Hydrothermal Synthesis of ZnO–doped Ceria Nanorods: Effect of ZnO Content on the Redox Properties and the CO Oxidation Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Synthesis
2.2. Materials Characterization
2.3. Catalytic Evaluation Studies
3. Results and Discussion
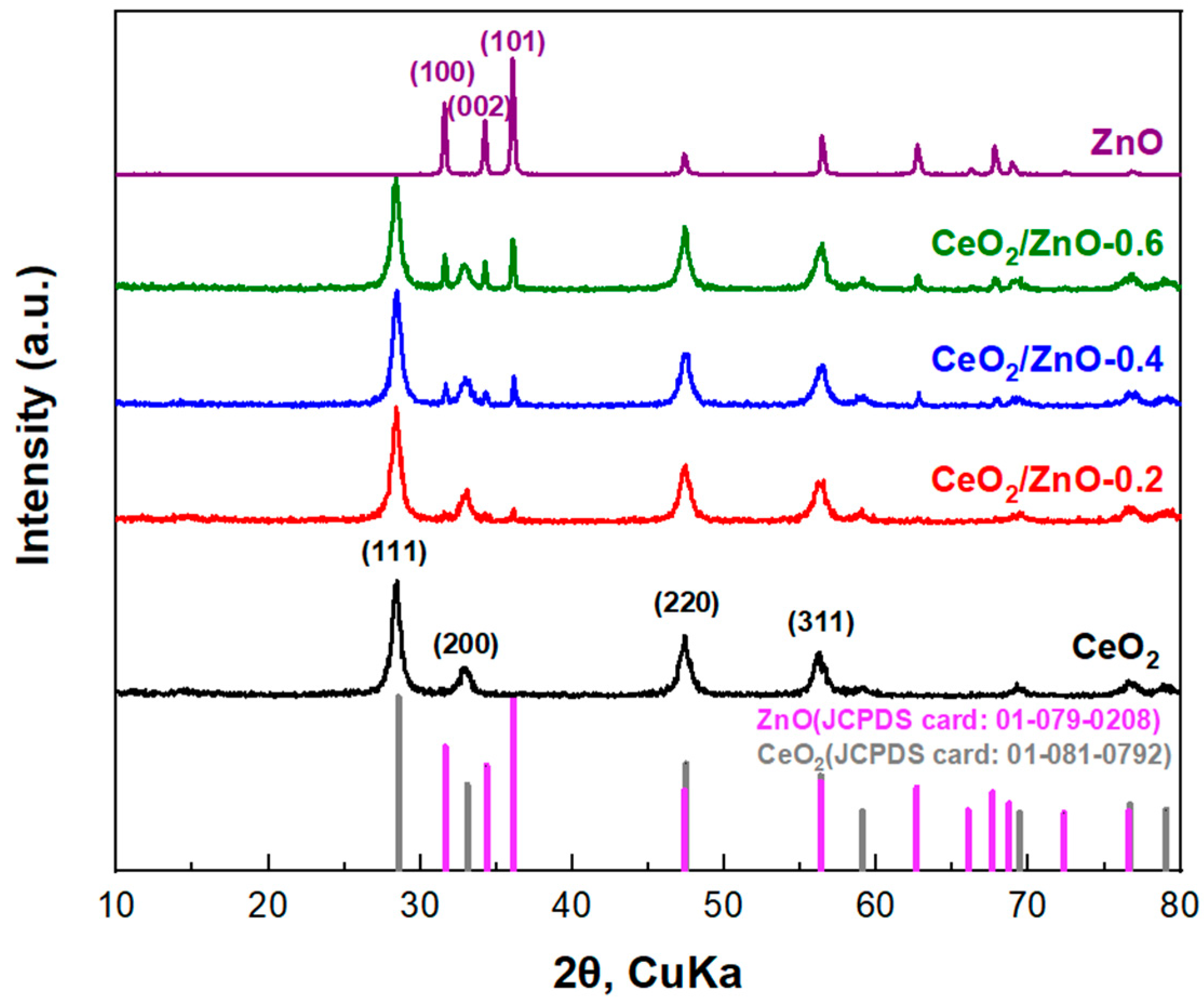
3.1. Textural and Structural Characterization
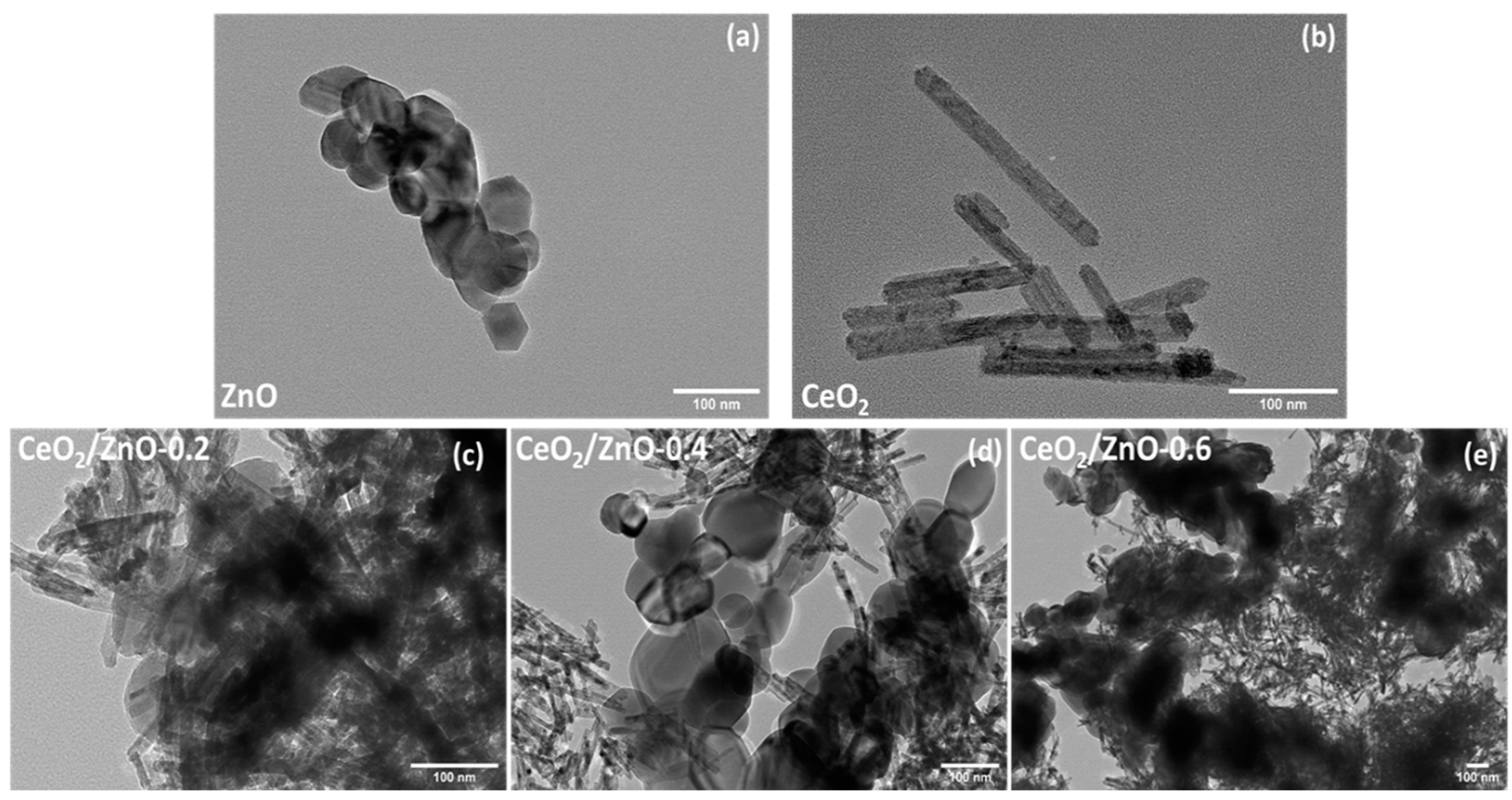
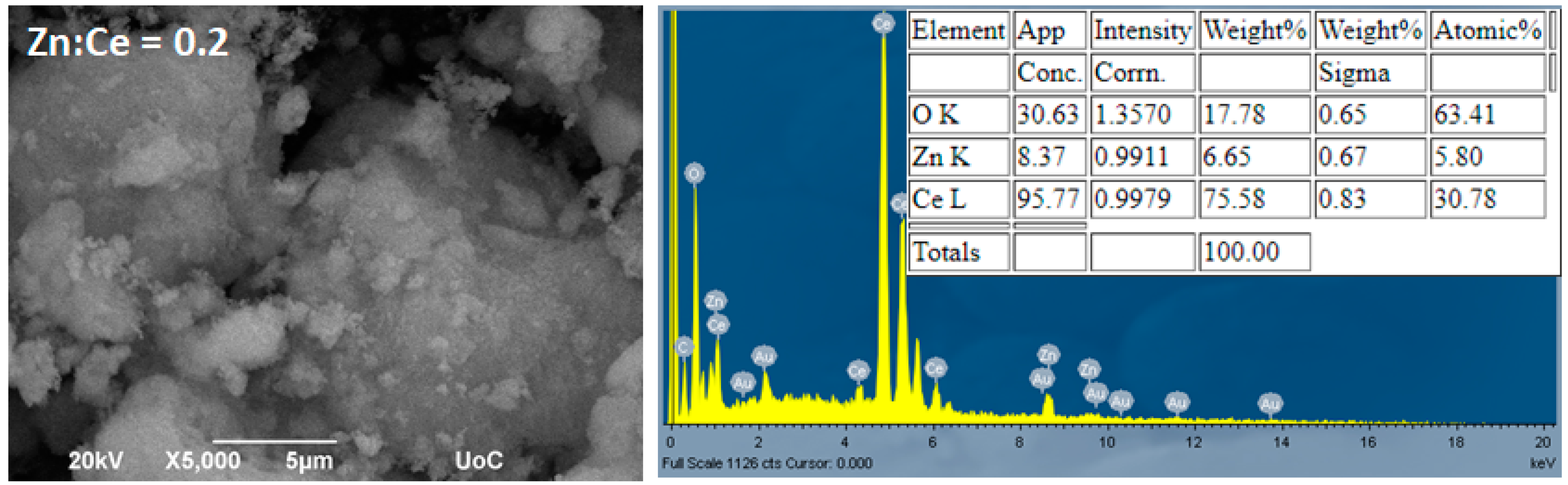
3.2. Morphological Characterization (TEM, SEM–EDS)
3.3. Redox Properties (H2–TPR)
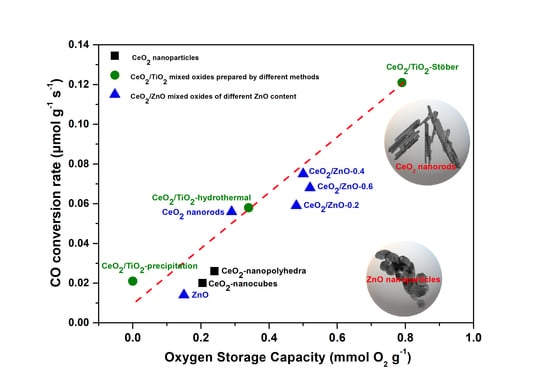
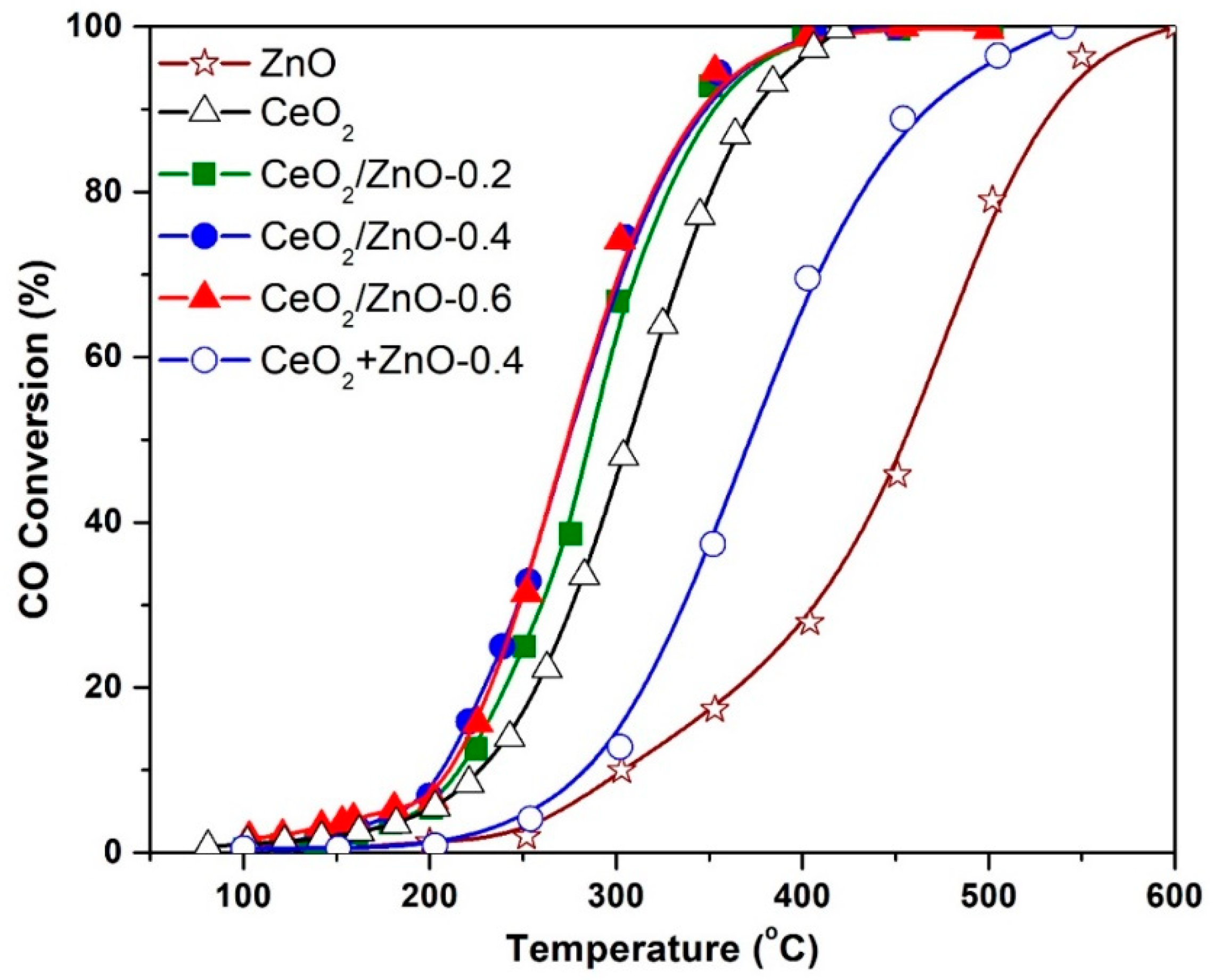
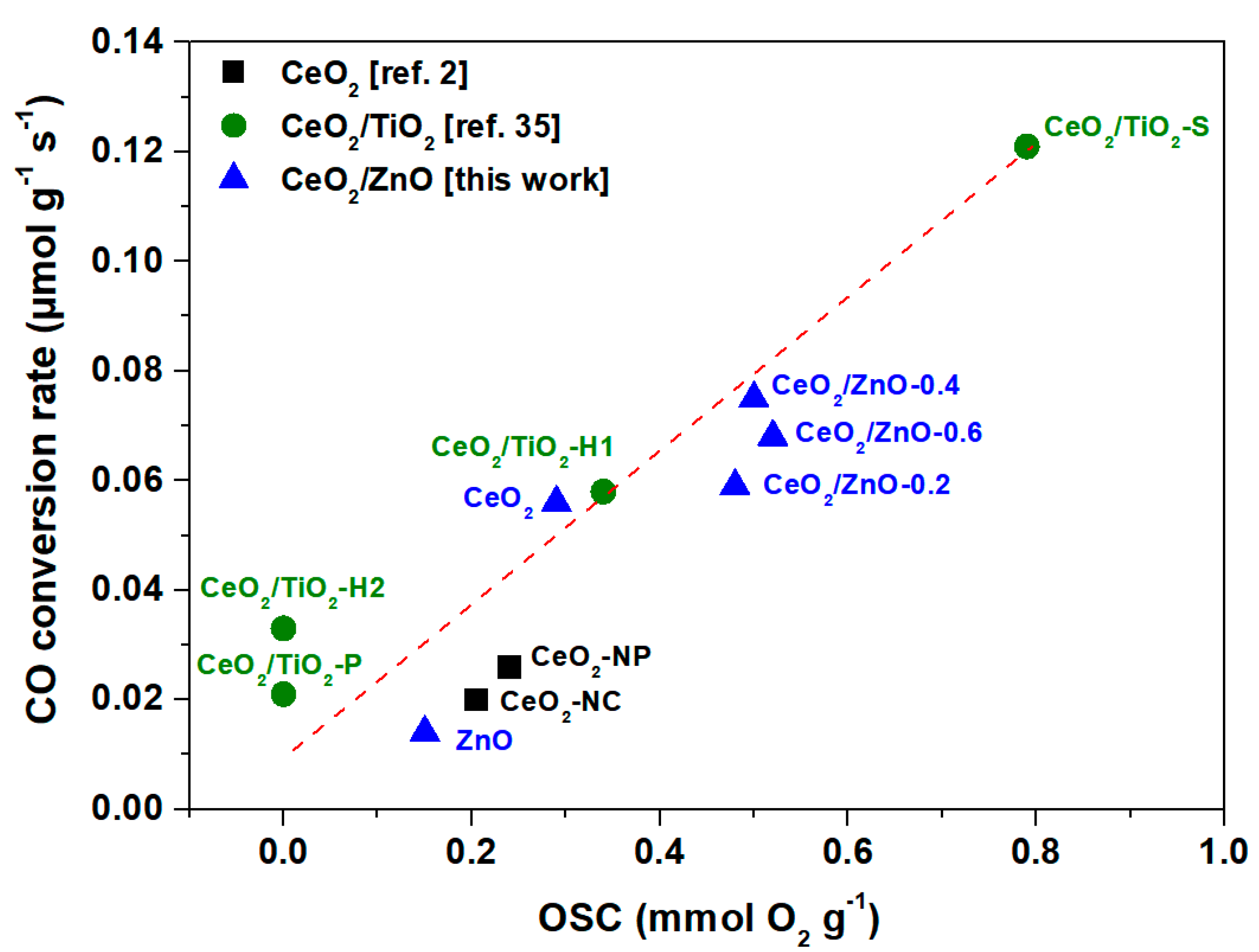
3.4. Catalytic Evaluation Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lykaki, M.; Pachatouridou, E.; Iliopoulou, E.; Carabineiro, S.A.C.; Konsolakis, M. Impact of the synthesis parameters on the solid state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv. 2017, 7, 6160–6169. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Stefa, S.; Carabineiro, S.A.C.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Facet-Dependent Reactivity of Fe2O3/CeO2 Nanocomposites: Effect of Ceria Morphology on CO Oxidation. Catalysts 2019, 9, 371. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Li, M.; Overbury, S.H. On the structure dependence of CO oxidation over CeO2 nanocrystals with well-defined surface planes. J. Catal. 2012, 285, 61–73. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Y.; Huang, J.; Lee, J.; Kim, D.H.; Frenkel, A.I.; Kim, T. Effects of Molecular and Electronic Structures in CoOx/CeO2 Catalysts on NO Reduction by CO. J. Phys. Chem. C 2019, 123, 7166–7177. [Google Scholar] [CrossRef]
- Zhang, S.; Lee, J.; Kim, D.H.; Kim, T. NO reduction by CO over CoOx/CeO2 catalysts: Effect of support calcination temperature on activity. Mol. Catal. 2020, 482, 110703. [Google Scholar] [CrossRef]
- Akter, N.; Zhang, S.; Lee, J.; Kim, D.H.; Boscoboinik, J.A.; Kim, T. Selective catalytic reduction of NO by ammonia and NO oxidation Over CoOx/CeO2 catalysts. Mol. Catal. 2020, 482, 110664. [Google Scholar] [CrossRef]
- Cámara, A.L.; Corberán, V.C.; Martínez-Arias, A.; Barrio, L.; Si, R.; Hanson, J.C.; Rodriguez, J.A. Novel manganese-promoted inverse CeO2/CuO catalyst: In situ characterization and activity for the water-gas shift reaction. Catal. Today 2020, 339, 24–31. [Google Scholar] [CrossRef]
- Chen, C.; Zhan, Y.; Zhou, J.; Li, D.; Zhang, Y.; Lin, X.; Jiang, L.; Zheng, Q. Cu/CeO2 Catalyst for Water-Gas Shift Reaction: Effect of CeO2 Pretreatment. ChemPhysChem 2018, 19, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Ronda-Lloret, M.; Rico-Francés, S.; Sepúlveda-Escribano, A.; Ramos-Fernandez, E.V. CuOx/CeO2 catalyst derived from metal organic framework for reverse water-gas shift reaction. Appl. Catal. A Gen. 2018, 562, 28–36. [Google Scholar] [CrossRef]
- Damaskinos, C.M.; Vasiliades, M.A.; Stathopoulos, V.N.; Efstathiou, A.M. The Effect of CeO2 Preparation Method on the Carbon Pathways in the Dry Reforming of Methane on Ni/CeO2 Studied by Transient Techniques. Catalysts 2019, 9, 621. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, A.M.; De Souza, K.R.; Jacobs, G.; Graham, U.M.; Davis, B.H.; Mattos, L.V.; Noronha, F.B. Steam and CO2 reforming of ethanol over Rh/CeO2 catalyst. Appl. Catal. B Environ. 2011, 102, 94–109. [Google Scholar] [CrossRef] [Green Version]
- Hou, T.; Yu, B.; Zhang, S.; Xu, T.; Wang, D.; Cai, W. Hydrogen production from ethanol steam reforming over Rh/CeO2 catalyst. Catal. Commun. 2015, 58, 137–140. [Google Scholar] [CrossRef]
- Kumar, P.A.; Tanwar, M.D.; Russo, N.; Pirone, R.; Fino, D. Synthesis and catalytic properties of CeO2 and Co/CeO2 nanofibres for diesel soot combustion. Catal. Today 2012, 184, 279–287. [Google Scholar] [CrossRef]
- Muroyama, H.; Hano, S.; Matsui, T.; Eguchi, K. Catalytic soot combustion over CeO2-based oxides. Catal. Today 2010, 153, 133–135. [Google Scholar] [CrossRef]
- Aneggi, E.; Wiater, D.; de Leitenburg, C.; Llorca, J.; Trovarelli, A. Shape-Dependent Activity of Ceria in Soot Combustion. ACS Catal. 2014, 4, 172–181. [Google Scholar] [CrossRef]
- Konsolakis, M.; Lykaki, M. Recent Advances on the Rational Design of Non-Precious Metal Oxide Catalysts Exemplified by CuOx/CeO2 Binary System: Implications of Size, Shape and Electronic Effects on Intrinsic Reactivity and Metal-Support Interactions. Catalysts 2020, 10, 160. [Google Scholar] [CrossRef] [Green Version]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2 -Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Paier, J.; Penschke, C.; Sauer, J. Oxygen Defects and Surface Chemistry of Ceria: Quantum Chemical Studies Compared to Experiment. Chem. Rev. 2013, 113, 3949–3985. [Google Scholar] [CrossRef]
- Cargnello, M.; Doan-Nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of Metal Nanocrystal Size Reveals Metal-Support Interface Role for Ceria Catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.-X.; Gao, P.-X. Nanostructured cerium oxide: Preparation, characterization, and application in energy and environmental catalysis. MRS Commun. 2016, 6, 311–329. [Google Scholar] [CrossRef] [Green Version]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Elias, J.S.; Risch, M.; Giordano, L.; Mansour, A.N.; Shao-Horn, Y. Structure, Bonding, and Catalytic Activity of Monodisperse, Transition-Metal-Substituted CeO2 Nanoparticles. J. Am. Chem. Soc. 2014, 136, 17193–17200. [Google Scholar] [CrossRef] [PubMed]
- Bion, N.; Epron, F.; Moreno, M.; Mariño, F.; Duprez, D. Preferential Oxidation of Carbon Monoxide in the Presence of Hydrogen (PROX) over Noble Metals and Transition Metal Oxides: Advantages and Drawbacks. Top. Catal. 2008, 51, 76–88. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Xiang, L.; Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: A review. Ceram. Int. 2018, 44, 7357–7377. [Google Scholar] [CrossRef]
- Sajjad, M.; Ullah, I.; Khan, M.I.; Khan, J.; Khan, M.Y.; Qureshi, M.T. Structural and optical properties of pure and copper doped zinc oxide nanoparticles. Results Phys. 2018, 9, 1301–1309. [Google Scholar] [CrossRef]
- Lee, C.-G.; Na, K.-H.; Kim, W.-T.; Park, D.-C.; Yang, W.-H.; Choi, W.-Y. TiO2/ZnO Nanofibers Prepared by Electrospinning and Their Photocatalytic Degradation of Methylene Blue Compared with TiO2 Nanofibers. Appl. Sci. 2019, 9, 3404. [Google Scholar] [CrossRef] [Green Version]
- Stathopoulos, V.N.; Papandreou, A.; Kanellopoulou, D.; Stournaras, C.J. Structural ceramics containing electric arc furnace dust. J. Hazard. Mater. 2013, 262, 91–99. [Google Scholar] [CrossRef]
- Rodwihok, C.; Wongratanaphisan, D.; Van Tam, T.; Choi, W.M.; Hur, S.H.; Chung, J.S. Cerium-Oxide-Nanoparticle-Decorated Zinc Oxide with Enhanced Photocatalytic Degradation of Methyl Orange. Appl. Sci. 2020, 10, 1697. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.-Y.; Yuan, Z.-Y.; Cao, J.-L. Hydrangea-Like Meso-/Macroporous ZnO-CeO2 Binary Oxide Materials: Synthesis, Photocatalysis and CO Oxidation. Eur. J. Inorg. Chem. 2010, 2010, 716–724. [Google Scholar] [CrossRef]
- Lv, Z.; Zhong, Q.; Ou, M. Utilizing peroxide as precursor for the synthesis of CeO2/ZnO composite oxide with enhanced photocatalytic activity. Appl. Surf. Sci. 2016, 376, 91–96. [Google Scholar] [CrossRef]
- De Lima, J.F.; Martins, R.F.; Neri, C.R.; Serra, O.A. ZnO:CeO2-based nanopowders with low catalytic activity as UV absorbers. Appl. Surf. Sci. 2009, 255, 9006–9009. [Google Scholar] [CrossRef]
- Liu, I.-T.; Hon, M.-H.; Teoh, L.G. The preparation, characterization and photocatalytic activity of radical-shaped CeO2/ZnO microstructures. Ceram. Int. 2014, 40, 4019–4024. [Google Scholar] [CrossRef]
- Xie, Q.; Zhao, Y.; Guo, H.; Lu, A.; Zhang, X.; Wang, L.; Chen, M.-S.; Peng, D.-L. Facile Preparation of Well-Dispersed CeO2–ZnO Composite Hollow Microspheres with Enhanced Catalytic Activity for CO Oxidation. ACS Appl. Mater. Interfaces 2014, 6, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Mu, G.; Liu, C.; Wei, Q.; Huang, Y. Three dimensionally ordered macroporous CeO2-ZnO catalysts for enhanced CO oxidation. Mater. Lett. 2016, 181, 161–164. [Google Scholar] [CrossRef]
- Stefa, S.; Lykaki, M.; Fragkoulis, D.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Effect of the Preparation Method on the Physicochemical Properties and the CO Oxidation Performance of Nanostructured CeO2/TiO2 Oxides. Processes 2020, 8, 847. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Meng, M.; Li, X.; Li, X.-G.; Zha, Y.-Q.; Hu, T.-D.; Xie, Y.-N.; Zhang, J. Mesoporous Co3O4–CeO2 and Pd/Co3O4–CeO2 catalysts: Synthesis, characterization and mechanistic study of their catalytic properties for low-temperature CO oxidation. J. Catal. 2008, 254, 310–324. [Google Scholar] [CrossRef]
- Liu, J.; Zhao, Z.; Wang, J.; Xu, C.; Duan, A.; Jiang, G.; Yang, Q. The highly active catalysts of nanometric CeO2-supported cobalt oxides for soot combustion. Appl. Catal. B Environ. 2008, 84, 185–195. [Google Scholar] [CrossRef]
- Yu, S.-W.; Huang, H.-H.; Tang, C.-W.; Wang, C.-B. The effect of accessible oxygen over Co3O4–CeO2 catalysts on the steam reforming of ethanol. Int. J. Hydrogen Energy 2014, 39, 20700–20711. [Google Scholar] [CrossRef]
- Subramanian, V.; Potdar, H.S.; Jeong, D.-W.; Shim, J.-O.; Jang, W.-J.; Roh, H.-S.; Jung, U.H.; Yoon, W.L. Synthesis of a Novel Nano-Sized Pt/ZnO Catalyst for Water Gas Shift Reaction in Medium Temperature Application. Catal. Lett. 2012, 142, 1075–1081. [Google Scholar] [CrossRef]
- Khan, A.; Smirniotis, P.G. Relationship between temperature-programmed reduction profile and activity of modified ferrite-based catalysts for WGS reaction. J. Mol. Catal. A Chem. 2008, 280, 43–51. [Google Scholar] [CrossRef]
- Mukherjee, D.; Rao, B.G.; Reddy, B.M. CO and soot oxidation activity of doped ceria: Influence of dopants. Appl. Catal. B Environ. 2016, 197, 105–115. [Google Scholar] [CrossRef]
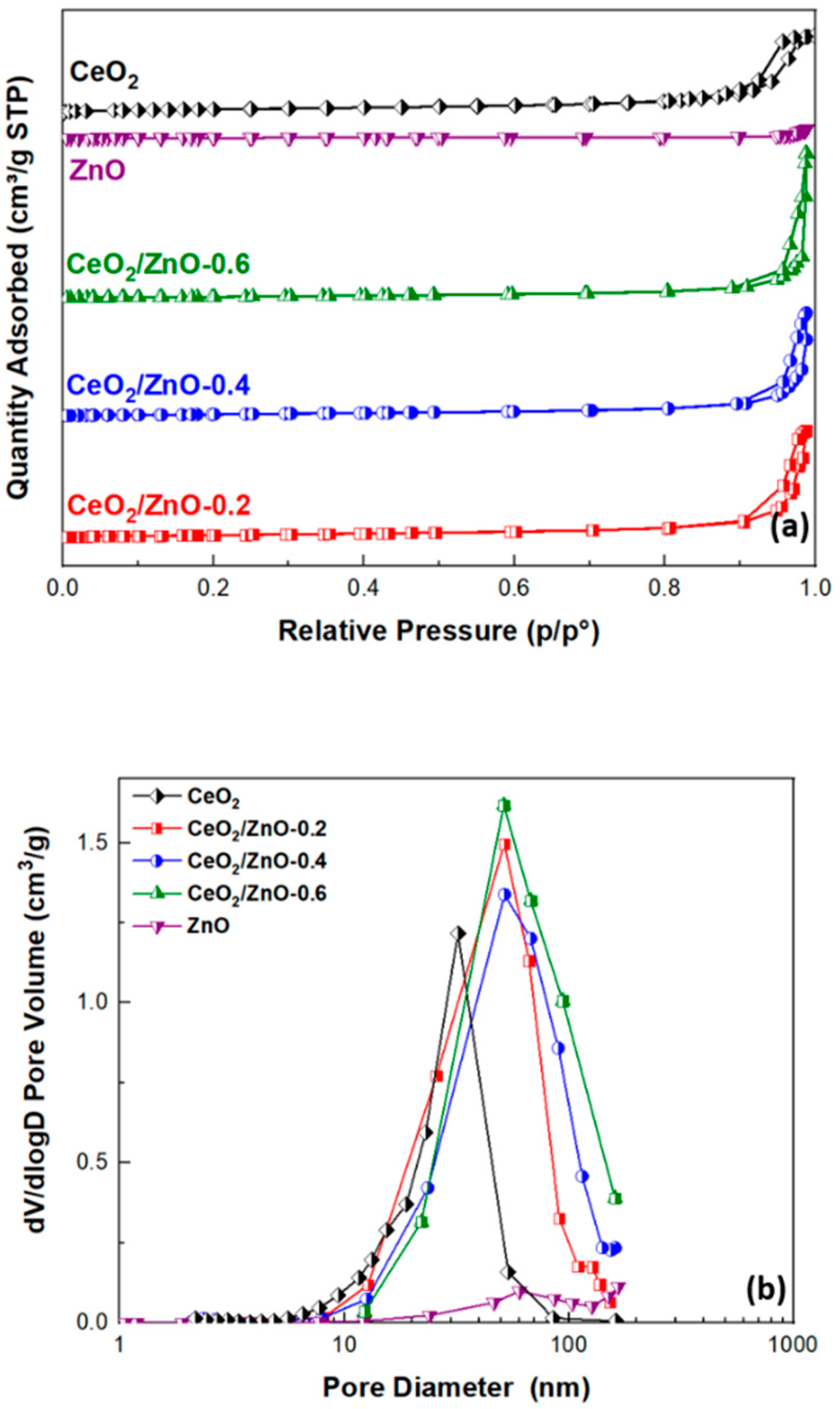

| Sample | BET Analysis | ||
|---|---|---|---|
| Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | |
| CeO2 | 79.31 ± 0.15 | 0.48 | 24.2 |
| CeO2/ZnO–0.2 | 76.22 ± 0.12 | 0.67 | 34.4 |
| CeO2/ZnO–0.4 | 62.21 ± 0.11 | 0.65 | 40.6 |
| CeO2/ZnO–0.6 | 56.12 ± 0.09 | 0.91 | 55.9 |
| ZnO | 7.05 ± 0.01 | 0.06 | 23.0 |
| Samples | XRD Analysis | |||
|---|---|---|---|---|
| Phase Detected | Average Crystallite Size, DXRD (nm) | Lattice Parameters (nm) | ||
| CeO2 | ZnO | |||
| CeO2 | Cerium (IV) oxide | 12.99 ± 0.01 | - | a = b = c = 0.5430 ± 0.0001 |
| CeO2/ZnO–0.2 | Cerium (IV) oxide Zincite | 12.14 ± 0.01 | 44.41 ± 0.05 | a = b = c = 0.5439 ± 0.0001 a = b = 0.3180 ± 0.0001; c = 0.5232 ± 0.0001 |
| CeO2/ZnO–0.4 | Cerium (IV) oxide Zincite | 11.92 ± 0.01 | 44.65 ± 0.05 | a = b = c = 0.5430 ± 0.0001 a = b = 0.3262 ± 0.0001; c = 0.5225 ± 0.0001 |
| CeO2/ZnO–0.6 | Cerium (IV) oxide Zincite | 11.56 ± 0.01 | 39.32 ± 0.05 | a = b = c = 0.5439 ± 0.0001 a = b = 0.3267 ± 0.0001; c = 0.523 ± 0.0001 |
| ZnO | Zincite | - | 34.50 ± 0.05 | a = b = 0.3272 ± 0.0001; c = 0.5233 ± 0.0001 |
| Sample | H2 Uptake (mmol H2/g) and OSC (mmol O2/g) | ||||
|---|---|---|---|---|---|
| Peak A | Peak B | Peak C | H2 uptake | OSC | |
| CeO2 | - | - | 0.59 | 0.59 | 0.29 |
| CeO2/ZnO–0.2 | 0.15 | 0.21 | 0.61 | 0.97 | 0.48 |
| CeO2/ZnO–0.4 | 0.15 | 0.23 | 0.63 | 1.01 | 0.50 |
| CeO2/ZnO–0.6 | 0.16 | 0.25 | 0.63 | 1.04 | 0.52 |
| ZnO | 0.14 | 0.17 | - | 0.31 | 0.15 |
| Sample | CO Conversion (%) | Specific Rate | |
|---|---|---|---|
| r (μmol g−1 s−1) | r (×100) (μmol m−2 s−1) | ||
| CeO2 | 5.1 | 0.056 | 0.070 |
| CeO2/ZnO–0.2 | 5.4 | 0.059 | 0.077 |
| CeO2/ZnO–0.4 | 6.9 | 0.075 | 0.121 |
| CeO2/ZnO–0.6 | 6.2 | 0.068 | 0.121 |
| ZnO | 1.3 | 0.014 | 0.201 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefa, S.; Lykaki, M.; Binas, V.; Pandis, P.K.; Stathopoulos, V.N.; Konsolakis, M. Hydrothermal Synthesis of ZnO–doped Ceria Nanorods: Effect of ZnO Content on the Redox Properties and the CO Oxidation Performance. Appl. Sci. 2020, 10, 7605. https://doi.org/10.3390/app10217605
Stefa S, Lykaki M, Binas V, Pandis PK, Stathopoulos VN, Konsolakis M. Hydrothermal Synthesis of ZnO–doped Ceria Nanorods: Effect of ZnO Content on the Redox Properties and the CO Oxidation Performance. Applied Sciences. 2020; 10(21):7605. https://doi.org/10.3390/app10217605
Chicago/Turabian StyleStefa, Sofia, Maria Lykaki, Vasillios Binas, Pavlos K. Pandis, Vassilis N. Stathopoulos, and Michalis Konsolakis. 2020. "Hydrothermal Synthesis of ZnO–doped Ceria Nanorods: Effect of ZnO Content on the Redox Properties and the CO Oxidation Performance" Applied Sciences 10, no. 21: 7605. https://doi.org/10.3390/app10217605