H-scan Subtraction Doppler Imaging: A Novel Ultrasound Small Blood Vessel Flow Characterization with Scattering and Reflection Identification
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Theory of H-Scan Ultrasound Imaging
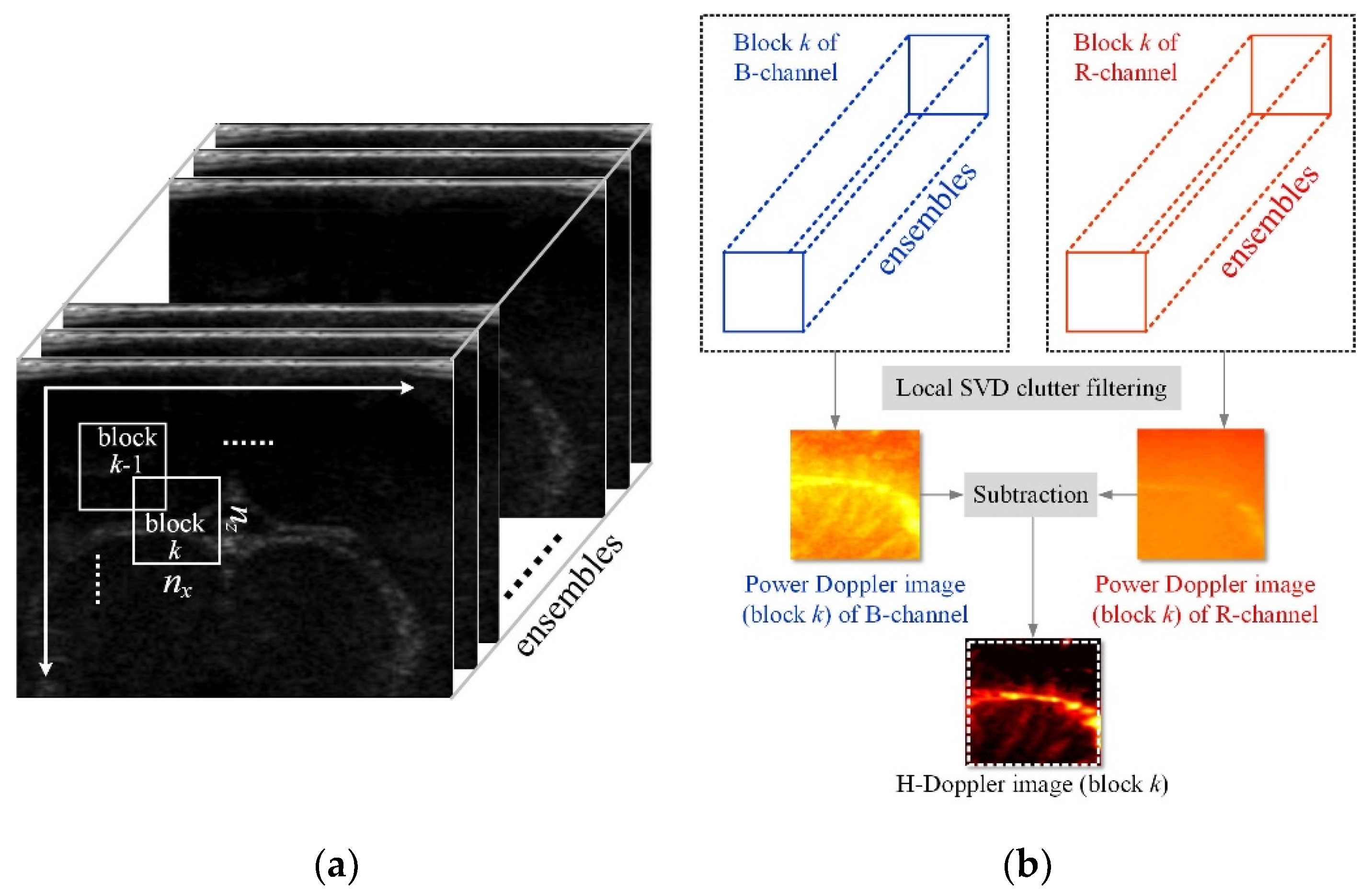
2.2. Principle of H-Doppler Flow Imaging
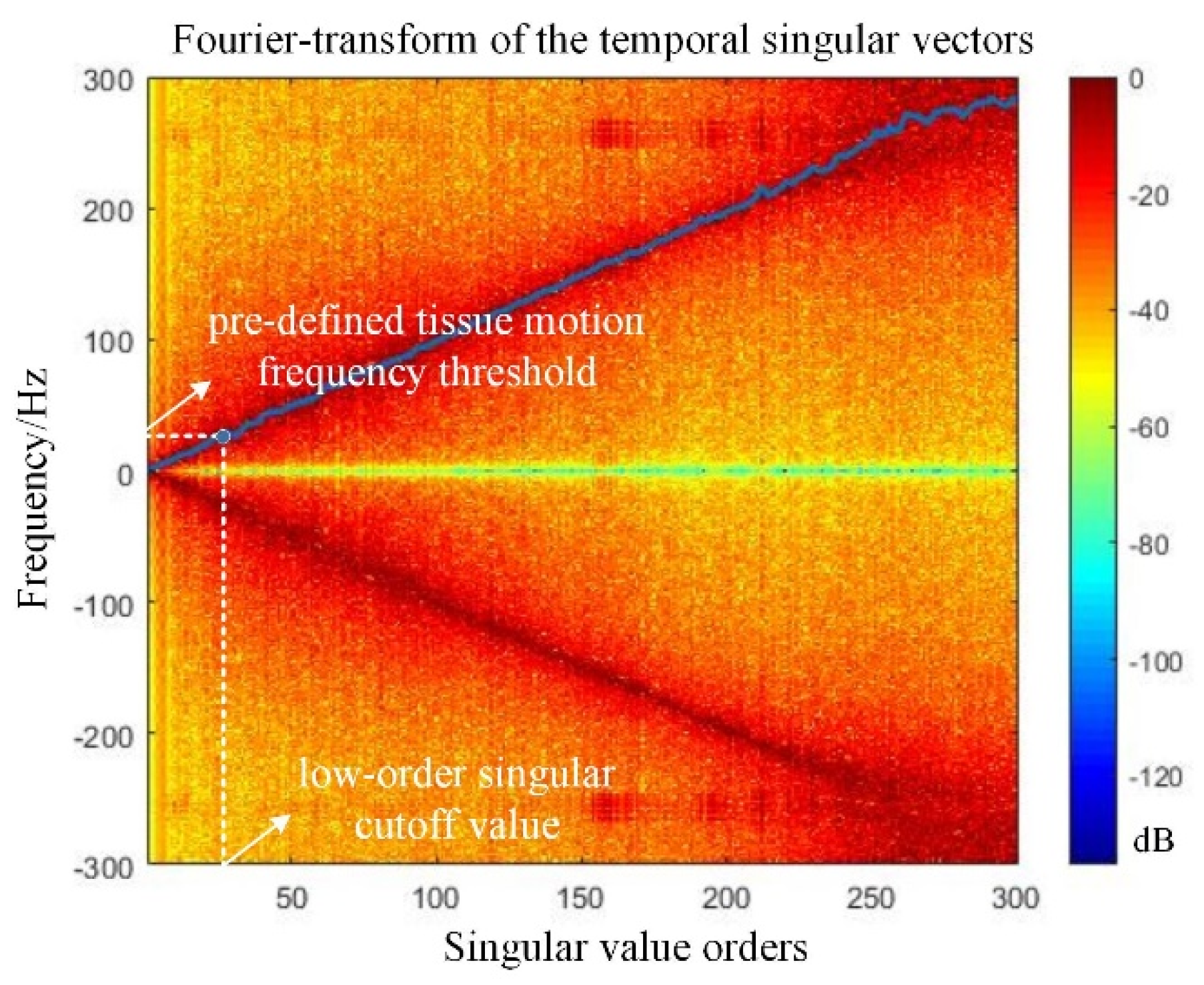
2.3. Block-Wise Local SVD Clutter Filtering for H-Doppler
2.4. System Setup
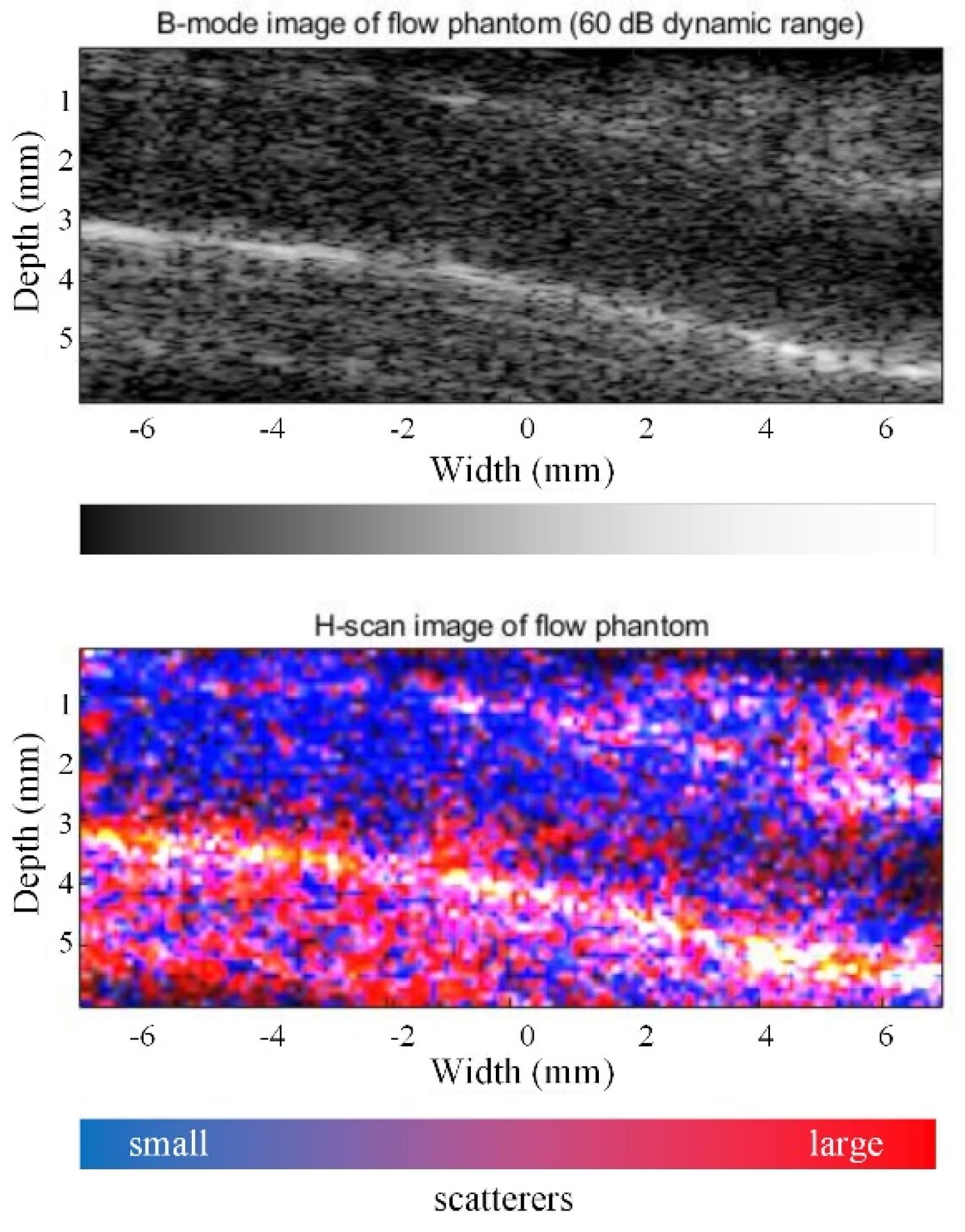
2.5. Flow Phantoms Production
2.6. Animal Preparation and Statistical Analysis
3. Results
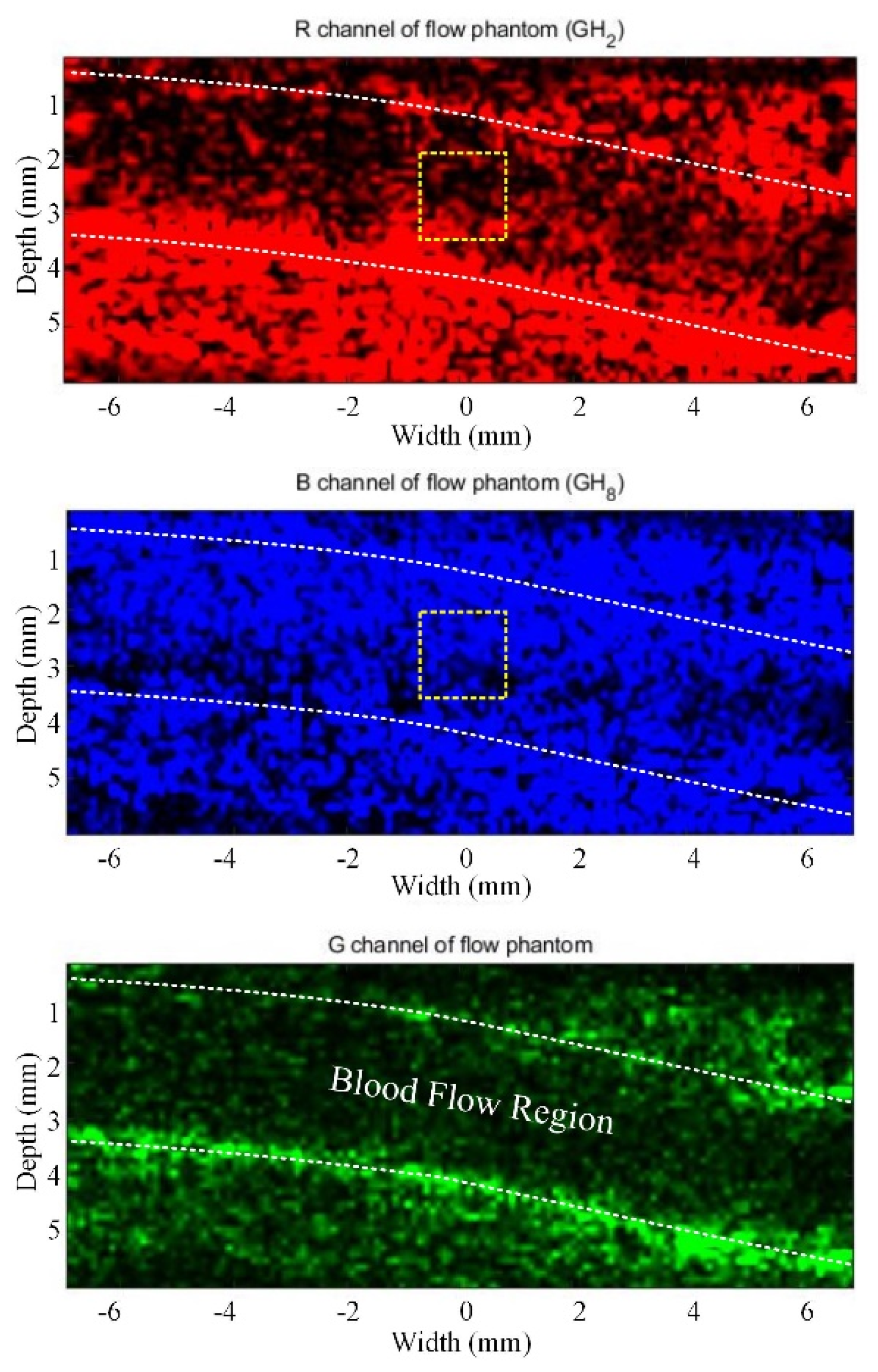
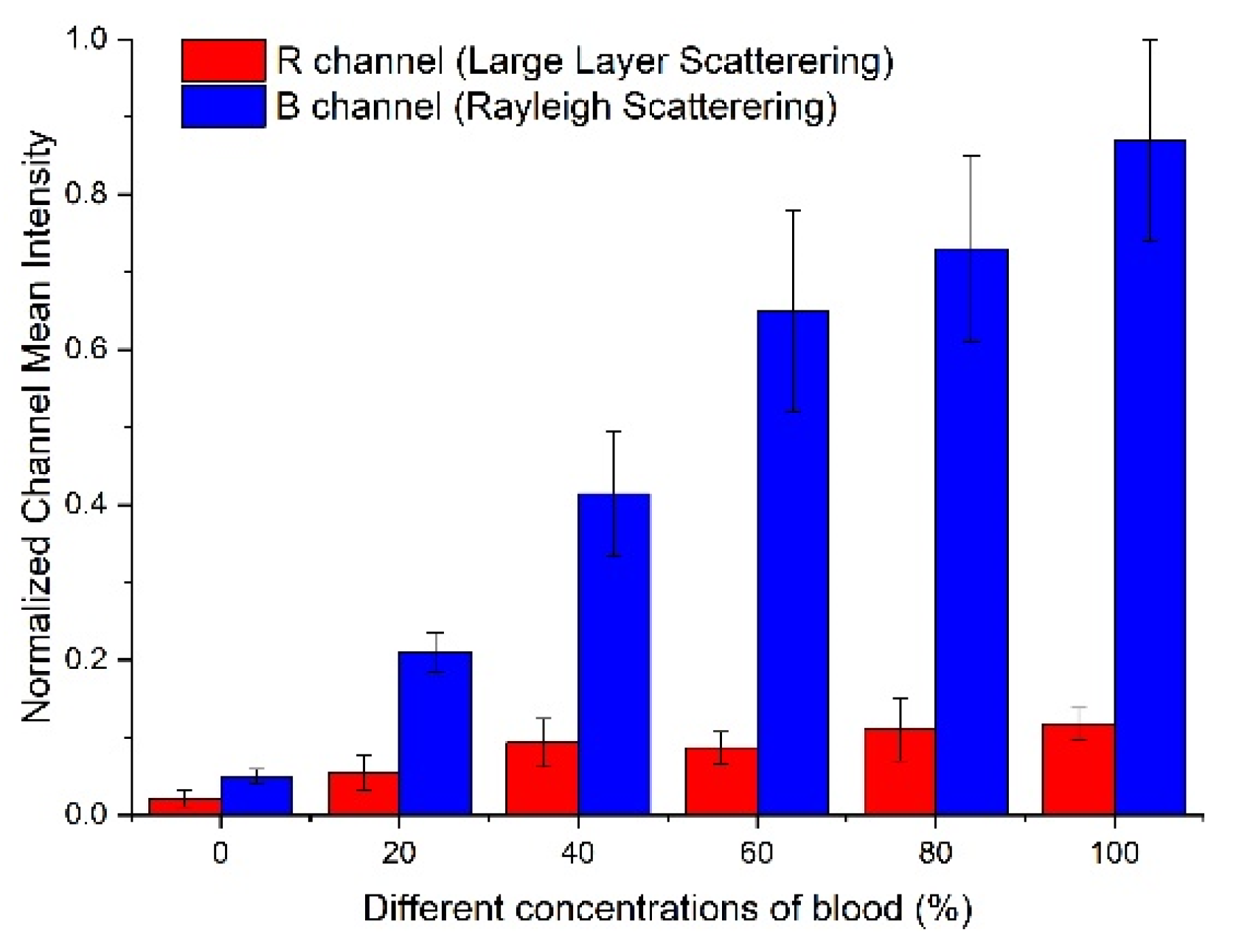
3.1. Flow Phantom Study
3.2. Rabbit Brain Imaging
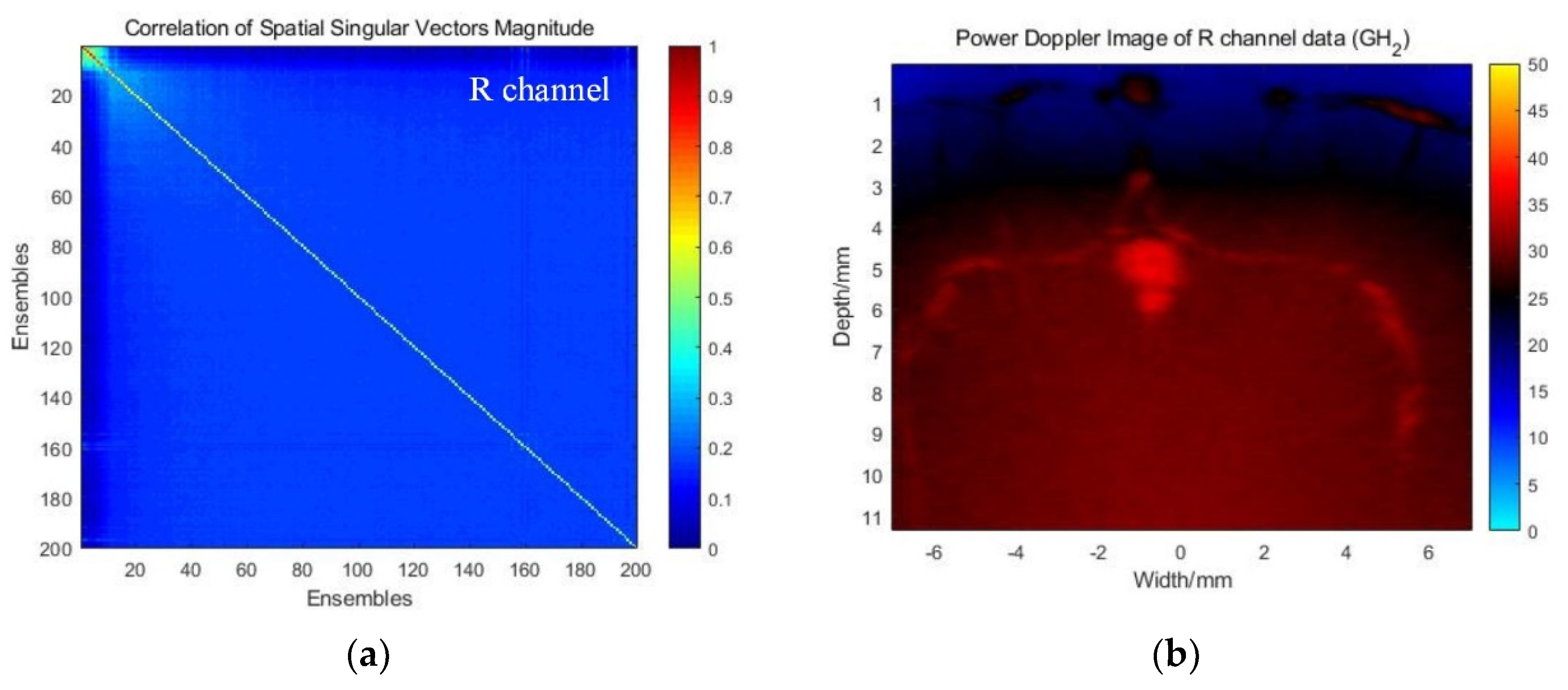
3.2.1. Correlation Analysis and SVD-Filtering Results in RGB Channels
3.2.2. Global SVD Clutter Filtering for H-Doppler
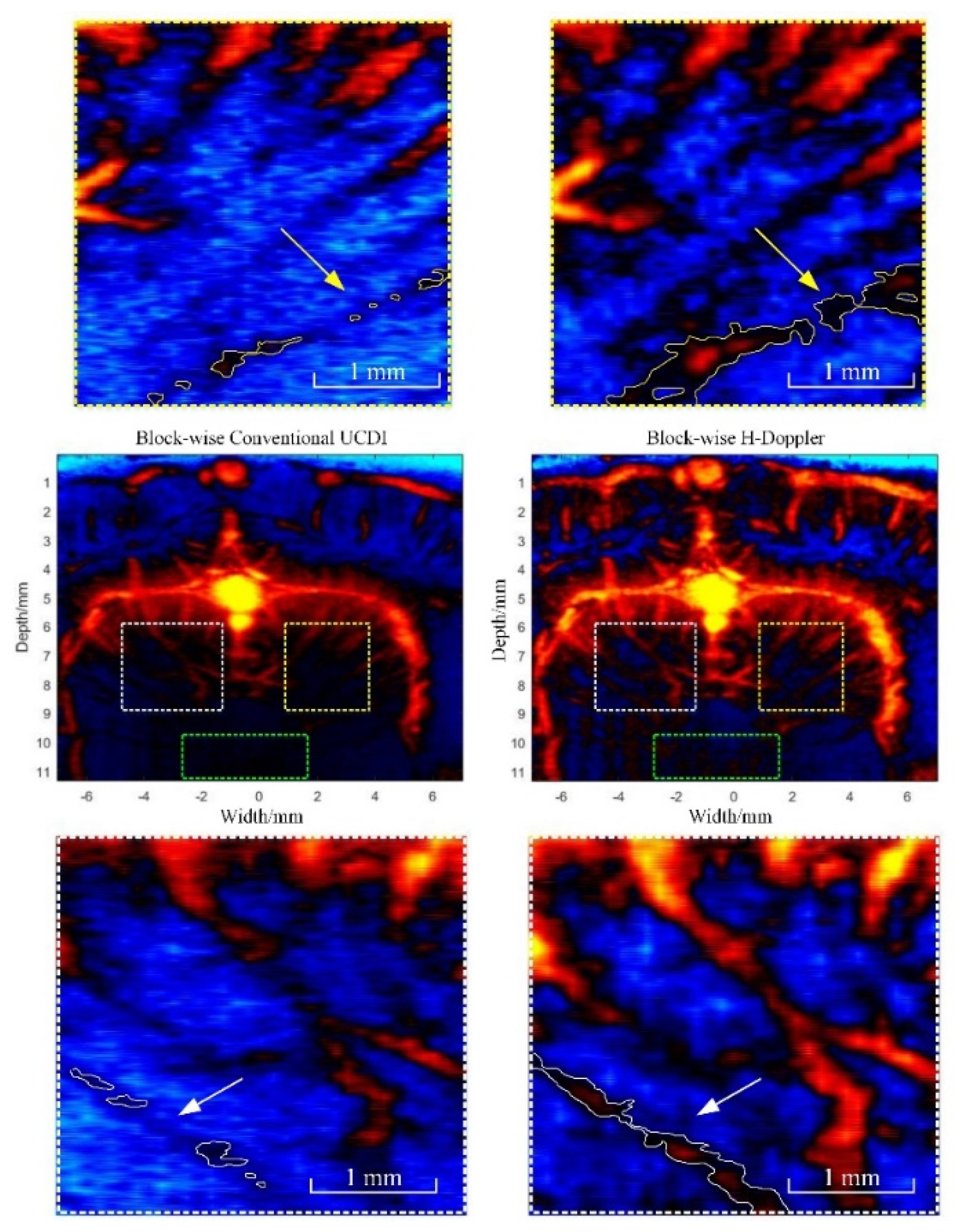
3.2.3. Block-Wise Local SVD Filtering for H-Doppler
4. Discussion
Limitations
- To successfully capture the signals encoded in the ultrasound data with Gaussian weighted Hermite polynomials kernels, the frequency bands of the ultrasound RF signal are assumed to contain the information on both large and small scattering objects. Therefore, the probe used in the H-Doppler method should be broadband.
- According to the basic assumption of the H-scan, the emission waveform needs to be approximated as a GH4 kernel (13). This is also why an arbitrary waveform generator module was adopted in the experiment to obtain the best performance of tissue scattering classification for the H-scan.
- Another limitation of the current H-scan method is that it cannot compensate for frequency-dependent attenuation. Since the GH2 and GH8 convolution outputs represent different frequency-dependent scattering, i.e., the B channel is contributed by higher frequency ultrasound signals reflected from small scatterers; the corresponding image quality will be diminished in deep tissue. These influences were ignored in this paper, but recently, some have proposed that the attenuation correction method can enhance the ability of tissue characterization for H-scan imaging, especially for deep tissue [33]. An adaptive attenuation correction that can match to the changing spectrum undergoing attenuation will be investigated in a future study to improve the visualization of the H-Doppler results in deep tissue.
- Additionally, there is a lack of a ground truth of the actual vessel sizes and distributions in the in vivo images for comparison to the ultrasound images. The future work also includes obtaining histological sections of the rabbit brain together with the ultrasound imaging plane to visually verify the size and location of blood vessels for comparison with different beamforming approaches and the H-Doppler method.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bercoff, J.; Montaldo, G.; Loupas, T.; Savery, D.; Mézière, F.; Fink, M.; Tanter, M. Ultrafast compound Doppler imaging: Providing full blood flow characterization. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2011, 58, 134–147. [Google Scholar] [CrossRef] [PubMed]
- Osmanski, B.-F.; Pernot, M.; Montaldo, G.; Bel, A.; Messas, E.; Tanter, M. Ultrafast Doppler imaging of blood flow dynamics in the myocardium. IEEE Trans. Med. Imaging 2012, 31, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Podkowa, A.S.; Oelze, M.L.; Ketterling, J.A. High-frame-rate Doppler ultrasound using a repeated transmit sequence. Appl. Sci. 2018, 8, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossi, S.; Ramalli, A.; Fool, F.; Tortoli, P. High-Frame-Rate 3-D Vector Flow Imaging in the Frequency Domain. Appl. Sci. 2020, 10, 5365. [Google Scholar] [CrossRef]
- Mace, E.; Montaldo, G.; Osmanski, B.-F.; Cohen, I.; Fink, M.; Tanter, M. Functional ultrasound imaging of the brain: Theory and basic principles. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Urs, R.; Ketterling, J.A.; Silverman, R.H. Ultrafast ultrasound imaging of ocular anatomy and blood flow. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3810–3816. [Google Scholar] [CrossRef]
- Urs, R.; Ketterling, J.A.; Alfred, C.; Lloyd, H.O.; Yiu, B.Y.; Silverman, R.H. Ultrasound imaging and measurement of choroidal blood flow. Transl. Vis. Sci. Technol. 2018, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Correas, J.-M.; Anglicheau, D.; Joly, D.; Gennisson, J.-L.; Tanter, M.; Hélénon, O. Ultrasound-based imaging methods of the kidney—Recent developments. Kidney Int. 2016, 90, 1199–1210. [Google Scholar] [CrossRef]
- Baranger, J.; Arnal, B.; Perren, F.; Baud, O.; Tanter, M.; Demené, C. Adaptive spatiotemporal SVD clutter filtering for ultrafast Doppler imaging using similarity of spatial singular vectors. IEEE Trans. Med. Imaging 2018, 37, 1574–1586. [Google Scholar] [CrossRef] [Green Version]
- Demené, C.; Deffieux, T.; Pernot, M.; Osmanski, B.-F.; Biran, V.; Gennisson, J.-L.; Sieu, L.-A.; Bergel, A.; Franqui, S.; Correas, J.-M. Spatiotemporal clutter filtering of ultrafast ultrasound data highly increases Doppler and fUltrasound sensitivity. IEEE Trans. Med. Imaging 2015, 34, 2271–2285. [Google Scholar] [CrossRef]
- Hur, B.Y.; Lee, J.Y.; Chu, A.J.; Kim, S.H.; Han, J.K.; Choi, B.I. UltraFast Doppler ultrasonography for hepatic vessels of liver recipients: Preliminary experiences. Ultrasonography 2015, 34, 58. [Google Scholar] [CrossRef] [PubMed]
- Alfred, C.; Cobbold, R.S. Single-ensemble-based eigen-processing methods for color flow imaging-Part I. The Hankel-SVD filter. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2008, 55, 559–572. [Google Scholar]
- Alfred, C.; Lovstakken, L. Eigen-based clutter filter design for ultrasound color flow imaging: A review. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2010, 57, 1096–1111. [Google Scholar]
- Lovstakken, L.; Bjaerum, S.; Kristoffersen, K.; Haaverstad, R.; Torp, H. Real-time adaptive clutter rejection filtering in color flow imaging using power method iterations. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2006, 53, 1597–1608. [Google Scholar] [CrossRef]
- Song, P.; Manduca, A.; Trzasko, J.D.; Chen, S. Ultrasound small vessel imaging with block-wise adaptive local clutter filtering. IEEE Trans. Med. Imaging 2016, 36, 251–262. [Google Scholar] [CrossRef]
- Marčenko, V.A.; Pastur, L.A. Distribution of eigenvalues for some sets of random matrices. Math. Ussr-Sb. 1967, 1, 457. [Google Scholar] [CrossRef]
- Shankar, P.M. Ultrasonic tissue characterization using a generalized Nakagami model. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2001, 48, 1716–1720. [Google Scholar] [CrossRef]
- Takiuchi, S.; Rakugi, H.; Honda, K.; Masuyama, T.; Hirata, N.; Ito, H.; Sugimoto, K.; Yanagitani, Y.; Moriguchi, K.; Okamura, A. Quantitative ultrasonic tissue characterization can identify high-risk atherosclerotic alteration in human carotid arteries. Circulation 2000, 102, 766–770. [Google Scholar] [CrossRef]
- Parker, K. Scattering and reflection identification in H-scan images. Phys. Med. Biol. 2016, 61, L20–L28. [Google Scholar] [CrossRef]
- Parker, K. The H-scan format for classification of ultrasound scattering. OMICS J. Radiol. 2016, 5, 1000236. [Google Scholar]
- Khairalseed, M.; Xiong, F.; Kim, J.-W.; Mattrey, R.F.; Parker, K.J.; Hoyt, K. Spatial angular compounding technique for H-scan ultrasound imaging. Ultrasound Med. Biol. 2018, 44, 267–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouyang, Y.; Tsui, P.-H.; Wu, S.; Wu, W.; Zhou, Z. Classification of Benign and Malignant Breast Tumors Using H-Scan Ultrasound Imaging. Diagnostics 2019, 9, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khairalseed, M.; Xiong, F.; Mattrey, R.; Parker, K.; Hoyt, K. Detection of early tumor response to Abraxane using H-scan imaging: Preliminary results in a small animal model of breast cancer. In Proceedings of the 2017 IEEE International Ultrasonics Symposium (IUS), Washington, DC, USA, 6–9 September 2017; pp. 1–4. [Google Scholar]
- Khairalseed, M.; Javed, K.; Jashkaran, G.; Kim, J.W.; Parker, K.J.; Hoyt, K. Monitoring Early Breast Cancer Response to Neoadjuvant Therapy Using H-Scan Ultrasound Imaging: Preliminary Preclinical Results. J. Ultrasound Med. 2019, 38, 1259–1268. [Google Scholar] [CrossRef] [PubMed]
- Gary, R.G.; Laimes, R.; Pinto, J.; Guerrero, J.; Chavez, H.; Salazar, C.; Lavarello, R.J.; Parker, K.J. H-scan analysis of thyroid lesions. J. Med. Imaging 2018, 5, 013505. [Google Scholar]
- Cobbold, R.S. Foundations of Biomedical Ultrasound; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Morse, P.M.; Ingard, K.U. Theoretical Acoustics; Princeton University Press: Princeton, NJ, USA, 1986. [Google Scholar]
- Huang, R.; Schmerr, L.; Sedov, A. Improving the Born approximation for the scattering of ultrasound in elastic solids. Ultrasonics 2006, 44, e981–e984. [Google Scholar] [CrossRef]
- Ruo, F. Ultrasonic Investigation of Blood. Acta Acust. 1989, 6, 170–177. [Google Scholar]
- Angelsen, B.A. Instantaneous frequency, mean frequency, and variance of mean frequency estimators for ultrasonic blood velocity Doppler signals. IEEE Trans. Biomed. Eng. 1981, 11, 733–741. [Google Scholar] [CrossRef]
- Shung, K.K.; Kuo, I.; Cloutier, G. Ultrasonic scattering properties of blood. In Intravascular Ultrasound; Springer: Berlin/Heidelberg, Germany, 1993; pp. 119–139. [Google Scholar]
- Montaldo, G.; Tanter, M.; Bercoff, J.; Benech, N.; Fink, M. Coherent plane-wave compounding for very high frame rate ultrasonography and transient elastography. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2009, 56, 489–506. [Google Scholar] [CrossRef]
- Tai, H.; Khairalseed, M.; Hoyt, K. Adaptive attenuation correction during H-scan ultrasound imaging using K-means clustering. Ultrasonics 2020, 102, 105987. [Google Scholar] [CrossRef]





| Order, n | GH(t)n | Energy(n) |
|---|---|---|
| 0 | (1)G | |
| 1 | (2t)G | |
| 2 | (4t2 − 2)G | |
| 3 | (8t3 − 12t)G | |
| 4 | (16t4 − 48t2 + 12)G | |
| 5 | (32t5 − 160t3 + 120t)G |
| Global SVD Filtering | BWL-SVD Filtering | |||
|---|---|---|---|---|
| UCDI | H-Doppler | UCDI | H-Doppler | |
| Low-order SVT | 22 | 22 | 22–31 | 22–31 |
| High-order SVT | 232 | / | 232–264 | / |
| CNR (white) | 15.5 dB | 19.6 dB | 20.1 dB | 23.2 dB |
| CNR (yellow) | 10.1 dB | 16.7 dB | 19.7 dB | 24.7 dB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, Y.; Zhang, D.; Xu, Y.; Chen, Y.; Wu, Z.; Cui, Y. H-scan Subtraction Doppler Imaging: A Novel Ultrasound Small Blood Vessel Flow Characterization with Scattering and Reflection Identification. Appl. Sci. 2020, 10, 7604. https://doi.org/10.3390/app10217604
Jiao Y, Zhang D, Xu Y, Chen Y, Wu Z, Cui Y. H-scan Subtraction Doppler Imaging: A Novel Ultrasound Small Blood Vessel Flow Characterization with Scattering and Reflection Identification. Applied Sciences. 2020; 10(21):7604. https://doi.org/10.3390/app10217604
Chicago/Turabian StyleJiao, Yang, Derong Zhang, Yiwen Xu, Yang Chen, Zhe Wu, and Yaoyao Cui. 2020. "H-scan Subtraction Doppler Imaging: A Novel Ultrasound Small Blood Vessel Flow Characterization with Scattering and Reflection Identification" Applied Sciences 10, no. 21: 7604. https://doi.org/10.3390/app10217604





