 | Oleuropein isomer c |
| 36 | Oleuropein isomer d |
| 37 | ligstroside |
| 38 | Luteolin |
| 39 | Quercetin |
| 40 | Oleuropein/oleuroside methyl ether |
| 41 | Resinoside |
| Total AMPk Bioactive Compounds | |||||
|---|---|---|---|---|---|
| Source | SS | DF | MS | F-Value | p-Value |
| X1 | 15.41 | 1 | 15.41 | 108.22 | 0.0091 * |
| X2 | 0.40 | 1 | 0.40 | 2.79 | 0.2366 |
| X3 | 0.16 | 1 | 0.16 | 1.11 | 0.4033 |
| X11 | 37.2 | 1 | 37.2 | 261.32 | 0.0038 * |
| X12 | 0.75 | 1 | 0.75 | 5.25 | 0.1490 |
| X13 | 0.07 | 1 | 0.07 | 0.48 | 0.5612 |
| X22 | 0.29 | 1 | 0.29 | 2.06 | 0.2874 |
| X23 | 0.10 | 1 | 0.10 | 0.70 | 0.4922 |
| X33 | 13.50 | 1 | 13.50 | 94.82 | 0.0104 * |
| Lack of fit | 0.71 | 3 | 0.24 | 1.67 | 0.3957 |
| Pure error | 0.28 | 2 | 0.14 | ||
| Total (corr.) | 65.46 | 14 | |||
| R2 | 98.47 | ||||
| Adj R2 | 95.73 | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín-García, B.; Pimentel-Moral, S.; Gómez-Caravaca, A.M.; Arráez-Román, D.; Segura-Carretero, A. A Box-Behnken Design for Optimal Green Extraction of Compounds from Olive Leaves That Potentially Activate the AMPK Pathway. Appl. Sci. 2020, 10, 4620. https://doi.org/10.3390/app10134620
Martín-García B, Pimentel-Moral S, Gómez-Caravaca AM, Arráez-Román D, Segura-Carretero A. A Box-Behnken Design for Optimal Green Extraction of Compounds from Olive Leaves That Potentially Activate the AMPK Pathway. Applied Sciences. 2020; 10(13):4620. https://doi.org/10.3390/app10134620
Chicago/Turabian StyleMartín-García, Beatriz, Sandra Pimentel-Moral, Ana María Gómez-Caravaca, David Arráez-Román, and Antonio Segura-Carretero. 2020. "A Box-Behnken Design for Optimal Green Extraction of Compounds from Olive Leaves That Potentially Activate the AMPK Pathway" Applied Sciences 10, no. 13: 4620. https://doi.org/10.3390/app10134620