Carbon Dioxide Uptake by MOC-Based Materials
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
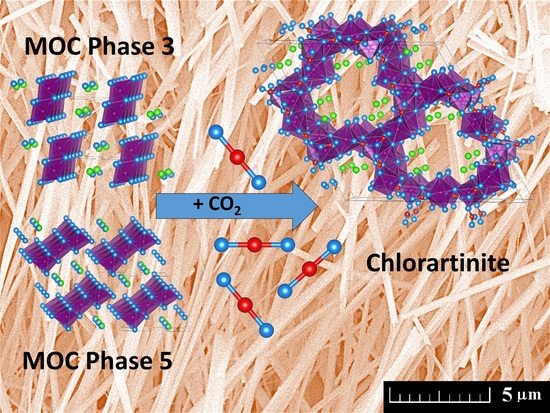
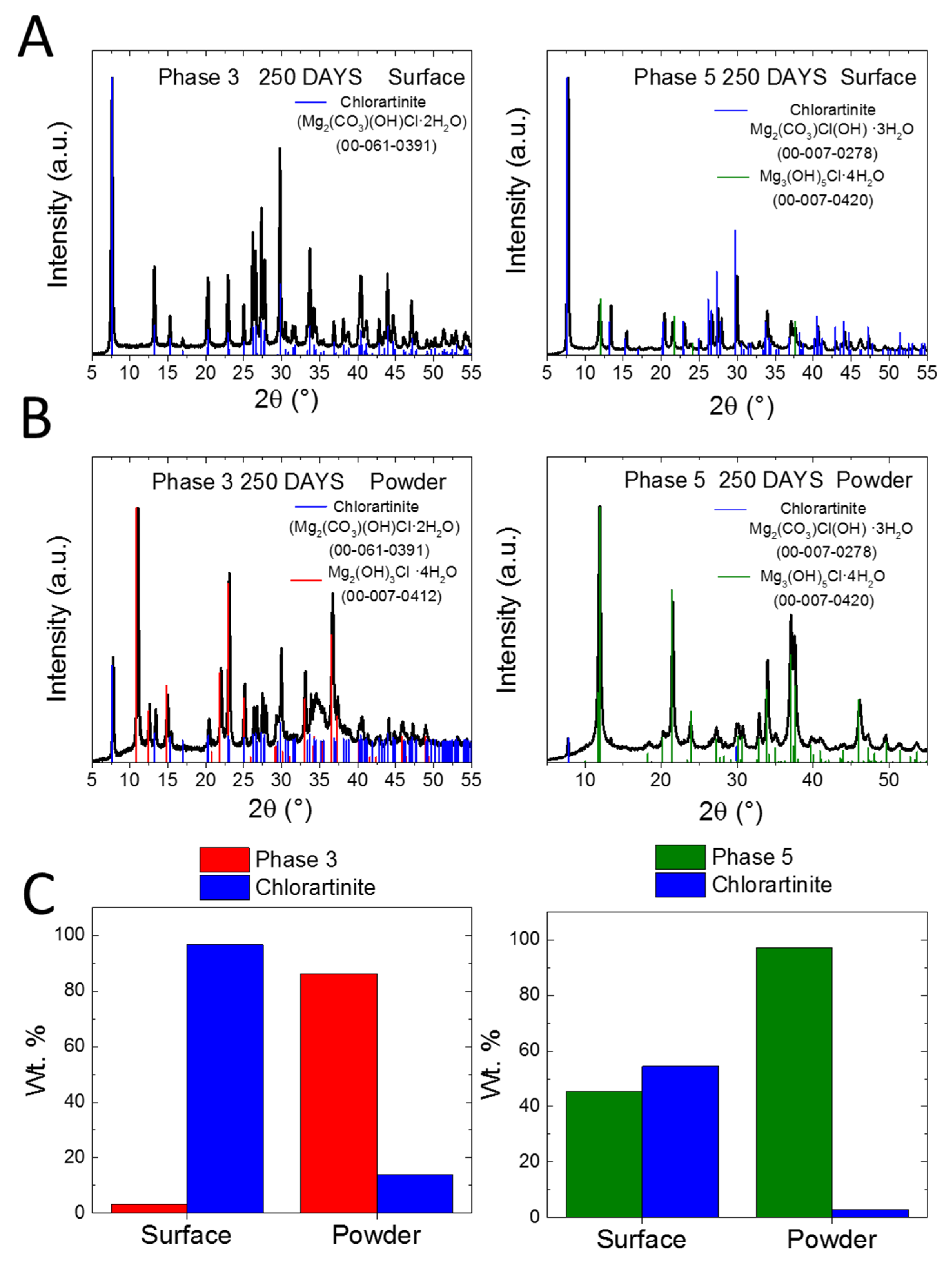
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sorel, S. On a new magnesium cement. CR Acad. Sci. 1867, 65, 102–104. [Google Scholar]
- Dinnebier, R.E.; Freyer, D.; Bette, S.; Oestreich, M. 9Mg(OH)2·MgCl2·4H2O, a high temperature phase of the magnesia binder system. Inorg. Chem. 2010, 49, 9770–9776. [Google Scholar] [CrossRef] [PubMed]
- Dinnebier, R.E.; Oestreich, M.; Bette, S.; Freyer, D. 2Mg(OH)2·MgCl2·2H2O and 2Mg(OH)2·MgCl2·4H2O, two high temperature phases of the magnesia cement system. Z. Anorganische Allgemeine Chemie 2012, 638, 628–633. [Google Scholar] [CrossRef]
- Matkovic, B.; Young, J. Microstructure of magnesium oxychloride cements. Nat. Phys. Sci. 1973, 246, 79–80. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Jiříčková, A.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlíková, M.; Záleská, M.; Pavlík, Z.; Jankovský, O. Synthesis, structure, and thermal stability of magnesium oxychloride 5Mg (OH)2∙MgCl2∙8H2O. Appl. Sci. 2020, 10, 1683. [Google Scholar] [CrossRef] [Green Version]
- Lojka, M.; Jankovský, O.; Jiříčková, A.; Lauermannová, A.-M.; Antončík, F.; Sedmidubský, D.; Pavlík, Z. Thermal Stability and Kinetics of Formation of Magnesium Oxychloride Phase 3Mg (OH) 2∙MgCl2∙8H2O. Materials 2020, 13, 767. [Google Scholar] [CrossRef] [Green Version]
- Demediuk, T.; Cole, W.; Hueber, H. Studies on magnesium and calcium oxychlorides. Aust. J. Chem. 1955, 8, 215–233. [Google Scholar] [CrossRef]
- Li, Z.; Chau, C. Influence of molar ratios on properties of magnesium oxychloride cement. Cem. Concr. Res. 2007, 37, 866–870. [Google Scholar] [CrossRef]
- Montle, J.; Mayhan, K. The role of magnesium oxychloride as a fire-resistive material. Fire Technol. 1974, 10, 201–210. [Google Scholar] [CrossRef]
- Qiao, H.X.; Zhu, B.R.; Shi, Y.Y.; Dong, J.M.; Elizabeth Wanjiru, M. Strength development and micro-mechanism of magnesium oxychloride cement concrete. Mater. Res. Innov. 2015, 19, S1–S185. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Z. Light-weight wood–magnesium oxychloride cement composite building products made by extrusion. Constr. Build. Mater. 2012, 27, 382–389. [Google Scholar] [CrossRef] [Green Version]
- El-Gammal, M.; El-Alfy, A.; Mohamed, N. Using magnesium oxide wallboard as an alternative building façade cladding material in modern cairo buildings. J. Appl. Sci. Res. 2012, 8, 2024–2032. [Google Scholar]
- Chau, C.K.; Chan, J.; Li, Z. Influences of fly ash on magnesium oxychloride mortar. Cem. Concr. Compos. 2009, 31, 250–254. [Google Scholar] [CrossRef]
- Ozturk, A.; Said, M.; Timuçin, M. Production and characterization of magnesium oxychloride cement bricks for fine polishing of porcelain stoneware tiles. Ind. Ceram. 2011, 31, 89–98. [Google Scholar]
- Karimi, Y.; Monshi, A. Effect of magnesium chloride concentrations on the properties of magnesium oxychloride cement for nano SiC composite purposes. Ceram. Int. 2011, 37, 2405–2410. [Google Scholar] [CrossRef]
- Yunsong, J. Study of the new type of light magnesium cement foamed material. Mater. Lett. 2001, 50, 28–31. [Google Scholar] [CrossRef]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Antončík, F.; Pivák, A.; Pavlík, Z. Influence of waste plastic aggregate and water-repellent additive on the properties of lightweight magnesium oxychloride cement composite. Appl. Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Záleská, M.; Pavlíková, M.; Jankovský, O.; Lojka, M.; Pivák, A.; Pavlík, Z. Experimental analysis of MOC composite with a waste-expanded polypropylene-based aggregate. Materials 2018, 11, 931. [Google Scholar] [CrossRef] [Green Version]
- Deng, D. The mechanism for soluble phosphates to improve the water resistance of magnesium oxychloride cement. Cem. Concr. Res. 2003, 33, 1311–1317. [Google Scholar] [CrossRef]
- He, P.; Poon, C.S.; Tsang, D.C.W. Effect of pulverized fuel ash and CO2 curing on the water resistance of magnesium oxychloride cement (MOC). Cem. Concr. Res. 2017, 97, 115–122. [Google Scholar] [CrossRef]
- Tang, S.; Hu, Y.; Ren, W.; Yu, P.; Huang, Q.; Qi, X.; Li, Y.; Chen, E. Modeling on the hydration and leaching of eco-friendly magnesium oxychloride cement paste at the micro-scale. Constr. Build. Mater. 2019, 204, 684–690. [Google Scholar] [CrossRef]
- Cannesson, E.; Manier, S.; Nicholson, J.W. The influence of Group I metal chlorides on water loss and chloride release from magnesium oxychloride cements. Ceram.-Silikáty 2011, 55, 183–187. [Google Scholar]
- Xu, K.; Xi, J.; Guo, Y.; Dong, S. Effects of a new modifier on the water-resistance of magnesite cement tiles. Solid State Sci. 2012, 14, 10–14. [Google Scholar] [CrossRef]
- Li, C.; Yu, H. Influence of fly ash and silica fume on water-resistant property of magnesium oxychloride cement. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2010, 25, 721–724. [Google Scholar] [CrossRef]
- Pade, C.; Guimaraes, M. The CO2 uptake of concrete in a 100 year perspective. Cem. Concr. Res. 2007, 37, 1348–1356. [Google Scholar] [CrossRef]
- Fernández-Carrasco, L.; Torréns-Martín, D.; Martínez-Ramírez, S. Carbonation of ternary building cementing materials. Cem. Concr. Compos. 2012, 34, 1180–1186. [Google Scholar] [CrossRef]
- Lee, S.-W.; Lee, T.-H.; Park, J.-W.; Park, C.-H.; Kim, H.-J.; Kim, S.-M.; Lee, S.-H.; Song, J.-Y.; Lee, J.-H. The effect of laser irradiation on peel strength of temporary adhesives for wafer bonding. Int. J. Adhes. Adhes. 2015, 57, 9–12. [Google Scholar] [CrossRef]
- Power, I.M.; Dipple, G.M.; Francis, P.S. Assessing the carbon sequestration potential of magnesium oxychloride cement building materials. Cem. Concr. Compos. 2017, 78, 97–107. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. Impact of hydrated magnesium carbonate additives on the carbonation of reactive MgO cements. Cem. Concr. Res. 2013, 54, 87–97. [Google Scholar] [CrossRef]
- Unluer, C.; Al-Tabbaa, A. The role of brucite, ground granulated blastfurnace slag, and magnesium silicates in the carbonation and performance of MgO cements. Constr. Build. Mater. 2015, 94, 629–643. [Google Scholar] [CrossRef] [Green Version]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci. 2016, 9, 880–883. [Google Scholar] [CrossRef] [Green Version]
- Mo, L.; Panesar, D.K. Accelerated carbonation—A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Pu, L.; Unluer, C. Investigation of carbonation depth and its influence on the performance and microstructure of MgO cement and PC mixes. Constr. Build. Mater. 2016, 120, 349–363. [Google Scholar] [CrossRef]
- Vandeperre, L.J.; Al-Tabbaa, A. Accelerated carbonation of reactive MgO cements. Adv. Cem. Res. 2007, 19, 67–79. [Google Scholar] [CrossRef]
- Cannistraro, G.; Cannistraro, M.; Piccolo, A.; Restivo, R. Potentials and limits of oxidative photocatalysisand possible applications in the field of cultural heritage. In Advanced Materials Research; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2013; pp. 111–117. [Google Scholar]
- Cannistraro, G.; Cannistraro, M.; Cannistraro, A.; Galvagno, A.; Engineer, F. Analysis of air pollution in the urban center of four cities Sicilian. Int. J. Heat Technol. 2016, 34, S219–S225. [Google Scholar] [CrossRef]
- Cannistraro, G.; Cannistraro, M.; Cao, J.; Ponterio, L. New technique monitoring and transmission environmental data with mobile systems. Instrum. Mes. Metrol. 2018, 17, 549. [Google Scholar] [CrossRef]
- Cannistraro, M.; Ponterio, L.; Cao, J. Experimental study of air pollution in the urban centre of the city of Messina. Model. Meas. Control C 2018, 79, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Chang, J. Rapid hardening β-C2S mineral and microstructure changes activated by accelerated carbonation curing. J. Therm. Anal. Calorim. 2017, 129, 681–689. [Google Scholar] [CrossRef]
- Jang, J.G.; Lee, H.K. Microstructural densification and CO2 uptake promoted by the carbonation curing of belite-rich Portland cement. Cem. Concr. Res. 2016, 82, 50–57. [Google Scholar] [CrossRef]
- Mo, L.; Zhang, F.; Deng, M. Mechanical performance and microstructure of the calcium carbonate binders produced by carbonating steel slag paste under CO2 curing. Cem. Concr. Res. 2016, 88, 217–226. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Mechanism for rapid hardening of cement pastes under coupled CO2-water curing regime. Cem. Concr. Compos. 2019, 97, 78–88. [Google Scholar] [CrossRef]
- Seo, J.H.; Amr, I.T.; Park, S.M.; Bamagain, R.A.; Fadhel, B.A.; Kim, G.M.; Hunaidy, A.S.; Lee, H.K. CO₂ uptake of carbonation-cured cement blended with ground volcanic Ash. Materials 2018, 11, 2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blunden, J.; Amdt, D.S.; Hartfield, G.; Sánchez-Lugo, A.; Scambos, T.A.; Schreck, C.J.I.; Stammerjohn, S.; Stanitski, D.M.; Willett, K.M.; Bissolli, P. State of Climate in 2017. Bull. Am. Meteorol. Soc. 2018, 99, S1–S310. [Google Scholar]
- Imbabi, M.S.; Carrigan, C.; McKenna, S. Trends and developments in green cement and concrete technology. Int. J. Sustain. Built Environ. 2012, 1, 194–216. [Google Scholar] [CrossRef] [Green Version]
- Kemp, R.; Barteková, E.; Türkeli, S. The innovation trajectory of eco-cement in the Netherlands: A co-evolution analysis. Int. Econ. Econ. Policy 2017, 14, 409–429. [Google Scholar] [CrossRef] [Green Version]
- Gartner, E.; Hirao, H. A review of alternative approaches to the reduction of CO2 emissions associated with the manufacture of the binder phase in concrete. Cem. Concr. Res. 2015, 78, 126–142. [Google Scholar] [CrossRef] [Green Version]
- Wisconsin Department of Health Services; Carbon Dioxide. Available online: https://www.dhs.wisconsin.gov/chemical/carbondioxide.htm (accessed on 17 February 2020).
- Thiery, M.; Villain, G.; Dangla, P.; Platret, G. Investigation of the carbonation front shape on cementitious materials: Effects of the chemical kinetics. Cem. Concr. Res. 2007, 37, 1047–1058. [Google Scholar] [CrossRef]
- Wang, J.; Xu, H.; Xu, D.; Du, P.; Zhou, Z.; Yuan, L.; Cheng, X. Accelerated carbonation of hardened cement pastes: Influence of porosity. Constr. Build. Mater. 2019, 225, 159–169. [Google Scholar] [CrossRef]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jankovský, O.; Lojka, M.; Lauermannová, A.-M.; Antončík, F.; Pavlíková, M.; Pavlík, Z.; Sedmidubský, D. Carbon Dioxide Uptake by MOC-Based Materials. Appl. Sci. 2020, 10, 2254. https://doi.org/10.3390/app10072254
Jankovský O, Lojka M, Lauermannová A-M, Antončík F, Pavlíková M, Pavlík Z, Sedmidubský D. Carbon Dioxide Uptake by MOC-Based Materials. Applied Sciences. 2020; 10(7):2254. https://doi.org/10.3390/app10072254
Chicago/Turabian StyleJankovský, Ondřej, Michal Lojka, Anna-Marie Lauermannová, Filip Antončík, Milena Pavlíková, Zbyšek Pavlík, and David Sedmidubský. 2020. "Carbon Dioxide Uptake by MOC-Based Materials" Applied Sciences 10, no. 7: 2254. https://doi.org/10.3390/app10072254