Integrated Multifunctional Laryngoscope for Medical Diagnosis and Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. System Design
2.2. Probe Design
3. Experiments and Results
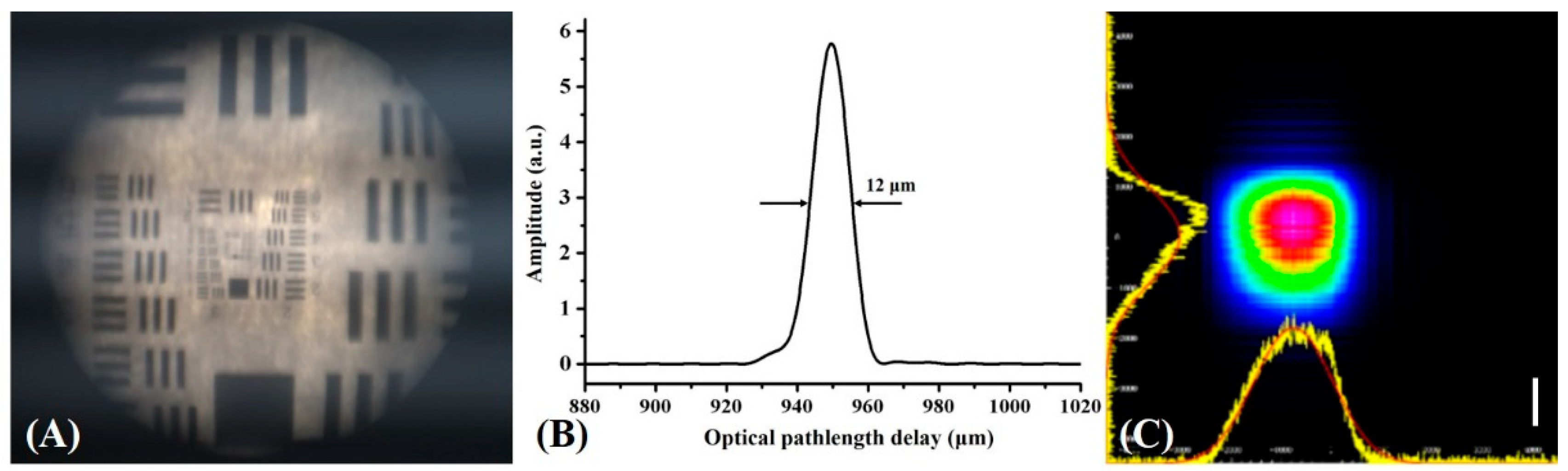
3.1. Evaluation of System Parameters
3.2. Ex Vivo Porcine Larynx Experiments:
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lewis, C.M.; Laccourreye, O.; Weber, R.S.; Holsinger, F.C. Cancers of the Larynx: Tis, T1, T2 Evaluation and Management. Head and Neck Cancer; Springer: New York, NY, USA, 2011. [Google Scholar]
- Rudolph, E.; Dyckhoff, G.; Becher, H.; Dietz, A.; Ramroth, H. Effects of tumour stage, comorbidity and therapy on survival of laryngeal cancer patients: A systematic review and a meta-analysis. Eur. Arch. Oto-Rhino-L 2011, 268, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhao, Q.; Ding, G.; Zhu, Y.; Li, W.; Chen, W. Incidence and mortality of laryngeal cancer in China, 2008–2012. Chin. J. Cancer Res. 2018, 30, 299–306. [Google Scholar] [CrossRef]
- Hoffman, H.T.; Porter, K.; Karnell, L.H.; Cooper, J.S.; Weber, R.S.; Langer, C.J.; Ang, K.; Gay, G.; Stewart, A.; Robinson, R.A. Laryngeal cancer in the United States: Changes in demographics, patterns of care, and survival. Laryngoscope 2006, 116, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.A.; Cheng, A.J.; Fang, T.J.; Huang, C.G.; Liao, C.T.; Chang, J.T.; Li, H.Y. High incidence of malignant transformation of laryngeal papilloma in Taiwan. Laryngoscope 2008, 118, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Chu, E.A.; Kim, Y.J. Laryngeal cancer: Diagnosis and preoperative work-up. Otolaryng Clin. N. Am. 2008, 41, 673–695. [Google Scholar] [CrossRef] [PubMed]
- Davaris, N.; Kropf, S.; Arens, C. Influence of prior laryngeal surgery on the diagnostic value of laryngeal endoscopy. Laryngo-Rhino-Otologie 2018, 97, 10263. [Google Scholar]
- Lin, K.; Zheng, W.; Lim, C.M.; Huang, Z. Real-time in vivo diagnosis of laryngeal carcinoma with rapid fiber-optic Raman spectroscopy. Biomed. Opt. Express 2016, 7, 3705–3715. [Google Scholar] [CrossRef] [Green Version]
- Song, P. Assessment of vocal cord function and voice disorders. In Principles and Practice of Interventional Pulmonology; Ernst, A., Herth, F., Eds.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Bergström, L.; Ward, E.C.; Finizia, C. The impact of laryngeal biopsy on voice outcomes: A pilot study. Otorhinolaryngol. Head Neck Surg. 2016, 1, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Wohl, D.L. Nonsurgical management of pediatric vocal fold nodules. Arch. Otolaryngol. Head Neck Surg. 2005, 131, 68–70. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Fercher, A.F.; Drexler, W.; Hitzenberger, C.K.; Lasser, T. Optical coherence tomography—Principles and applications. Rep. Prog. Phys. 2003, 66, 239–303. [Google Scholar] [CrossRef]
- Costa, R.A.; Skaf, M.; Melo, L.A.S.; Calucci, D.; Cardillo, J.A.; Castro, J.C.; Huang, D.; Wojtkowski, M. Retinal assessment using optical coherence tomography. Prog. Retin. Eye Res. 2006, 25, 325–353. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.H.; Lee, H.C.; Ahsen, O.O.; Liang, K.; Giacomelli, M.G.; Potsaid, B.M.; Tao, Y.K.; Jayaraman, V.; Figueiredo, M.; Huang, Q.; et al. Ultrahigh speed endoscopic optical coherence tomography for gastroenterology. Biomed. Opt. Express 2014, 5, 4387–4404. [Google Scholar] [CrossRef] [Green Version]
- Sinclair, H.; Bourantas, C.; Bagnall, A.; Mintz, G.S.; Kunadian, V. OCT for the identification of vulnerable plaque in acute coronary syndrome. JACC Cardiovasc. Imaging 2015, 8, 198–209. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, A.; Gelikonov, V.; Gelikonov, G.; Feldchtein, F.; Kuranov, R.; Gladkova, N.; Shakhova, N.; Snopova, L.; Shakhov, A.; Kuznetzova, I.; et al. In vivo endoscopic OCT imaging of precancer and cancer states of human mucosa. Opt. Express 1997, 1, 432–440. [Google Scholar] [CrossRef] [PubMed]
- Gelikonov, V.M.; Gelikonov, G.V.; Gladkova, N.D.; Shahova, N.M.; Feldchtein, F.I.; Sergeev, A.M.; Imalux, C. Optical Coherent Tomography Apparatus, Fiberoptic Lateral Scanner and Method for Studying Biological Tissues In Vivo. U.S. Patent No. 6,608,684, 19 August 2003. [Google Scholar]
- Guo, S.; Hutchison, R.; Jackson, R.P.; Kohli, A.; Sharp, T.; Orwin, E.; Haskell, R.; Chen, Z.; Wong, B.J.F. Office-based optical coherence tomographic imaging of human vocal cords. J. Biomed. Opt. 2006, 11, 30501–30503. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Liu, G.; Rubinstein, M.; Saidi, A.; Wong, B.J.; Chen, Z. Office-based dynamic imaging of vocal cords in awake patients with swept-source optical coherence tomography. J. Biomed. Opt. 2009, 14, 64020. [Google Scholar] [CrossRef] [Green Version]
- Wisweh, H.; Rohrbeck, N.; Krüger, A.; Kraft, M.; Aleksandrov, K.; Lubatschowski, H. In a laryngoscope for office-based imaging of human vocal folds using OCT. In Proceedings of the European Conference on Biomedical Optics, Munich, Germany, 14–18 June 2009; p. 73720I. [Google Scholar]
- Donner, S.; Bleeker, S.; Ripken, T.; Ptok, M.; Jungheim, M.; Krueger, A. Automated working distance adjustment enables optical coherence tomography of the human larynx in awake patients. J. Med. Imaging 2015, 2, 26003. [Google Scholar] [CrossRef] [Green Version]
- Torkian, B.A.; Guo, S.; Jahng, A.W.; Liaw, L.H.; Chen, Z.; Wong, B.J. Noninvasive measurement of ablation crater size and thermal injury after CO2 laser in the vocal cord with optical coherence tomography. Otolaryngol. Head Neck Surg. 2006, 134, 86–91. [Google Scholar] [CrossRef] [Green Version]
- Fleming, C.P.; Quan, K.J.; Rollins, A.M. Toward guidance of epicardial cardiac radiofrequency ablation therapy using optical coherence tomography. J. Biomed. Opt. 2010, 15, 41510. [Google Scholar] [CrossRef]
- Murgu, S.D.; Colt, H.G.; Mukai, D.; Brenner, M. Multimodal imaging guidance for laser ablation in tracheal stenosis. Laryngoscope 2010, 120, 1840–1846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.H.; Zhou, C.; Tao, Y.K.; Lee, H.C.; Ahsen, O.O.; Figueiredo, M.; Kirtane, T.; Adler, D.C.; Schmitt, J.M.; Huang, Q.; et al. Structural markers observed with endoscopic 3-dimensional optical coherence tomography correlating with Barrett’s esophagus radiofrequency ablation treatment response (with videos). Gastrointest. Endosc. 2012, 76, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boppart, S.A.; Herrmann, J.; Pitris, C.; Stamper, D.L.; Brezinski, M.E.; Fujimoto, J.G. High-Resolution optical coherence tomography-guided laser ablation of surgical tissue. J. Surg. Res. 1999, 82, 275–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shakhov, A.V.; Terentjeva, A.B.; Kamensky, V.A.; Snopova, L.B.; Gelikonov, V.M.; Feldchtein, F.I.; Sergeev, A.M. Optical coherence tomography monitoring for laser surgery of laryngeal carcinoma. J. Surg. Oncol. 2001, 77, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.E.; Tearney, G.J.; Bouma, B.E.; Izatt, J.A.; Hee, M.R.; Swanson, E.A.; Southern, J.F.; Fujimoto, J.G. Optical coherence tomography for optical biopsy. Properties and demonstration of vascular pathology. Circulation 1996, 93, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Parsa, P.; Jacques, S.L.; Nishioka, N.S. Optical properties of rat liver between 350 and 2200 nm. Appl. Opt. 1989, 28, 2325. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.; Glanz, H.; von Gerlach, S.; Wisweh, H.; Lubatschowski, H.; Arens, C. Clinical value of optical coherence tomography in laryngology. Head Neck 2008, 30, 1628–1635. [Google Scholar] [CrossRef]
- Wong, B.J.; Jackson, R.P.; Guo, S.; Ridgway, J.M.; Mahmood, U.; Su, J.; Shibuya, T.Y.; Crumley, R.L.; Gu, M.; Armstrong, W.B.; et al. In vivo optical coherence tomography of the human larynx: Normative and benign pathology in 82 patients. Laryngoscope 2005, 115, 1904–1911. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.C.; Liu, F.C.; Li, A.H.; Yu, H.P. Video laryngoscopy-assisted tracheal intubation in airway management. Expert Rev. Med. Devices 2018, 15, 265–275. [Google Scholar] [CrossRef]
- Gora, M.J.; Suter, M.J.; Tearney, G.J.; Li, X. Endoscopic optical coherence tomography: Technologies and clinical applications [Invited]. Biomed. Opt. Express 2017, 8, 2405. [Google Scholar] [CrossRef] [Green Version]
- Kraus, M.F.; Potsaid, B.; Mayer, M.A.; Bock, R.; Baumann, B.; Liu, J.J.; Hornegger, J.; Fujimoto, J.G. Motion correction in optical coherence tomography volumes on a per A-scan basis using orthogonal scan patterns. Biomed. Opt. Express 2012, 3, 1182. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Li, X.; Kang, J.; Zou, J.; Liang, F.; Zhang, J. Integrated Multifunctional Laryngoscope for Medical Diagnosis and Treatment. Appl. Sci. 2020, 10, 7491. https://doi.org/10.3390/app10217491
Liang S, Li X, Kang J, Zou J, Liang F, Zhang J. Integrated Multifunctional Laryngoscope for Medical Diagnosis and Treatment. Applied Sciences. 2020; 10(21):7491. https://doi.org/10.3390/app10217491
Chicago/Turabian StyleLiang, Shanshan, Xinyu Li, Jiajing Kang, Jiebin Zou, Faya Liang, and Jun Zhang. 2020. "Integrated Multifunctional Laryngoscope for Medical Diagnosis and Treatment" Applied Sciences 10, no. 21: 7491. https://doi.org/10.3390/app10217491





