Recent Technical Progression in Photoacoustic Imaging—Towards Using Contrast Agents and Multimodal Techniques
Abstract
:1. Introduction
1.1. PAI Technique and Its Categories
1.2. Key Devices in PAI
2. Achievements of PAI Techniques in the Past Five Years
3. Exogenous Reagents for PAI
4. PAI in Combination with Other Imaging Techniques
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yoko Hoshi, M.D.; Yukio, Y. Overview of diffuse optical tomography and its clinical applications. J. Biomed. Opt. 2016, 21, 091312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gunther, J.; Andersson-Engels, S. Review of current methods of acousto-optical tomography for biomedical applications. Front. Optoelectron. 2017, 10, 211–238. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.H.; Xia, F.; Sawan, M. Photoacoustic imaging for monitoring of stroke diseases: A review. Photoacoustics 2021, 23, 100287. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, E.H.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Karlas, A.; Fasoula, N.-A.; Paul-Yuan, K.; Reber, J.; Kallmayer, M.; Bozhko, D.; Seeger, M.; Eckstein, H.-H.; Wildgruber, M.; Ntziachristos, V. Cardiovascular optoacoustics: From mice to men—A review. Photoacoustics 2019, 14, 19–30. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z. Multimodal intravascular photoacoustic and ultrasound imaging. Biomed. Eng. Lett. 2018, 8, 193–201. [Google Scholar] [CrossRef]
- Bauer, A.Q.; Nothdurft, R.E.; Erpelding, T.N.; Wang, L.V.; Culver, J.P. Quantitative, high-resolution photoacoustic spectroscopy by combining photoacoustic imaging with diffuse optical tomography. Proc. SPIE 2011, 7899, 78993O. [Google Scholar]
- Vu, T.; Razansky, D.; Yao, J. Listening to tissues with new light: Recent technological advances in photoacoustic imaging. J. Opt. 2019, 21, 1003001. [Google Scholar] [CrossRef]
- Das, D.; Sharma, A.; Rajendran, P.; Pramanik, M. Another decade of photoacoustic imaging. Phys. Med. Biol. 2021, 66, 05TR01. [Google Scholar] [CrossRef]
- Subochev, P.; Smolina, E.; Sergeeva, E.; Kirillin, M.; Orlova, A.; Kurakina, D.; Emyanov, D.; Razansky, D. Toward whole-brain in vivo optoacoustic angiography of rodents: Modeling and experimental observations. Biomed. Opt. Exp. 2020, 11, 1477–1488. [Google Scholar] [CrossRef]
- Li, L.; Zhu, L.; Ma, C.; Lin, L.; Yao, J.; Wang, L.; Maslov, K.; Zhang, R.; Chen, W.; Shi, J.; et al. Single-impulse panoramic photoacoustic computed tomography of small-animal whole-body dynamics at high spatiotemporal resolution. Nat. Biomed. Eng. 2017, 1, 0071. [Google Scholar] [CrossRef]
- Liu, C.; Gong, X.; Lin, R.; Liu, F.; Chen, J.; Wang, Z.; Song, L.; Chu, J. Advances in imaging techniques and genetically encoded probes for photoacoustic imaging. Theranostics 2016, 6, 2414–2430. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, R.; Liu, C.; Zhao, J.; Si, G.; Song, L. Quantitative analysis on in vivo tumour-microvascular images from optical-resolution photoacoustic microscopy. J. Biophotonics 2019, 12, e201800421. [Google Scholar] [CrossRef]
- Liu, C.; Liang, Y.; Wang, L. Single-shot photoacoustic microscopy of hemoglobin concentration, oxygen saturation, and blood flow in sub-microseconds. Photoacoustics 2020, 17, 100156. [Google Scholar] [CrossRef]
- Wang, L.; Maslov, K.; Wang, L.V. Single-cell label-free photoacoustic flowoxigraphy in vivo. Proc. Natl. Acad. Sci. USA 2013, 110, 5759–5764. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Liang, Y.; Wang, L. Optical-resolution photoacoustic microscopy oxygen saturation with nonlinear compensation. Biomed. Opt. Exp. 2019, 10, 3061–3069. [Google Scholar] [CrossRef]
- Rebling, J.; Estrada, H.; Gottschalk, S.; Sela, G.; Zwack, M.; Wissmeyer, G.; Ntziachristos, V.; Razansky, D. Dual-wavelength hybrid optoacoustic-ultrasound biomicroscopy for functional imaging of large-scale cerebral vascular networks. J. Biophotonics 2018, 11, e201800057. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Maslov, K.I.; Zhang, Y.; Xia, Y.; Wang, L.V. Label-free oxygen-metabolic photoacoustic microscopy in vivo. J. Biomed. Opt. 2011, 16, 076003. [Google Scholar] [CrossRef]
- Fang, H.; Maslov, K.; Wang, L.V. Photoacoustic doppler effect from flowing small light-absorbing particles. Phys. Rev. Lett. 2007, 99, 184501. [Google Scholar] [CrossRef] [Green Version]
- Strohm, E.M.; Kolios, M.C. Classification of blood cells and tumour cells using label-free ultrasound and photoacoustics. Cytom. Part A 2015, 87, 741–749. [Google Scholar] [CrossRef]
- Galanzha, E.I.; Kokoska, M.S.; Shashkov, E.V.; Kim, J.W.; Tuchin, V.V.; Zharov, V.V. In vivo fiber-based multicolor photoacoustic detection and photothermal purging of metastasis in sentinel lymph nodes targeted by nanoparticles. J. Biophoton. 2009, 2, 528–539. [Google Scholar] [CrossRef] [Green Version]
- Allen, T.J.; Hall, A.; Dhillon, A.P.; Owen, J.S.; Beard, P.C. Spectroscopic photoacoustic imaging of lipid-rich plaques in the human aorta in the 740 to 1400 nm wavelength range. J. Biomed. Opt. 2012, 17, 061209. [Google Scholar] [CrossRef]
- Jansen, K.; van Soest, G.; van der Steen, A.F.W. Intravascular photoacoustic imaging: A new tool for vulnerable plaque identification. Ultrasound Med. Biol. 2014, 40, 1037–1048. [Google Scholar] [CrossRef] [Green Version]
- Tsang, V.T.C.; Li, X.; Wong, T.T.W. A review of endogenous and exogenous contrast agents used in photoacoustic tomography with different sensing configurations. Sensors 2020, 20, 5595. [Google Scholar] [CrossRef]
- Wang, D.; Wu, Y.; Xia, J. Review on photoacoustic imaging of the brain using nanoprobes. Neurophoton 2016, 3, 010901. [Google Scholar] [CrossRef] [Green Version]
- Cox, B.T.; Arridge, S.R.; Köstlia, K.P.; and Beard, P.C. Quantitative photoacoustic imaging: Fitting a model of light transport to the initial pressure distri-bution. Proc. SPIE 2005, 5697, 49–55. [Google Scholar]
- Costa, M.M.; Shah, A.; Rivens, I.; Box, C.; O’Shea, T.; Papaevangelou, E.; Bamber, J.; ter Haar, G. Quantitative photoacoustic imaging study of tumours in vivo: Baseline variations in quantitative measurements. Photoacoustics 2019, 13, 53–65. [Google Scholar] [CrossRef]
- Govinahallisathyanarayana, S.; Ning, B.; Cao, R.; Hu, S.; Hossack, J.A. Dictionary learning-based reverberation removal enables depth-resolved photoacoustic microscopy of cortical microvasculature in the mouse brain. Sci. Rep. 2018, 8, 985. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Maslov, K.; Yao, J.; Rao, B.; Wang, L.V. Fast voice-coil scanning optical-resolution photoacoustic microscopy. Opt. Lett. 2011, 36, 139–141. [Google Scholar] [CrossRef]
- Yao, J.; Huang, C.-H.; Wang, L.; Yang, J.-M.; Gao, L.; Maslov, K.I.; Zou, J.; Wang, L.V. Wide-field fast-scanning photoacoustic microscopy based on a water-immersible MEMS scanning mirror. J. Biomed. Opt. 2012, 17, 080505. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, C.; Park, K.; Lim, G.; Kim, C. A PDMS-based 2-axis waterproof scanner for photoacoustic microscopy. Sensors 2015, 15, 9815–9826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Y.; Shi, J.; Maslo, K.I.; Cao, R.; Wang, L.V. Wave of single-impulse-stimulated fast initial dip in single vessels of mouse brains imaged by high-speed functional photoacoustic microscopy. J. Biomed. Opt. 2020, 25, 066501. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Beard, P.C. Pulsed near-infrared laser diode excitation system for biomedical photoacoustic imaging. Opt. Lett. 2006, 31, 3462–3464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, T.J.; Beard, P.C. Dual wavelength laser diode excitation source for 2D photoacoustic imaging. Proc. SPIE 2007, 6437, 64371U. [Google Scholar]
- Upputuri, P.K.; Pramanik, M. Dynamic in vivo imaging of small animal brain using pulsed laser diode-based photoacoustic tomography system. J. Biomed. Opt. 2017, 22, 090501. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Periyasamy, V.; Kalva, S.K.; Pramanik, M. A high-performance compact photoacoustic tomography system for in vivo small-animal brain imaging. J. Vis. Exp. 2017, 124, e55811. [Google Scholar] [CrossRef]
- Rajendran, P.; Sahu, S.; Dienzo, R.A.; Pramanik, M. In vivo detection of venous sinus distension due to intracranial hypotension in small animal using pulsed-laser-diode photoacoustic tomography. J. Biophoton. 2020, 13, e201960162. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shiget, Y.; Wang, X. Light emitting diodes based photoacoustic imaging and potential clinical applications. Sci. Rep. 2018, 8, 9885. [Google Scholar] [CrossRef] [Green Version]
- Najafzadeh, E.; Ghadiri, H.; Alimohamadi, M.; Farnia, P.; Mehrmohammadi, M.; Ahmadian, A. Evaluation of multi-wavelengths LED-based photoacoustic imaging for maximum safe resection of glioma: A proof of concept study. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 1053–1062. [Google Scholar] [CrossRef]
- Zhong, H.; Duan, T.; Lan, H.; Zhou, M.; Gao, F. Rview of low-cost photoacoustic sensing and imaging based on laser diode and light-emitting diode. Sensors 2018, 18, 2264. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Ding, Y.; Liu, G.; Zeng, L. Low-cost photoacoustic imaging systems based on laser diode and light-emitting diode excitation. J. Innov. Opt. Health Sci. 2017, 10, 1730003. [Google Scholar] [CrossRef] [Green Version]
- Singh, M.K.A.; Xia, W. Portable and affordable light source-based photoacoustic tomography. Sensors 2020, 20, 6173. [Google Scholar] [CrossRef]
- Aytac-Kipergil, E.; Demirkiran, A.; Uluc, N.; Yavas, S.; Kayikcioglu, T.; Salman, S.; Karamuk, S.G.; Ilday, F.O.; Unlu, M.B. Development of a fiber laser with independently adjustable properties for optical resolution photoacoustic microscopy. Sci. Rep. 2016, 6, 1–10. [Google Scholar]
- Allen, T.J.; Spurrell, J.; Berendt, M.O.; Ogunlade, O.; Alam, S.U.; Zhang, E.Z.; Richardson, D.J.; Beard, P.C. Ultrafast laser-scanning optical resolution photoacoustic microscopy at up to 2 million A-lines per second. J. Biomed. Opt. 2018, 23, 126502. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Liang, Y.; Jin, L.; Wang, L. Dual-polarized fiber laser sensor for photoacoustic microscopy. Sensors 2019, 19, 4632. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Alam, S.; Kang, Q.; Shepherd, D.P.; Richardson, D.J. Raman-shifted wavelength-selectable pulsed fiber laser with high repetition rate and high pulse energy in the visible. Opt. Exp. 2017, 25, 351–356. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; He, L.; Liang, Y.; Wang, L. Wide-field polygon-scanning photoacoustic microscopy of oxygen saturation at 1-MHz A-line rate. Photoacoustics 2020, 20, 100195. [Google Scholar] [CrossRef]
- Allen, T.J.; Berendt, M.; Lin, D.; Alam, S.U.; Huynh, N.T.; Zhang, E.; Richardson, D.J.; Beard, P.C. High pulse energy fibre laser as an excitation source for photoa-coustic tomograph. Opt. Exp. 2020, 28, 34255–34265. [Google Scholar] [CrossRef]
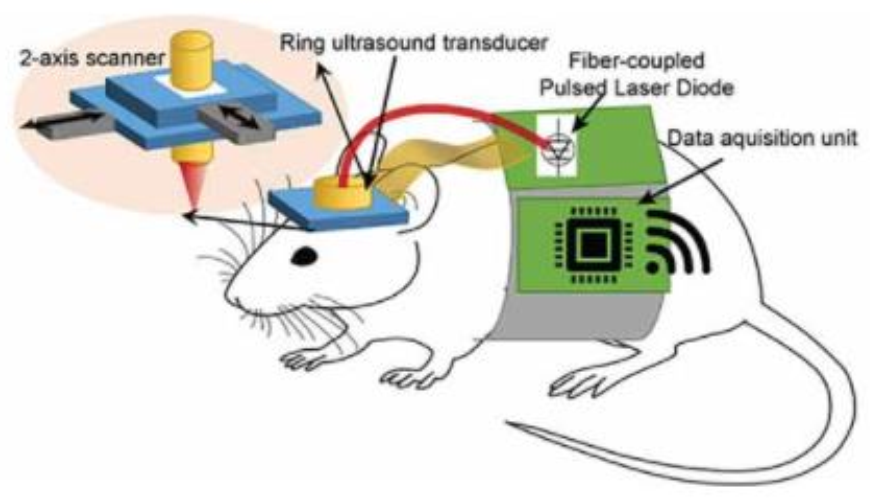
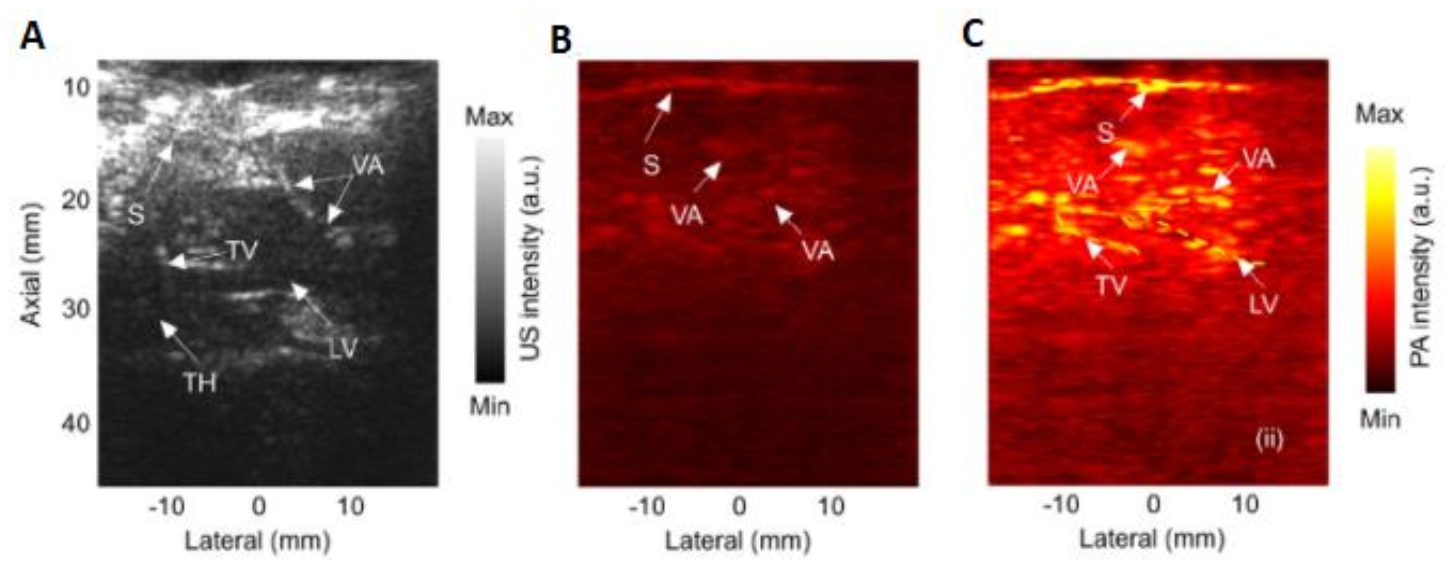
- Tang, J.; Dai, X.; Jiang, H. Wearable scanning photoacoustic brain imaging in behaving rats. J. Biophotonics 2016, 9, 570–575. [Google Scholar] [CrossRef]
- Tang, J.; Coleman, J.E.; Dai, X.; Jiang, H. Wearable 3-D photoacoustic tomography for functional brain im-aging in behaving rats. Sci. Rep. 2016, 6, 25470. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Xie, H.; Xi, L. Wearable optical resolution photoacoustic microscopy. J. Biophoton. 2019, 12, e201900066. [Google Scholar] [CrossRef]
- Dangi, A.; Agrawal, S.; Datta, G.R.; Srinivasan, V.; Kothapalli, S.R. Towards a low-cost and portable photoacoustic microscope for point-of-care and wearable applications. IEEE Sens. J. 2020, 20, 6881–6888. [Google Scholar] [CrossRef]
- Dangi, A.; Roy, K.; Agrawal, S.; Chen, H.; Ashok, A.; Wible, C.; Osman, M.; Pratap, R.; Kothapalli, S.R. A modular approach to neonatal whole-brain photoacoustic imaging. Proc. SPIE 2020, 11240, 1124057. [Google Scholar]
- Greenberg, D.S.; Houweling, A.R.; Kerr, J.N.D. Population imaging of ongoingneuronal activity in the visual cortex of awake rats. Nat. Neurosci. 2008, 11, 749–751. [Google Scholar] [CrossRef]
- Kaisti, K.K.; Langsjo, J.W.; Aalto, S.; Oikonen, V.; Sipila, H.; Teras, M.; Hinkka, S.; Metsahonkala, L.; Scheinin, H. Effects of sevoflurane, propofol and adjunct-nitrous oxide on regional cerebral blood flow, oxygen consumption, and blood volume in humans. Anesthesiology 2003, 99, 603–613. [Google Scholar] [CrossRef]
- Shtoyerman, E.; Arieli, A.; Slovin, H.; Vanzetta, I.; Grinvald, A. Long-term optical imaging and spectroscopy reveal mechanisms underlying the intrinsic signal and stability of cortical maps in V1 of behaving monkeys. J. Neurosci. 2000, 20, 8111–8121. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Li, J.; Ning, B.; Sun, N.; Wang, T.; Zuo, Z.; Hu, S. Functional and oxygen-metabolic photoacoustic microscopy of the awake mouse brain. NeuroImage 2017, 150, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Yao, J. 3D Monte Carlo simulation of light distribution in mouse brain in quantitative photoacoustic computed tomography. Quant. Imaging Med. Surg. 2021, 11, 1046–1059. [Google Scholar] [CrossRef]
- Manwar, R.; Li, X.; Mahmoodkalayeh, S.; Asano, E.; Zhu, D.; Avanaki, K. Deep learning protocol for improved photoacoustic brain imaging. J. Biophoton. 2020, 13, e202000212. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Recent advances in photoacoustic contrast agents for in vivo imaging. Nanomed. Nanobiotechnol. 2020, 12, e1618. [Google Scholar] [CrossRef]
- Liu, J.; Cai, X.; Pan, H.-C.; Bandla, A.; Chuan, C.K.; Wang, S.; Thakor, N.; Liao, L.-D.; Liu, B. Molecular engineering of photoacoustic performance by chalcogenide variation in conjugated polymer nanoparticles for brain vascular imaging. Small 2018, 14, 1703732. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, C.; Hu, D.; Wang, F.; Wu, H.; Gong, X.; Liu, X.; Song, L.; Sheng, Z.; Zheng, H. Single-layer MoS2 nanosheets with amplified photoacoustic effect for highly sensitive photoacoustic imaging of orthotopic brain tumours. Adv. Funct. Mater. 2016, 26, 8715–8725. [Google Scholar] [CrossRef]
- Liu, C.; Chen, J.; Zhu, Y.; Gong, X.; Zheng, R.; Chen, N.; Chen, D.; Yan, H.; Zhang, P.; Zheng, H.; et al. Highly sensitive MoS2-indocyanine green hybrid for photoacoustic imaging of orthotopic brain glioma at deep site. Nanomicro Lett. 2018, 10, 48. [Google Scholar] [PubMed] [Green Version]
- Guo, B.; Sheng, Z.; Kenry; Hu, D.; Lin, X.; Xu, S.; Liu, C.; Zheng, H.; Liu, B. Biocompatible conjugated polymer nanoparticles for highly efficient photoacoustic imaging of orthotopic brain tumours in the second near-infrared window. Mater. Horiz. 2017, 4, 1151–1156. [Google Scholar] [CrossRef]
- Guo, B.; Sheng, Z.; Hu, D.; Liu, C.; Zheng, H.; Liu, B. Through Scalp and Skull NIR-II Photothermal Therapy of Deep Orthotopic Brain Tumours with Precise Photoacoustic Imaging Guidance. Adv. Mater. 2018, 30, 1802591. [Google Scholar] [CrossRef]
- Sheng, Z.; Guo, B.; Hu, D.; Xu, S.; Wu, W.; Liew, W.H.; Yao, K.; Jiang, J.; Liu, C.; Zheng, H.; et al. Bright aggregation-induced-emission dots for targeted synergetic NIR-II fluorescence and NIR-I photoacoustic imaging of orthotopic brain tumours. Adv. Mater. 2018, 30, 1800766. [Google Scholar] [CrossRef]
- Guo, B.; Feng, Z.; Hu, D.; Xu, S.; Middha, E.; Pan, Y.; Liu, C.; Zheng, H.; Qian, J.; Sheng, Z.; et al. Precise Deciphering of brain vasculatures and microscopic tumours with dual NIR-II fluorescence and photoacoustic imaging. Adv. Mater. 2019, 31, 1902504. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Yan, H.; Liu, Y.; Zhang, J.; Shan, W.; Lai, P.; Li, H.; Ren, L.; Li, Z.; et al. Aggregation-induced absorption enhancement for deep near-infrared II photoacoustic imaging of brain gliomas in vivo. Adv. Sci. 2019, 6, 1801615. [Google Scholar] [CrossRef]
- Du, L.; Qin, H.; Ma, T.; Zhang, T.; Xing, D. In vivo imaging-guided photothermal/photoacoustic synergistic therapy with bioorthogonal metabolic glycoengineering-activated tumour targeting nanoparticles. ACS Nano 2017, 11, 8930–8943. [Google Scholar] [CrossRef]
- Liu, L.; Chen, Q.; Wen, L.; Li, C.; Qin, H.; Xing, D. Photoacoustic therapy for precise eradication of glioblastoma with a tumour site blood brain barrier permeability upregulating nanoparticle. Adv. Funct. Mater. 2019, 29, 1808601. [Google Scholar] [CrossRef]
- Wang, S.C.; Sheng, Z.; Yang, Z.; Hu, D.; Long, X.; Feng, G.; Liu, Y.; Yuan, Z.; Zhang, J.; Zheng, H.; et al. Activatable small-molecule photoacoustic probes that cross the blood-brain barrier for visualization of copper(II) in mice with alzheimer’s disease. Angew. Chem. Int. Ed. 2019, 58, 12415–12419. [Google Scholar] [CrossRef]
- Rao, B.; Zhang, R.; Li, L.; Shao, J.-Y.; Wang, L.V. Photoacoustic imaging of voltage responses beyond the optical diffusion limit. Sci. Rep. 2017, 7, 2560. [Google Scholar] [CrossRef]
- Kang, J.; Zhang, H.K.; Kadam, S.D.; Fedorko, J.; Valentine, H.; Malla, A.P.; Yan, P.; Harraz, M.M.; Kang, J.U.; Rahmim, A.; et al. Transcranial recording of electrophysiological neural activity in the rodent brain in vivo using functional photoacoustic imaging of near-infrared voltage-sensitive dye. Front. Neurosci. 2019, 13, 597. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Kadam, S.D.; Elmore, J.S.; Sullivan, B.J.; Valentine, H.; Malla, A.P.; Harraz, M.M.; Rahmim, A.; Kang, J.U.; Loew, L.M.; et al. Transcranial photoacoustic imaging of NMDA-evoked focal circuit dynamics in rat hippocampus. J. Neural Eng. 2020, 17, 025001. [Google Scholar] [CrossRef]
- Li, W.T.; Chen, R.; Lv, J.; Wang, H.; Liu, Y.; Peng, Y.; Qian, Z.; Fu, G.; Nie, L. In vivo photoacoustic imaging of brain injury and rehabilitation by high-efficient near-infrared dye labeled mesen-chymal stem cells with enhanced brain barrier permeability. Adv. Sci. 2017, 5, 1700277. [Google Scholar] [CrossRef]
- Le Floc’h, J.; Lu, H.D.; Lim, T.L.; Démoré, C.; Prud’homme, R.K.; Hynynen, K.; Foster, F.S. Transcranial photoacoustic detection of blood-brain barrier disruption following focused ultrasound-mediated nanoparticle delivery. Mol. Imag. Biol. 2020, 22, 324–334. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Sun, M.; Cui, M.; Fu, Y.; Lin, Y.; Li, Z.; Nie, L. Highly specific noninvasive photoacoustic and positron emission tomography of brain plaque with functionalized croconium dye labeled by a radiotracer. Chem. Sci. 2017, 8, 2710–2716. [Google Scholar] [CrossRef] [Green Version]
- Jia, X.; Fan, K.; Zhang, R.; Zhang, D.; Zhang, J.; Gao, Y.; Zhang, T.; Li, W.; Li, J.; Yan, X.; et al. Precise visual distinction of brain glioma from normal tissues via targeted photoacoustic and fluorescence navigation. Nanomedicine 2020, 27, 102204. [Google Scholar] [CrossRef]
- Qiao, Y.; Gumin, J.; MacLellan, C.J.; Gao, F.; Bouchard, R.; Lang, F.F.; Stafford, R.J.; Melancon, M.P. Magnetic resonance and photoacoustic imaging of brain tumour mediated by mesenchymal stem cell labeled with multifunctional nanoparticle introduced via carotid artery injection. Nanotechnology 2018, 29, 165101. [Google Scholar] [CrossRef]
- Duan, Y.; Hu, D.; Guo, B.; Shi, Q.; Wu, M.; Xu, S.; Kenry; Liu, X.; Jiang, J.; Sheng, Z.; et al. Nanostructural control enables optimized photoacoustic-fluorescence-magnetic resonance multimodal imaging and photothermal therapy of brain tumour. Adv. Funct. Mater. 2020, 30, 1907077. [Google Scholar] [CrossRef]
- Raikwar, S.P.; Thangavel, R.; Ahmed, M.E.; Selvakumar, G.P.; Kempuraj, D.; Wu, K.; Khan, O.; Bazley, K.; Bussinger, B.; Kukulka, K.; et al. Real-time noninvasive bioluminescence, ultrasound and photoacoustic imaging in NFκB-RE-Luc transgenic mice reveal glia maturation factor-mediated immediate and sustained spatio-temporal activation of NFκB signaling post-traumatic brain injury in a gender-specific manner. Cell Mol. Neurobiol. 2021, 41, 1687–1706. [Google Scholar]
- Hartman, R.K.; Hallam, K.A.; Donnelly, E.M.; Emelianov, S.Y. Photoacoustic imaging of gold nanorods in the brain delivered via microbubble- assisted focused ultrasound: A tool for in vivo molecular neuroimaging. Laser Phys. Lett. 2019, 16, 025603. [Google Scholar] [CrossRef]
- Liang, B.; Liu, W.; Zhan, Q.; Li, M.; Zhuang, M.; Liu, Q.H.; Yao, J. Impacts of the murine skull on high-frequency transcranial photoacoustic brain imaging. J. Biophoton. 2019, 12, e201800466. [Google Scholar] [CrossRef]
- Anastasio, M.A.; Zhang, J.; Pan, X.; Zou, Y.; Ku, G.; Wang, L.V. Half-time image reconstruction in thermoacoustic tomography. IEEE Trans. Med. Imag. 2005, 24, 199–210. [Google Scholar] [CrossRef]
- Xi, L.; Jin, T.; Zhou, J.; Carney, P.; Jiang, H. Hybrid and electrophysiological recording of neurovascular communications in freely-moving rats. Neuroimage 2017, 161, 232–240. [Google Scholar] [CrossRef]
- Tsytsarev, V.; Rao, B.; Maslov, K.I.; Li, L.; Wang, L.V. Photoacoustic and optical coherence tomography of epilepsy with high temporal and spatial resolution and dual optical contrasts. J. Neurosci. Methods 2013, 216, 142–145. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Zhou, J.; Carney, P.; Jiang, H. A novel detachable head-mounted device for simultaneous EEG and photoacoustic monitoring of epilepsy in freely moving rats. Neurosci. Res. 2015, 91, 57–62. [Google Scholar] [CrossRef]
- Liu, Y.; Lv, X.; Liu, H.; Zhou, Z.; Huang, J.; Lei, S.; Cai, S.; Chen, Z.; Guo, Y.; Chen, Z.; et al. Porous gold nanocluster-decorated manganese monoxide nanocomposites for microenvironment-activatable MR/photoacoustic/CT tumour imaging. Nanoscale 2018, 10, 3631–3638. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Zhao, K.; Zhao, Y.; Liu, Y.; Gong, H.; Luo, Q.; Zhu, D. Skull optical clearing solution for enhancing ultrasonic and photoacoustic imaging. IEEE Trans. Med. Imag. 2016, 35, 1903–1906. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Myllylä, T. Recent Technical Progression in Photoacoustic Imaging—Towards Using Contrast Agents and Multimodal Techniques. Appl. Sci. 2021, 11, 9804. https://doi.org/10.3390/app11219804
Zhao Z, Myllylä T. Recent Technical Progression in Photoacoustic Imaging—Towards Using Contrast Agents and Multimodal Techniques. Applied Sciences. 2021; 11(21):9804. https://doi.org/10.3390/app11219804
Chicago/Turabian StyleZhao, Zuomin, and Teemu Myllylä. 2021. "Recent Technical Progression in Photoacoustic Imaging—Towards Using Contrast Agents and Multimodal Techniques" Applied Sciences 11, no. 21: 9804. https://doi.org/10.3390/app11219804





