Measurements of N2, CO2, Ar, O2 and Air Pressure Broadening Coefficients of the HCl P(5) Line in the 1–0 Band Using an Interband Cascade Laser
Abstract
:1. Introduction
2. Methods and Experiment
2.1. Methodology
2.2. Experimental Details
3. Results and Discussion
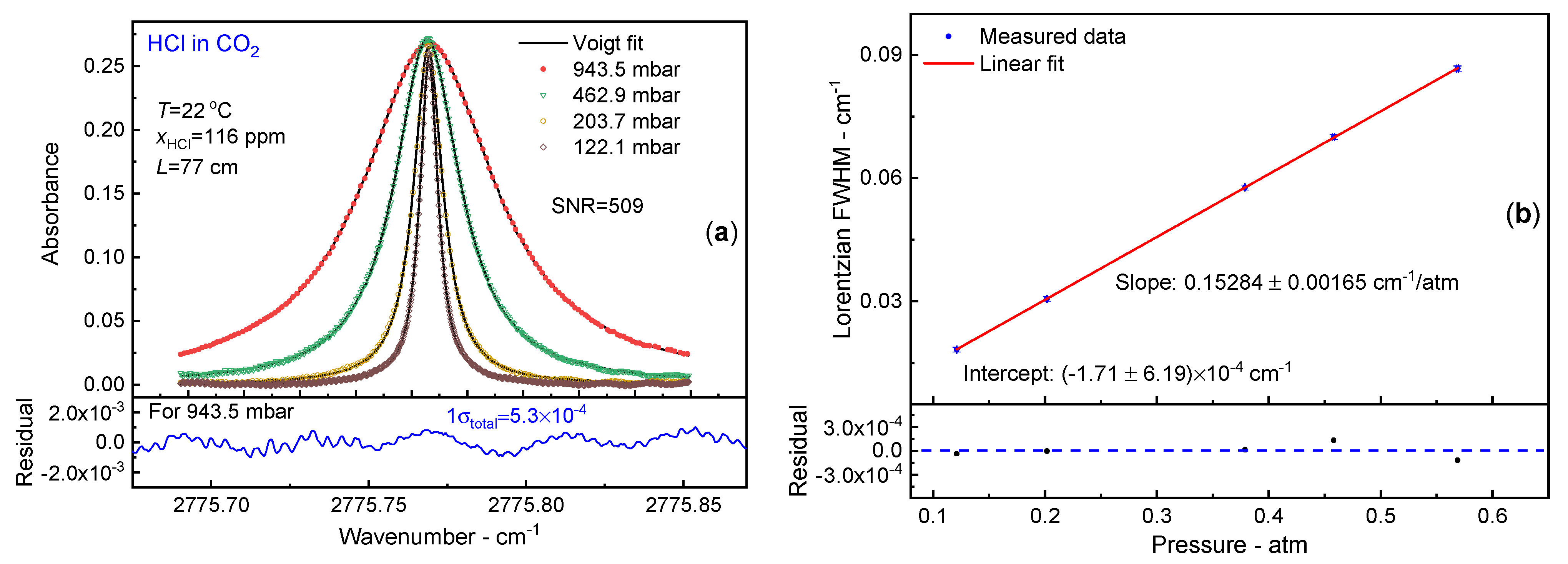
3.1. CO2 Broadening Coefficient
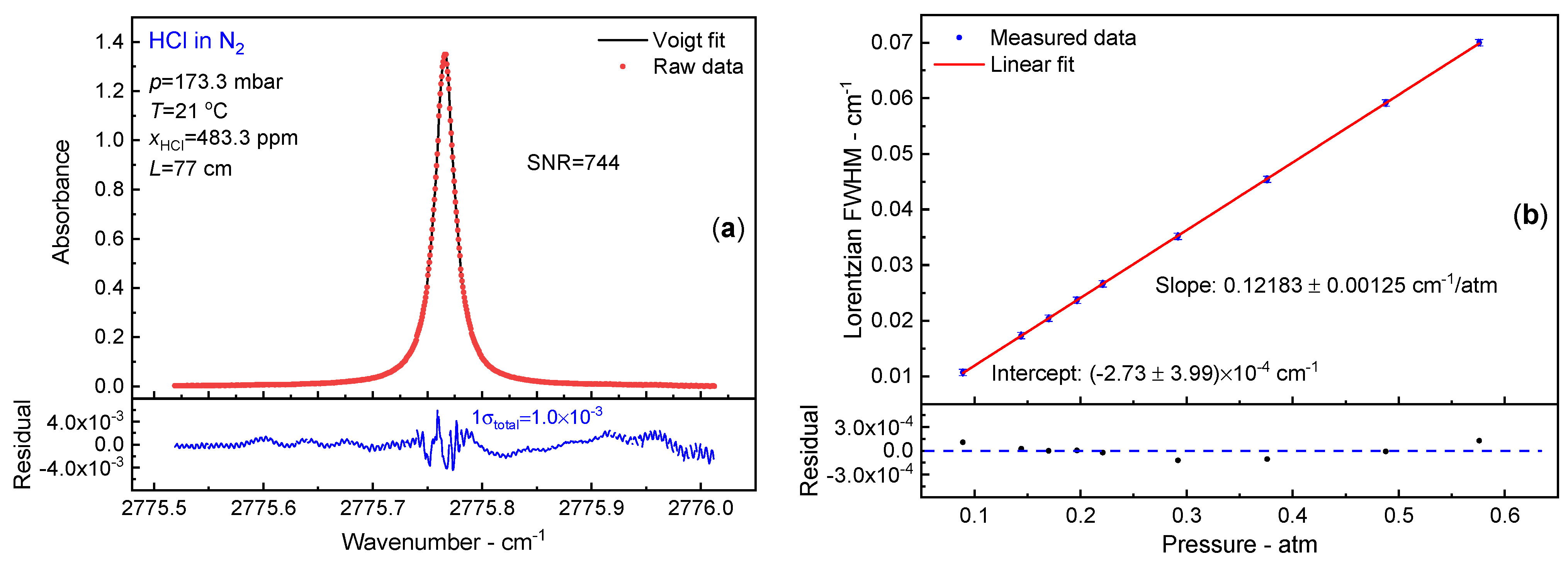
3.2. N2 Broadening Coefficient
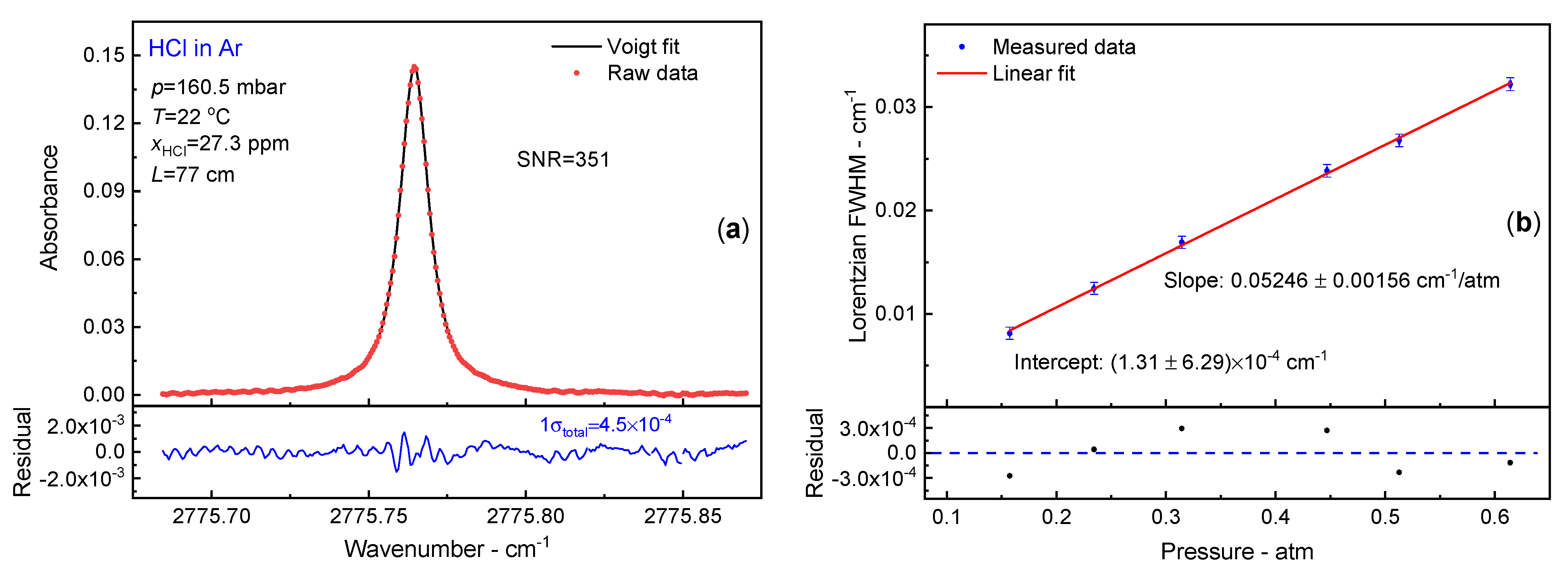
3.3. Ar Broadening Coefficient
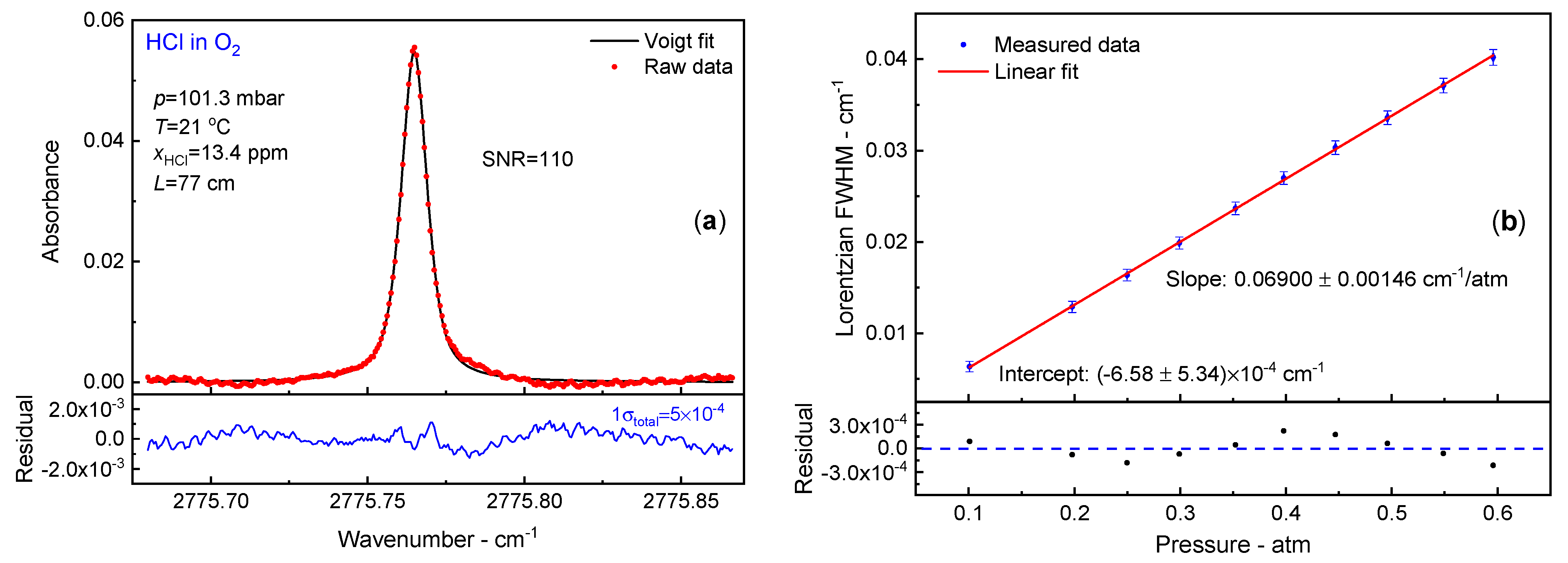
3.4. O2 Broadening Coefficient
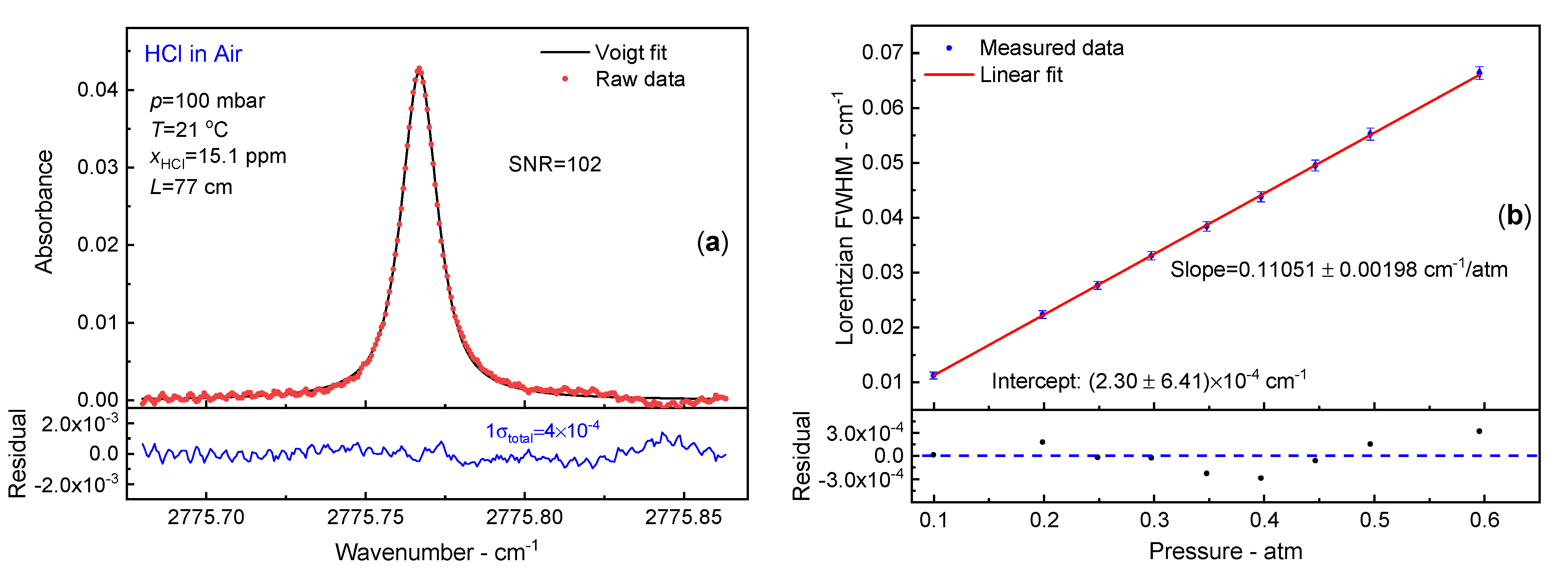
3.5. Air Broadening Coefficien
3.6. Comparison with Literature Values
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thierry, L.; Ferrería de laFuente, J.F.; Neuwahl, F.; Canova, M.; Pinasseau, A.; Jankov, I.; Brinkmann, T.; Roudier, S.; Sancho, L.D. BAT Reference Document for Large Combustion Plants. Industrial Emissions Directive 2010/75/EU (Integrated PollutionPrevention and Control); Publications Office of the European Union: Luxembourg, 2016; ISBN 9789279743030. [Google Scholar] [CrossRef]
- EMPIR Project: IMPRESS2. Available online: http://empir.npl.co.uk/impress/ (accessed on 25 April 2021).
- Abdoulmoumine, N.; Adhikari, S.; Kulkarni, A.; Chattanathan, S. A review on biomass gasification syngas cleanup. Appl. Energy 2015, 155, 294–307. [Google Scholar] [CrossRef]
- Bjoroey, O.; Haugholt, K.H.; Jaeger, T. Diode laser spectroscopy of gaseous HCl. Quantum Electron. 1996, 26, 1090–1092. [Google Scholar] [CrossRef]
- Leibold, H.; Hornung, A.; Seifert, H. HTHP syngas cleaning concept of two stage biomass gasification for FT synthesis. Powder Technol. 2008, 180, 265–270. [Google Scholar] [CrossRef]
- Ortwein, P.; Woiwode, W.; Fleck, S.; Eberhard, M.; Kolb, T.; Wagner, S.; Gisi, M.; Ebert, V. Absolute diode laser-based in situ detection of HCl in gasification processes. Exp. Fluids 2010, 49, 961–968. [Google Scholar] [CrossRef]
- International Technology Roadmap for Semiconductors. Available online: http://www.itrs2.net/itrs-reports.html (accessed on 25 April 2021).
- Wu, B.J.; Bai, H.; Lin, I.K.; Liu, S.S. Al-Cu pattern wafer study on metal corrosion due to chloride ion contaminants. IEEE Trans. Semicond. Manuf. 2010, 23, 553–558. [Google Scholar] [CrossRef]
- EMPIR Project: MetAMC2. Available online: http://empir.npl.co.uk/metamcii/ (accessed on 25 April 2021).
- Molina, M.J.; Rowland, F.S. Stratospheric sink of chlorofluoromrthanes: Chlorine atom-catalyzed destruction of ozone. Nature 1974, 249, 810. [Google Scholar] [CrossRef]
- Keene, W.C.; Khalil, M.A.K.; Erickson, D.J.; McCulloch, A.; Graedel, T.E.; Lobert, J.M.; Aucott, M.L.; Gong, S.L.; Harper, D.B.; Kleiman, G.; et al. Composite global emissions of reactive chlorine from anthropogenic and natural sources: Reactive chlorine emissions inventory. J. Geophys. Res. Atmos. 1999, 104, 8429–8440. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Huey, L.G.; Stickel, R.E.; Pierce, R.B.; Chen, G.; Avery, M.A.; Dibb, J.E.; Diskin, G.S.; Sachse, G.W.; McNaughton, C.S.; et al. Airborne measurements of HCl from the marine boundary layer to the lower stratosphere over the North Pacific Ocean during INTEX-B. Atmos. Chem. Phys. Discuss. 2008, 8, 3563–3595. [Google Scholar] [CrossRef] [Green Version]
- Keene, W.C.; Stutz, J.; Pszenny, A.A.P.; Maben, J.R.; Fischer, E.V.; Smith, A.M.; von Glasow, R.; Pechtl, S.; Sive, B.C.; Varner, R.K. Inorganic chlorine and bromine in coastal New England air during summer. J. Geophys. Res. Atmos. 2007, 112, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Serdyukov, A.; Gisi, M.; Werhahn, O.; Ebert, V. FTIR-based measurements of self-broadening and self-shift coefficients as well as line strength in the first overtone band of HCl at 1.76μM. J. Quant. Spectrosc. Radiat. Transf. 2015, 165, 76–87. [Google Scholar] [CrossRef]
- Li, G.; Domanskaya, A.V.; Tran, H.; Gisi, M.; Ebert, V. Broadening and shift coefficients for the (2 ← 0) overtone band of HCl (1.76 µm) induced by exhaust gases CO and CO2. J. Quant. Spectrosc. Radiat. Transf. 2017, 203, 434–439. [Google Scholar] [CrossRef]
- Ortwein, P.; Woiwode, W.; Wagner, S.; Gisi, M.; Ebert, V. Laser-based measurements of line strength, self- and pressure-broadening coefficients of the H35Cl R(3) absorption line in the first overtone region for pressures up to 1 MPa. Appl. Phys. B 2010, 100, 341–347. [Google Scholar] [CrossRef]
- Nwaboh, J.A.; Meuzelaar, H.; Liu, J.; Persijn, S.; Li, J.; Van Der Veen, A.M.H.; Chatellier, N.; Papin, A.; Qu, Z.; Werhahn, O.; et al. Accurate analysis of HCl in biomethane using laser absorption spectroscopy and ion-exchange chromatography. Analyst 2021, 146, 1402–1413. [Google Scholar] [CrossRef]
- Domanskaya, A.V.; Li, G.; Tran, H.; Gisi, M.; Ebert, V. Collision-induced line parameters for the (2 ← 0) overtone band of HCl (1.76 µm) in binary mixtures with H2 and CH4. J. Quant. Spectrosc. Radiat. Transf. 2017, 199, 71–76. [Google Scholar] [CrossRef]
- Tran, H.; Li, G.; Ebert, V.; Hartmann, J.-M. Super-and sub-Lorentzian effects in the Ar-broadened line wings of HCl gas. J. Chem. Phys. 2017, 146, 194305. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Nwaboh, J.; Werhahn, O.; Ebert, V. Towards a dTDLAS-Based Spectrometer for Absolute HCl Measurements in Combustion Flue Gases and a Better Evaluation of Thermal Boundary Layer Effects-of-sight measurements HCl measurement Spatial heterogeneity Thermal boundary layer. Flow Turbul. Combust. 2021, 106, 533–546. [Google Scholar] [CrossRef]
- Ebert, V.; Wolfrum, J. Absorption Spectroscopy. In Optical Measurements—Techniques and Applications, (Heat and Mass Transfer), 2nd ed.; Mayinger, F., Feldmann, O., Eds.; Springer: Heidelberg, Germany, 2001. [Google Scholar]
- Qu, Z.; Steinvall, E.; Ghorbani, R.; Schmidt, F.M. Tunable Diode Laser Atomic Absorption Spectroscopy for Detection of Potassium under Optically Thick Conditions. Anal. Chem. 2016, 88, 3754–3760. [Google Scholar] [CrossRef] [Green Version]
- Schulz, C.; Dreizler, A.; Ebert, V.; Wolfrum, J. Combustion Diagnostics. In Springer Handbook of Experimental Fluid Dynamics, 1st ed.; Tropea, C., Foss, J., Yarin, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; ISBN 9783662491621. [Google Scholar]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Chris Benner, D.; Bernath, P.F.; Birk, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R.; et al. The HITRAN2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 4–50. [Google Scholar] [CrossRef] [Green Version]
- Tudorie, M.; Földes, T.; Vandaele, A.C.; Vander Auwera, J. CO2 pressure broadening and shift coefficients for the 1-0 band of HCl and DCl. J. Quant. Spectrosc. Radiat. Transf. 2012, 113, 1092–1101. [Google Scholar] [CrossRef]
- Pine, A.S.; Looney, J.P. N2 and air broadening in the fundamental bands of HF and HCl. J. Mol. Spectrosc. 1987, 122, 41–55. [Google Scholar] [CrossRef]
- Boulet, C.; Flaud, P.M.; Hartmann, J.M. Infrared line collisional parameters of HCl in argon, beyond the impact approximation: Measurements and classical path calculations. J. Chem. Phys. 2004, 120, 11053–11061. [Google Scholar] [CrossRef]
- Asfin, R.E.; Domanskaya, A.V.; Maul, C. Broadening and shifting coefficients of rotation-vibrational lines in the fundamental and first overtone bands of HCl and HBr induced by oxygen and air. J. Quant. Spectrosc. Radiat. Transf. 2013, 130, 296–303. [Google Scholar] [CrossRef]
- Houdeau, J.P.; Larvor, M.; Haeusler, C. Etude basse temprature des largeurs et des dplacements des raies rovibrationnelles de la bande fondamentale de H35Cl comprim par N2, O2, D2 et H2. Can. J. Phys. 1980, 58, 318–324. [Google Scholar] [CrossRef]
- Asfin, R.E.; Domanskaya, A.V.; Maul, C.; Bulanin, M.O. Nitrogen-induced broadening and shift coefficients of rotation-vibrational lines in the fundamental and first overtone bands of HCl and HBr. J. Mol. Spectrosc. 2012, 282, 9–13. [Google Scholar] [CrossRef]
- Houdeau, J.P.; Larvor, M.; Haeusler, C. Widths and shifts of H35Cl lines in the fundamental band induced by argon and xenon at low temperatures. J. Quant. Spectrosc. Radiat. Transf. 1976, 16, 457–465. [Google Scholar] [CrossRef]


| This Work | References | Scaled to 296 K (This Work/References) | ||
|---|---|---|---|---|
| γCO2/103 cm−1/atm | 76.42 ± 0.83 | 74.2 ± 2.2 [25] | 76.23/74.2 [24] | |
| Pressure/atm | 0.1–0.6 | 0.2–0.93 | ||
| Temperature/K | 295 | 296 | ||
| Relative uncertainty | 1.09% | 3% | ||
| γN2/103 cm−1/atm | 60.92 ± 0.63 | 60.9 ± 0.5 [26] | 59.2 ± 0.3 [30] | 60.71/60.8 [25], 59.4 [26] |
| Pressure/atm | 0.1–0.6 | 0.026–0.27 | ≤11 | |
| Temperature/K | 294 | 295 | 298 | |
| Relative uncertainty | 1.03% | 0.8% | 0.5% | |
| γAr/103 cm−1/atm | 26.23 ± 0.78 | 29.4 ± 2 (meas.) 25.6 (cal.) [27] | 27.8 [31] | 26.19/27.89 [28] |
| Pressure/atm | 0.1–0.6 | 10–50 | 3–5 | |
| Temperature /K | 295 | Room T | 298 | |
| Relative uncertainty | 2.97% | 6.8% | – | |
| γO2/103 cm−1/atm | 34.50 ± 0.73 | 31.9 ± 0.3 [28] | 33.4 [29] | 34.38/32.01 [29], 33.51 [30] |
| Pressure/atm | 0.1–0.6 | 2–11 | ≤5 | |
| Temperature/K | 294 | 298 | 298 | |
| Relative uncertainty | 2.12% | 0.9% | – | |
| γAir/103 cm−1/atm | 55.26 ± 0.99 | 52.4 ± 0.5 [28] | 54.1 ± 0.3 [26] | 55.09/52.56 [29], 54.02 [25] |
| Pressure/atm | 0.1–0.6 | 2–11 | 0.026–0.27 | |
| Temperature/K | 294 | 298 | 295 | |
| Relative uncertainty | 1.79% | 1% | 0.6% | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qu, Z.; Nwaboh, J.A.; Li, G.; Werhahn, O.; Ebert, V. Measurements of N2, CO2, Ar, O2 and Air Pressure Broadening Coefficients of the HCl P(5) Line in the 1–0 Band Using an Interband Cascade Laser. Appl. Sci. 2021, 11, 5190. https://doi.org/10.3390/app11115190
Qu Z, Nwaboh JA, Li G, Werhahn O, Ebert V. Measurements of N2, CO2, Ar, O2 and Air Pressure Broadening Coefficients of the HCl P(5) Line in the 1–0 Band Using an Interband Cascade Laser. Applied Sciences. 2021; 11(11):5190. https://doi.org/10.3390/app11115190
Chicago/Turabian StyleQu, Zhechao, Javis A. Nwaboh, Gang Li, Olav Werhahn, and Volker Ebert. 2021. "Measurements of N2, CO2, Ar, O2 and Air Pressure Broadening Coefficients of the HCl P(5) Line in the 1–0 Band Using an Interband Cascade Laser" Applied Sciences 11, no. 11: 5190. https://doi.org/10.3390/app11115190







