Application of Metal Magnetic Memory Testing Technology to the Detection of Stress Corrosion Defect
Abstract
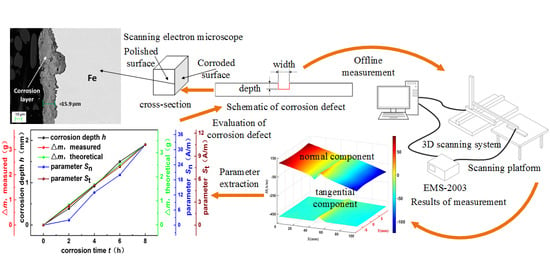
:1. Introduction
2. Experimental Section
2.1. Specimen Preparation
2.2. Description of Corrosion and Detection System
2.3. Testing Method
2.3.1. Static Tension Experiment
2.3.2. Corrosion Experiment without Loading
2.3.3. Corrosion Experiment after Loading
2.3.4. Surface Characterization
3. Results and Discussion
3.1. Experimental Results
3.2. Comparison of Experimental Results
3.3. Characterization of the Corrosion Layer
3.4. Analysis and Discussion
4. Quantitative Evaluation Parameters
5. Conclusions
- (1)
- The normal and tangential components of the MFL signals in corrosion experiments without and after loading presented a “trough-peak”, and “peak” shape, respectively. It is known from experimental results that when the specimen was corroded without and after loading, the normal and tangential components both showed the same signal characteristics, and a more substantial amplitude value could be found in the corrosion experiment after loading.
- (2)
- The normal and tangential components of the MFL signals in the static tensile experiment presented a “peak-trough”, and “trough” shape, respectively. It is found that the normal and tangential components showed different signal characteristics under different working conditions: When the specimen was subjected to tensile load and corroded, respectively, the normal and tangential components both exhibited opposite signal characteristics. According to the analysis, the critical reason for different signal characteristics is that the leakage magnetic field Hc generated at the defect after the specimen has been corroded is opposite to the leakage magnetic field Hm caused by force.
- (3)
- The characterization parameters of MFL signals, Sn and St, are capable of evaluating the corrosion degree of the specimen, and St is better. The characterization parameter of the tangential component of the MFL signals is more sensitive to the corrosion, which is different from the traditional research results on the MFL signals caused by stress where the characterization parameter of the normal component of the MFL signals is more sensitive to the force. The direct dependence of corrosion depth (h, theoretical) on the parameter St, was developed and the average error rates between the predicted and measured values are 8.94% under the same working condition. Therefore, the expression can be used to evaluate the corrosion degree of the specimen quantitatively.
Author Contributions
Funding
Conflicts of Interest
References
- Xue, H.B.; Cheng, Y. Passivity and Pitting Corrosion of X80 Pipeline Steel in Carbonate/Bicarbonate Solution Studied by Electrochemical Measurements. J. Mater. Eng. Perform. 2010, 19, 1311–1317. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zhang, J.; Li, X.; Zhou, J. Failure analysis of high strength pipeline with single and multiple corrosions. Mater. Des. 2015, 67, 552–557. [Google Scholar] [CrossRef]
- Ilman, M. Kusmono Analysis of internal corrosion in subsea oil pipeline. Case Stud. Eng. Fail. Anal. 2014, 2, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Coelho, D.; Linares, O.A.C.; Oliveira, A.L.S.; Andrade, M.A.S., Jr.; Mascaro, L.H.; Neto, J.E.S.B.; Bruno, O.M.; Pereira, E.C. Introducing a low-cost tool for 3D characterization of pitting corrosion in stainless steel. J. Solid State Electrochem. 2020, 24, 1909–1919. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Zhang, J.; Liu, X.; Li, X.; Zhou, J. Failure assessment of X80 pipeline with interacting corrosion defects. Eng. Fail. Anal. 2015, 47, 67–76. [Google Scholar] [CrossRef]
- Zhao, W.; Xin, R.; He, Z.; Wang, Y. Contribution of anodic dissolution to the corrosion fatigue crack propagation of X80 steel in 3.5 wt.% NaCl solution. Corros. Sci. 2012, 63, 387–392. [Google Scholar] [CrossRef]
- Mansfeld, F.; Sun, Z.; Hsu, C. Electrochemical noise analysis (ENA) for active and passive systems in chloride media. Electrochim. Acta 2001, 46, 3651–3664. [Google Scholar] [CrossRef]
- Guo, X.P.; Chen, Z.Y.; Qu, J.; Dong, Z.H. Novel quantitative method for evaluation pitting corrosion and pitting corrosion inhibition of carbon steel using electrochemical noise analysis. J. Mater. Sci. 2005, 40, 4469–4473. [Google Scholar] [CrossRef]
- Girija, S.; Mudali, U.K. Electrochemical Noise Analysis of Pitting Corrosion of Type 304L Stainless Steel. Corrosion 2014, 70, 283–293. [Google Scholar] [CrossRef]
- Chen, A.; Cao, F.; Liao, X.; Liu, W.; Zheng, L.; Zhang, J.; Cao, C. Study of pitting corrosion on mild steel during wet–dry cycles by electrochemical noise analysis based on chaos theory. Corros. Sci. 2013, 66, 183–195. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, G. Quantitative evaluation of pit sizes for high strength steel: Electrochemical noise, 3-D measurement, and image-recognition-based statistical analysis. Mater. Des. 2016, 94, 176–185. [Google Scholar] [CrossRef]
- Ahmad, A.; Bond, J. Non-Destructive Evaluation and Quality Control, ASM Handbook; ASM International: Almere, The Netherlands, 1989; Volume 17. [Google Scholar]
- Blitz, J. Electrical and Magnetic Methods of Non-Destructive Testing; Adam Hilger IOP Publishing Ltd.: Bristol, UK, 1991. [Google Scholar]
- Jiles, D.C. Introduction to Magnetism and Magnetic Materials, 2nd ed; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Chen, F.X. Magnetic Particle Detection by the Portable Electromagnetic Yoke. Nondestruct. Test. 2015, 37, 64–66. [Google Scholar]
- Liu, B.P.; Zhang, Y.M.; Zhou, G.Q. Magnetic particle testing for fillet welds of vertical cylindrical steel storage tank. Pet. Eng. Constr. 2015, 41, 76–79. [Google Scholar]
- Jagadish, C.; Clapham, L.; Atherton, D. Influence of uniaxial elastic stress on power spectrum and pulse height distribution of surface Barkhausen noise in pipeline steel. IEEE Trans. Magn. 1990, 26, 1160–1163. [Google Scholar] [CrossRef]
- Lindgren, M.; Lepistö, T. Relation between residual stress and Barkhausen noise in a duplex steel. NDT E Int. 2003, 36, 279–288. [Google Scholar] [CrossRef]
- Kim, H.M.; Rho, Y.W.; Yoo, H.R.; Cho, S.H.; Kim, D.K.; Koo, S.J.; Park, G.S. A study on the measurement of axial cracks in the Magnetic Flux Leakage NDT system. In Proceedings of the 2012 IEEE International Conference on Automation Science and Engineering (CASE), Seoul, Korea, 20–24 August 2012; pp. 624–629. [Google Scholar]
- Sablik, M.J.; Augustyniak, B. Modeling the magnetic field dependence of magnetoacoustic emission and its dependence on creep damage. Mater. Eval. 2000, 58, 655–660. [Google Scholar]
- Kim, H.M.; Park, G.S. A Study on the Estimation of the Shapes of Axially Oriented Cracks in CMFL Type NDT System. IEEE Trans. Magn. 2014, 50, 109–112. [Google Scholar] [CrossRef]
- Neslušan, M.; Bahleda, F.; Minárik, P.; Zgútová, K.; Jambor, M. Non-destructive monitoring of corrosion extent in steel rope wires via Barkhausen noise emission. J. Magn. Magn. Mater. 2019, 484, 179–187. [Google Scholar] [CrossRef]
- Dubov, A.A. A study of metal properties using the method of magnetic memory. Met. Sci. Heat Treat. 1997, 39, 401–405. [Google Scholar] [CrossRef]
- Wilson, J.; Tian, G.Y.; Barrans, S. Residual magnetic field sensing for stress measurement. Sens. Actuators A Phys. 2007, 135, 381–387. [Google Scholar] [CrossRef]
- Wang, Z.; Yao, K.; Deng, B.; Ding, K. Quantitative study of metal magnetic memory signal versus local stress concentration. NDT E Int. 2010, 43, 513–518. [Google Scholar] [CrossRef]
- Dubov, A.; Dubov, A.; Kolokolnikov, S. Application of the metal magnetic memory method for detection of defects at the initial stage of their development for prevention of failures of power engineering welded steel structures and steam turbine parts. Weld. World 2013, 58, 225–236. [Google Scholar] [CrossRef]
- Roskosz, M. Metal magnetic memory testing of welded joints of ferritic and austenitic steels. NDT E Int. 2011, 44, 305–310. [Google Scholar] [CrossRef]
- Roskosz, M.; Bieniek, M. Evaluation of residual stress in ferromagnetic steels based on residual magnetic field measurements. NDT E Int. 2012, 45, 55–62. [Google Scholar] [CrossRef]
- Roskosz, M.; Bieniek, M. Analysis of the universality of the residual stress evaluation method based on residual magnetic field measurements. NDT E Int. 2013, 54, 63–68. [Google Scholar] [CrossRef]
- Moonesan, M.; Kashefi, M. Effect of sample initial magnetic field on the metal magnetic memory NDT result. J. Magn. Magn. Mater. 2018, 460, 285–291. [Google Scholar] [CrossRef]
- Leng, J.; Liu, Y.; Zhou, G.; Gao, Y. Metal magnetic memory signal response to plastic deformation of low carbon steel. NDT E Int. 2013, 55, 42–46. [Google Scholar] [CrossRef]
- Ren, S.; Ren, X. Studies on laws of stress-magnetization based on magnetic memory testing technique. J. Magn. Magn. Mater. 2018, 449, 165–171. [Google Scholar] [CrossRef]
- Wu, L.; Wang, Y.-S.; Shi, P.; Zhao, B.; Wang, Y.-S. Influence of inhomogeneous stress on biaxial 3D magnetic flux leakage signals. NDT E Int. 2020, 109, 102178. [Google Scholar] [CrossRef]
- Li, X.M.; Ding, H.S.; Bai, S.W. Research on the stress-magnetism effect of ferromagnetic materials based on three-dimensional magnetic flux leakage testing. NDT E Int. 2014, 62, 50–54. [Google Scholar]
- Huang, H.; Yang, C.; Qian, Z.; Han, G.; Liu, Z. Magnetic memory signals variation induced by applied magnetic field and static tensile stress in ferromagnetic steel. J. Magn. Magn. Mater. 2016, 416, 213–219. [Google Scholar] [CrossRef]
- Yao, K.; Wang, Z.D.; Deng, B.; Shen, K. Experimental Research on Metal Magnetic Memory Method. Exp. Mech. 2011, 52, 305–314. [Google Scholar] [CrossRef]
- Ni, C.; Hua, L.; Wang, X. Crack propagation analysis and fatigue life prediction for structural alloy steel based on metal magnetic memory testing. J. Magn. Magn. Mater. 2018, 462, 144–152. [Google Scholar] [CrossRef]
- Huang, H.; Han, G.; Qian, Z.; Liu, Z. Characterizing the magnetic memory signals on the surface of plasma transferred arc cladding coating under fatigue loads. J. Magn. Magn. Mater. 2017, 443, 281–286. [Google Scholar] [CrossRef]
- Li, C.C.; Dong, L.H.; Wang, H.D.; Li, G.L.; Xu, B.S. Metal magnetic memory technique used to predict the fatigue crack propagation behavior of 0.45%C steel. J. Magn. Magn. Mater. 2016, 405, 150–157. [Google Scholar]
- Coughlin, C.; Clapham, L.; Atherton, D. Effects of stress on MFL responses from elongated corrosion pits in pipeline steel. NDT E Int. 2000, 33, 181–188. [Google Scholar] [CrossRef]
- Zhang, H.; Liao, L.; Zhao, R.; Zhang, H.; Yang, M.; Xia, R. The Non-Destructive Test of Steel Corrosion in Reinforced Concrete Bridges Using a Micro-Magnetic Sensor. Sensors 2016, 16, 1439. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Zhou, J.; Zhang, H.; Zhou, D.; Zhang, Z. Experimental Study on Corrosion of Unstressed Steel Strand based on Metal Magnetic Memory. KSCE J. Civ. Eng. 2019, 23, 1320–1329. [Google Scholar] [CrossRef]
- Richard, H.M.; Harvey, R.; Robert, J.S. Magnetostrictive phenomena in metallic materials and some of their device applications. IEEE Trans. Mag. 1971, 7, 29–48. [Google Scholar]
- Gatelier-Rothea, C.; Chicois, J.; Fougeres, R.; Fleischmann, P. Characterization of pure iron and (130p.p.m.) carbon–iron binary alloy by Barkhausen noise measurements: Study of the influence of stress and microstructure. Acta Mater. 1998, 46, 4873–4882. [Google Scholar] [CrossRef]
- Singh, V.; Lloyd, G.M.; Wang, M.L. Effects of temperature and corrosion thickness and composition on magnetic measurements of structural steel wires. NDT E Int. 2004, 37, 525–538. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Zuo, X. Research on methods of defect classification based on metal magnetic memory. NDT E Int. 2017, 92, 82–87. [Google Scholar] [CrossRef]
















| Material | |||
|---|---|---|---|
| 0.45% C steel | ≥355 | ≥600 | 16 |
| Specimen | Corrosion Stage | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Ⅳ | |
| Current I (A) | 0.4 | 0.4 | 0.4 | 0.4 |
| Corrosion time t (h) | 2 | 4 | 6 | 8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, B.; Yao, K.; Wu, L.; Li, X.; Wang, Y.-S. Application of Metal Magnetic Memory Testing Technology to the Detection of Stress Corrosion Defect. Appl. Sci. 2020, 10, 7083. https://doi.org/10.3390/app10207083
Zhao B, Yao K, Wu L, Li X, Wang Y-S. Application of Metal Magnetic Memory Testing Technology to the Detection of Stress Corrosion Defect. Applied Sciences. 2020; 10(20):7083. https://doi.org/10.3390/app10207083
Chicago/Turabian StyleZhao, Bingxun, Kai Yao, Libo Wu, Xinglong Li, and Yue-Sheng Wang. 2020. "Application of Metal Magnetic Memory Testing Technology to the Detection of Stress Corrosion Defect" Applied Sciences 10, no. 20: 7083. https://doi.org/10.3390/app10207083