Decomposition of Flavonols in the Presence of Saliva
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Collection of Saliva Samples
2.3. Influence of Saliva/H2O2 Solution on Stability of Flavonols
2.4. Influence of Microbiota
2.5. Apparatus
2.6. Statistical Analysis
3. Results and Discussion
3.1. Oxidation of Flavonols with Hydrogen Peroxide
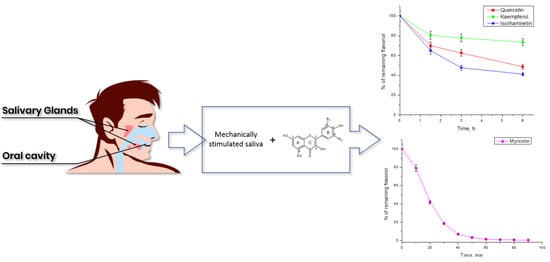
3.2. Stability of Flavonols in Saliva Solution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dai, Q.; Borenstein, A.R.; Wu, Y.; Jackson, J.C.; Larson, E.B. Fruit and Vegetable Juices and Alzheimer’s Disease: The Kame Project. Am. J. Med. 2006, 119, 751–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knekt, P.; Kumpulainen, J.; Järvinen, R.; Rissanen, H.; Heliövaara, M.; Reunanen, A.; Hakulinen, T.; Aromaa, A. Flavonoid intake and risk of chronic diseases. Am. J. Clin. Nutr. 2002, 76, 560–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Lafuente, A.; Guillamón, E.; Villares, A.; Rostagno, M.A.; Martínez, J.A. Flavonoids as anti-inflammatory agents: Implications in cancer and cardiovascular disease. Inflamm. Res. 2009, 58, 537–552. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Peng, I.W.; Kuo, S.M. Research Communication: Flavonoid Structure Affects the Inhibition of Lipid Peroxidation in Caco-2 Intestinal Cells at Physiological Concentrations. J. Nutr. 2003, 133, 2184–2187. [Google Scholar] [CrossRef] [Green Version]
- Lotito, S.B.; Frei, B. Relevance of apple polyphenols as antioxidants in human plasma: Contrasting in vitro and in vivo effects. Free. Radic. Biol. Med. 2004, 36, 201–211. [Google Scholar]
- Filipe, P.; Morlière, P.; Patterson, L.K.; Hug, G.L.; Mazière, J.C.; Mazière, C.; Freitas, J.P.; Fernandes, A.; Santus, R. Repair of Amino Acid Radicals of Apolipoprotein B100 of Low-Density Lipoproteins by Flavonoids. A Pulse Radiolysis Study with Quercetin and Rutin†. Biochemistry 2002, 41, 11057–11064. [Google Scholar] [CrossRef]
- Perez-Vizcaino, F.; Duarte, J. Flavonols and cardiovascular disease. Mol. Asp. Med. 2010, 31, 478–494. [Google Scholar] [CrossRef]
- Liao, W.; Chen, L.; Ma, X.; Jiao, R.; Li, X.; Wang, Y. Protective effects of kaempferol against reactive oxygen species-induced hemolysis and its antiproliferative activity on human cancer cells. Eur. J. Med. Chem. 2016, 114, 24–32. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.; Bast, A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Hirota, S.; Nishioka, T.; Shimoda, T.; Miura, K.; Ansai, T.; Takahama, U. Quercetin Glucosides are Hydrolyzed to Quercetin in Human Oral Cavity to Participate in Peroxidase-Dependent Scavenging of Hydrogen Peroxide. Food Sci. Technol. Res. 2001, 7, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid Glucosides are Hydrolyzed and Thus Activated in the Oral Cavity in Humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Truchado, P.; Ferreres, F.; Tomas-Barberan, F. Liquid chromatography–Tandem mass spectrometry reveals the widespread occurrence of flavonoid glycosides in honey, and their potential as floral origin markers. J. Chromatogr. A 2009, 1216, 7241–7248. [Google Scholar] [CrossRef] [PubMed]
- Walle, T.; Walle, U.K. The β-D-glucoside and sodium-dependent glucose transporter 1 (SGLT1)-inhibitor phloridzin is transported by both sglt1 and multidrug resistance-associated proteins 1/2. Drug Metab. Dispos. 2003, 31, 1288–1291. [Google Scholar] [CrossRef] [Green Version]
- Walgren, R.A.; Karnaky, K.J.; Lindenmayer, G.E.; Walle, T. Efflux of Dietary Flavonoid Quercetin 4′-β-Glucoside across Human Intestinal Caco-2 Cell Monolayers by Apical Multidrug Resistance-Associated Protein-2. J. Pharmacol. Exp. Ther. 2000, 294, 830–836. [Google Scholar]
- Tenovuo, J.O. Human Saliva: Clinical Chemistry and Microbiology; CRC Press: Boca Raton, FL, USA, 1998; pp. 55–91. [Google Scholar]
- Quijada-Morín, N.; Crespo-Expósito, C.; Rivas-Gonzalo, J.C.; García-Estévez, I.; Escribano-Bailón, M. Effect of the addition of flavan-3-ols on the HPLC-DAD salivary-protein profile. Food Chem. 2016, 207, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Cala, O.; Fabre, S.; Pinaud, N.; Dufourc, E.J.; Laguerre, M.; Fouquet, E.; Pianet, I. Towards a Molecular Interpretation of Astringency: Synthesis, 3D Structure, Colloidal State, and Human Saliva Protein Recognition of Procyanidins. Planta Med. 2011, 77, 1116–1122. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Seo, S.; Alli, I.; Chang, Y.W. Interactions of caseins with phenolic acids found in chocolate. Food Res. Int. 2015, 74, 177–184. [Google Scholar] [CrossRef]
- Pascal, C.; Poncet-Legrand, C.; Imberty, A.; Gautier, C.; Sarni-Manchado, P.; Cheynier, V.; Vernhet, A. Interactions between a non Glycosylated Human Proline-Rich Protein and Flavan-3-ols are Affected by Protein Concentration and Polyphenol/Protein Ratio. J. Agric. Food Chem. 2007, 55, 4895–4901. [Google Scholar] [CrossRef]
- Ginsburg, I.; Koren, E.; Shalish, M.; Kanner, J.; Kohen, R. Saliva increases the availability of lipophilic polyphenols as antioxidants and enhances their retention in the oral cavity. Arch. Oral Biol. 2012, 57, 1327–1334. [Google Scholar] [CrossRef]
- Yang, C.S.; Lee, M.J.; Chen, L. Human salivary tea catechin levels and catechin esterase activities: Implication in human cancer prevention studies. Cancer Epidemiol. Biomark. Prev. 1999, 8, 83–89. [Google Scholar]
- Siebert, K.J.; Maekawa, A.A.; Lynn, P. The effects of green tea drinking on salivary polyphenol concentration and perception of acid astringency. Food Qual. Prefer. 2011, 22, 157–164. [Google Scholar] [CrossRef]
- Mager, D.L.; Ximenez-Fyvie, L.A.; Haffajee, A.D.; Socransky, S.S. Distribution of selected bacterial species on intraoral surfaces. J. Clin. Periodontol. 2003, 30, 644–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Saeki, K.; Utsumi, K. Isolation of human salivary polymorphonuclear leukocytes and their stimulation-coupled responses. Arch. Biochem. Biophys. 1991, 289, 76–82. [Google Scholar] [CrossRef]
- Thomas, E.L.; Pera, K.A. Oxygen metabolism of Streptococcus mutans: Uptake of oxygen and release of superoxide and hydrogen peroxide. J. Bacteriol. 1983, 154, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Ramešová, Š.; Sokolova, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the stability of the bioactive flavonoids quercetin and luteolin under oxygen-free conditions. Anal. Bioanal. Chem. 2011, 402, 975–982. [Google Scholar] [CrossRef]
- Maini, S.; Hodgson, H.L.; Krol, E.S. The UVA and Aqueous Stability of Flavonoids is Dependent on B-Ring Substitution. J. Agric. Food Chem. 2012, 60, 6966–6976. [Google Scholar] [CrossRef]
- Zhou, A.; Sadik, O.A. Comparative Analysis of Quercetin Oxidation by Electrochemical, Enzymatic, Autoxidation, and Free Radical Generation Techniques: A Mechanistic Study. J. Agric. Food Chem. 2008, 56, 12081–12091. [Google Scholar] [CrossRef]
- Gwiazdon, M.; Biesaga, M. Stability of Quercetin and its Glycosides in the Presence of Saliva. In Quercetin: Food Sources, Antioxidant Properties and Health Effects; Nova Science Publishers: Hauppauge, NY, USA, 2015; pp. 209–222. ISBN 978-1-63483-595-4. [Google Scholar]
- Biesaga, M.; Pyrzynska, K. Liquid chromatography/tandem mass spectrometry studies of the phenolic compounds in honey. J. Chromatogr. A 2009, 1216, 6620–6626. [Google Scholar] [CrossRef]
- Krishnamachari, V.; Levine, L.H.; Paré, P.W. Flavonoid Oxidation by the Radical Generator AIBN: A Unified Mechanism for Quercetin Radical Scavenging. J. Agric. Food Chem. 2002, 50, 4357–4363. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, B.M.; Krol, E.S. UVA and UVB radiation-induced oxidation products of quercetin. J. Photochem. Photobiol. B Biol. 2009, 97, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Rossiter, J.T. Hydroxyl Free Radical-Mediated Oxidative Degradation of Quercetin and Morin: A Preliminary Investigation. J. Food Compos. Anal. 2002, 15, 103–113. [Google Scholar] [CrossRef]
- Jungbluth, G.; Rühling, I.; Ternes, W. Oxidation of flavonols with Cu(II), Fe(II) and Fe(III) in aqueous media. J. Chem. Soc. Perkin Trans. 2 2000, 1946–1952. [Google Scholar] [CrossRef]
- Pękal, A.; Biesaga, M.; Pyrzynska, K. Interaction of quercetin with copper ions: Complexation, oxidation and reactivity towards radicals. BioMetals 2010, 24, 41–49. [Google Scholar] [CrossRef]
- Pannala, A.S.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C. Flavonoid B-Ring Chemistry and Antioxidant Activity: Fast Reaction Kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction potentials of flavonoid and model phenoxyl radicals. Which ring in flavonoids is responsible for antioxidant activity? J. Chem. Soc. Perkin Trans. 2 1996, 2497–2504. [Google Scholar] [CrossRef]
- Krishnamachari, V.; Levine, L.H.; Zhou, C.; Paré, P.W. In Vitro Flavon-3-ol Oxidation Mediated by a B Ring Hydroxylation Pattern. Chem. Res. Toxicol. 2004, 17, 795–804. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; de Groot, M.J.; Berg, D.J.V.D.; Tromp, M.N.J.L.; Kelder, G.D.O.D.; van der Vijgh, A.W.J.F.; Bast, A. A Quantum Chemical Explanation of the Antioxidant Activity of Flavonoids. Chem. Res. Toxicol. 1996, 9, 1305–1312. [Google Scholar] [CrossRef]
- Weinert, E.E.; Dondi, R.; Colloredo-Melz, S.; Frankenfield, K.N.; Mitchell, C.H.; Freccero, M.; Rokita, S.E. Substituents on Quinone Methides Strongly Modulate Formation and Stability of Their Nucleophilic Adducts. J. Am. Chem. Soc. 2006, 128, 11940–11947. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Abraham, T.E.; Zakaria, Z.A. Reactivity of phenolic compounds towards free radicals under in vitro conditions. J. Food Sci. Technol. 2015, 52, 5790–5798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]





| Compound | Retention Time, Min | Q1 Mass | Q3 Mass | DP, V | CE, V |
|---|---|---|---|---|---|
| Gallic acid | 3.0 | 169 | 125 | −45 | −20 |
| 97 | −45 | −26 | |||
| Protocatechuic acid | 4.4 | 153 | 109 | −15 | −18 |
| 66 | −15 | −26 | |||
| 4-hydroxybenzoic acid | 7.1 | 137 | 93 | −25 | −18 |
| 119 | −25 | −8 | |||
| Vanillic acid | 9.8 | 167 | 152 | −30 | −16 |
| 107 | −30 | −24 | |||
| Hyperoside | 16.6 | 463 | 300 | −80 | −32 |
| 271 | −80 | −56 | |||
| Spiraeoside | 18.4 | 463 | 300 | −80 | −32 |
| 151 | −80 | −48 | |||
| Rutin | 18.9 | 609 | 300 | −65 | −32 |
| 270 | −65 | −74 | |||
| Myricetin | 20.1 | 317 | 151 | −20 | −26 |
| 179 | −20 | −22 | |||
| Quercitrin | 20.4 | 447 | 300 | −60 | −26 |
| 270 | −60 | −56 | |||
| Quercetin | 22.8 | 301 | 151 | −40 | −30 |
| 179 | −40 | −24 | |||
| Kaempferol | 25.0 | 285 | 151 | −45 | −25 |
| 117 | −45 | −53 | |||
| Isorhamnetin | 25.6 | 315 | 300 | −35 | −24 |
| 199 | −35 | −32 |
| Kaempferol | Quercetin | Isorhamnetin | Myricetin | |||||
|---|---|---|---|---|---|---|---|---|
| H2O2 | Saliva | H2O2 | Saliva | H2O2 | Saliva | H2O2 | Saliva | |
| Depside 1 | − | − | + | + | − | − | − | − |
| Depside 2 | − | − | − | + | − | − | + | + |
| 2,4,6−trihydroxybenzoic acid | − | − | + | − | − | − | + | − |
| Benzoic acid derivative | + | + | + | + | + | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogozinska, M.; Biesaga, M. Decomposition of Flavonols in the Presence of Saliva. Appl. Sci. 2020, 10, 7511. https://doi.org/10.3390/app10217511
Rogozinska M, Biesaga M. Decomposition of Flavonols in the Presence of Saliva. Applied Sciences. 2020; 10(21):7511. https://doi.org/10.3390/app10217511
Chicago/Turabian StyleRogozinska, Malgorzata, and Magdalena Biesaga. 2020. "Decomposition of Flavonols in the Presence of Saliva" Applied Sciences 10, no. 21: 7511. https://doi.org/10.3390/app10217511