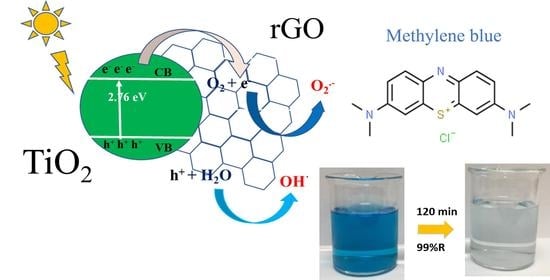
Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Graphene Oxide (GO)
2.3. Preparation of TiO2 Colloidal Solution
2.4. Preparation of TiO2@rGO Nanocomposites
2.5. Characterization of Photocatalysts
2.6. Photodegradation Experiments
3. Results and Discussion
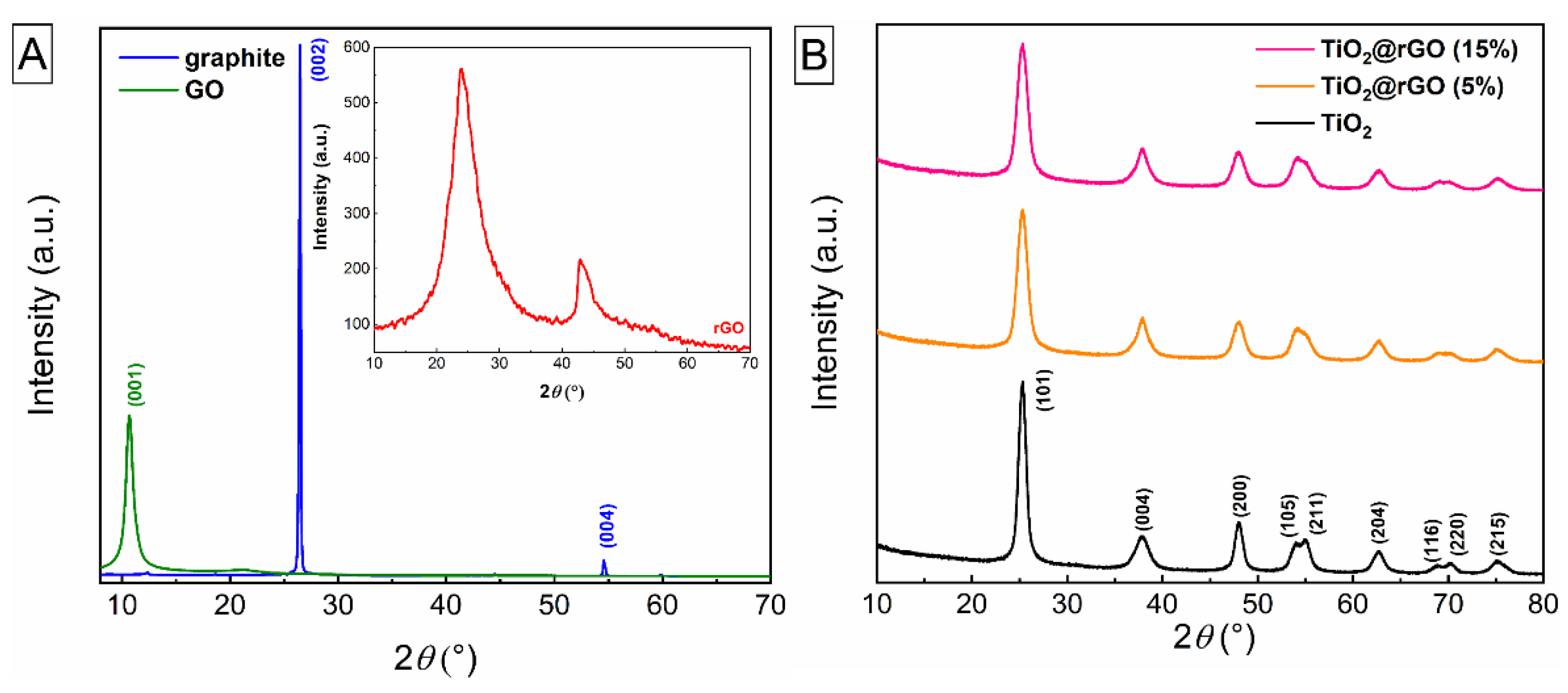
3.1. Characterization of Photocatalyst
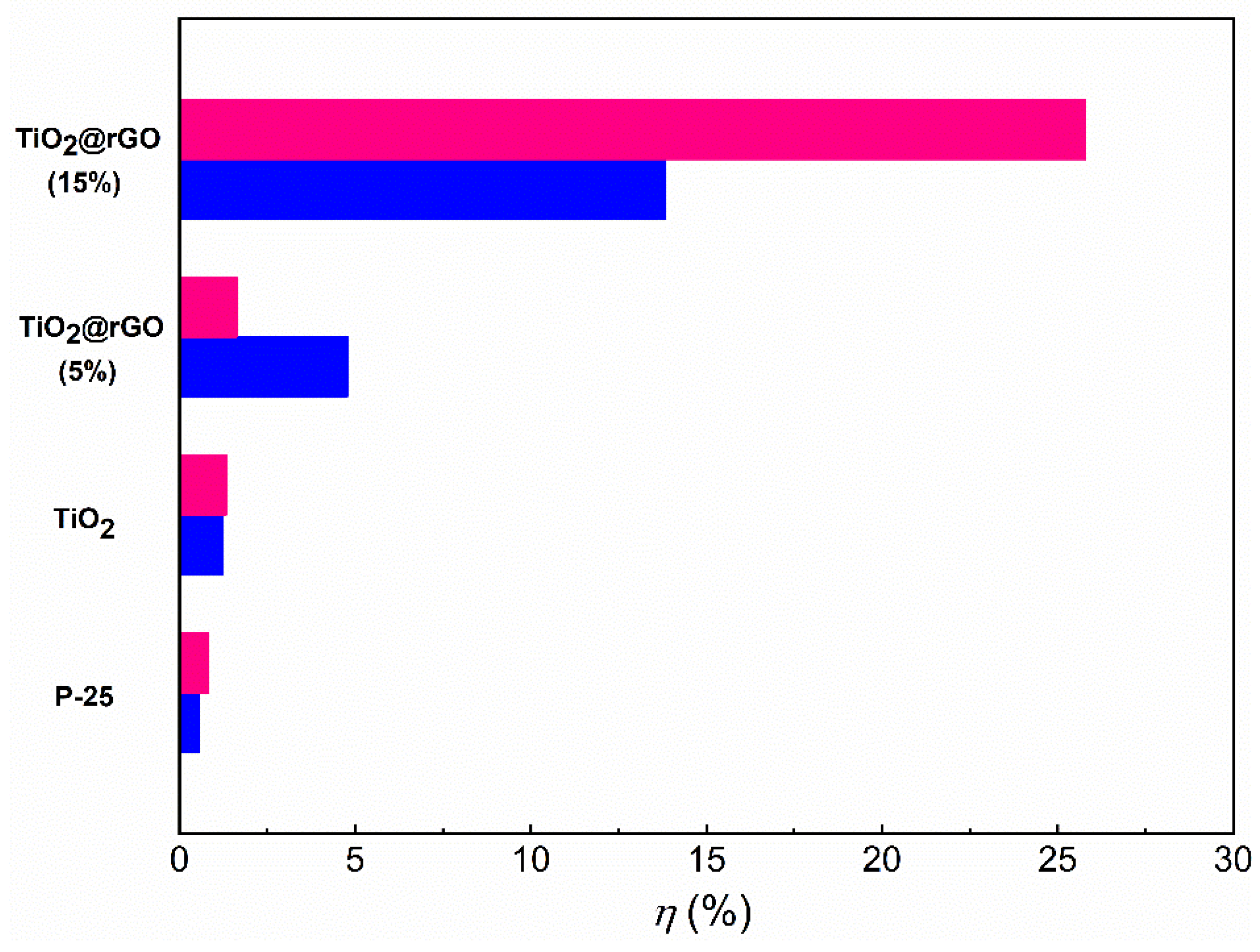
3.2. Photocatalytic Activity of TiO2@rGO Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef] [PubMed]
- Zangeneh, H.; Zinatizadeh, A.A.L.; Habibi, M.; Akia, M.; Hasnain Isa, M. Photocatalytic oxidation of organic dyes and pollutants in wastewater using different modified titanium dioxides: A comparative review. J. Ind. Eng. Chem. 2015, 26, 1–36. [Google Scholar] [CrossRef]
- Khan, S.A.; Arshad, Z.; Shahid, S.; Arshad, I.; Rizwan, K.; Sher, M.; Fatima, U. Synthesis of TiO2/Graphene oxide nanocomposites for their enhanced photocatalytic activity against methylene blue dye and ciprofloxacin. Compos. Part B Eng. 2019, 175, 107120. [Google Scholar] [CrossRef]
- Martins, P.M.; Ferreira, C.G.; Silva, A.R.; Magalhães, B.; Alves, M.M.; Pereira, L.; Marques, P.A.A.P.; Melle-Franco, M.; Lanceros-Méndez, S. TiO2/graphene and TiO2/graphene oxide nanocomposites for photocatalytic applications: A computer modeling and experimental study. Compos. Part B Eng. 2018, 145, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Akpan, U.G.; Hameed, B.H. Parameters affecting the photocatalytic degradation of dyes using TiO2-based photocatalysts: A review. J. Hazard. Mater. 2009, 170, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Luna-Sanguino, G.; Tolosana-Moranchel, A.; Duran-Valle, C.; Faraldos, M.; Bahamonde, A. Optimizing P25-rGO composites for pesticides degradation: Elucidation of photo-mechanism. Catal. Today 2019, 328, 172–177. [Google Scholar] [CrossRef]
- Sun, X.; Ji, S.; Wang, M.; Dou, J.; Yang, Z.; Qiu, H.; Kou, S.; Ji, Y.; Wang, H. Fabrication of porous TiO2-RGO hybrid aerogel for high-efficiency, visible-light photodegradation of dyes. J. Alloys Compd. 2020, 819, 153033. [Google Scholar] [CrossRef]
- Dashairya, L.; Sharma, M.; Basu, S.; Saha, P. SnS2/RGO based nanocomposite for efficient photocatalytic degradation of toxic industrial dyes under visible-light irradiation. J. Alloys Compd. 2019, 774, 625–636. [Google Scholar] [CrossRef]
- Tolosana-Moranchel, Á.; Manassero, A.; Satuf, M.L.; Alfano, O.M.; Casas, J.A.; Bahamonde, A. Influence of TiO2-rGO optical properties on the photocatalytic activity and efficiency to photodegrade an emerging pollutant. Appl. Catal. B Environ. 2019, 1–11. [Google Scholar] [CrossRef]
- Lu, F.; Astruc, D. Nanocatalysts and other nanomaterials for water remediation from organic pollutants. Coord. Chem. Rev. 2020, 408, 213180. [Google Scholar] [CrossRef]
- Kiwaan, H.A.; Atwee, T.M.; Azab, E.A.; El-Bindary, A.A. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide. J. Mol. Struct. 2020, 1200, 127115. [Google Scholar] [CrossRef]
- Du, X.; Wu, Y.; Kou, Y.; Mu, J.; Yang, Z.; Hu, X.; Teng, F. Amorphous carbon inhibited TiO2 phase transition in aqueous solution and its application in photocatalytic degradation of organic dye. J. Alloys Compd. 2019, 810, 151917. [Google Scholar] [CrossRef]
- Mehmood, Z.; Aamir, M.; Sher, M.; Sohail, M. A facile approach to synthesis graphene oxide/bismuth oxide nanocomposites and their superior sunlight driven photocatalytic activity. Optik 2019, 197, 163035. [Google Scholar] [CrossRef]
- Lin, X.; Sun, M.; Sun, S.; Zhang, Z. Graphene promoted triphasic N/Ti3+-TiO2 heterostructures: In-situ hydrothermal synthesis and enhanced photocatalytic performance. J. Alloys Compd. 2019, 785, 732–741. [Google Scholar] [CrossRef]
- Pedrosa, M.; Sampaio, M.J.; Horvat, T.; Nunes, O.C.; Dražić, G.; Rodrigues, A.E.; Figueiredo, J.L.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Visible-light-induced self-cleaning functional fabrics using graphene oxide/carbon nitride materials. Appl. Surf. Sci. 2019, 497, 101145. [Google Scholar] [CrossRef]
- Jung, S.C.; Bang, H.J.; Lee, H.; Kim, H.; Ha, H.H.; Yu, Y.H.; Park, Y.K. Degradation behaviors of naproxen by a hybrid TiO2 photocatalyst system with process components. Sci. Total Environ. 2019, 135216. [Google Scholar] [CrossRef]
- Chanu, A.L.; Singh, W.J.; Singh, K.J.; Devi, K.N. Effect of operational parameters on the photocatalytic degradation of Methylene blue dye solution using manganese doped ZnO nanoparticles. Results Phys. 2019, 12, 1230–1237. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Y.; Shi, X.; Li, K. Fast photocatalytic degradation of methylene blue dye using a low-power diode laser. J. Hazard. Mater. 2015, 283, 267–275. [Google Scholar] [CrossRef]
- Andreozzi, M.; Álvarez, M.G.; Contreras, S.; Medina, F.; Clarizia, L.; Vitiello, G.; Llorca, J.; Marotta, R. Treatment of saline produced water through photocatalysis using rGO-TiO2 nanocomposites. Catal. Today 2018, 315, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Li, B.R.; Wu, L.G.; Yin, Y.B.; Jiang, B.Q.; Lou, J.Q. Enhanced performance of TiO2/reduced graphene oxide doped by rare-earth ions for degrading phenol in seawater excited by weak visible light. Adv. Powder Technol. 2019, 30, 1920–1931. [Google Scholar] [CrossRef]
- Qian, R.; Zong, H.; Schneider, J.; Zhou, G.; Zhao, T.; Li, Y.; Yang, J.; Bahnemann, D.W.; Pan, J.H. Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: An overview. Catal. Today 2019, 335, 78–90. [Google Scholar] [CrossRef]
- Das, S.; Mahalingam, H. Dye degradation studies using immobilized pristine and waste polystyrene-TiO2/rGO/g-C3N4 nanocomposite photocatalytic film in a novel airlift reactor under solar light. J. Environ. Chem. Eng. 2019, 7, 103289. [Google Scholar] [CrossRef]
- Mouhat, F.; Coudert, F.X.; Bocquet, M.L. Structure and chemistry of graphene oxide in liquid water from first principles. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Keshavarz, M.; Kassanos, P.; Tan, B.; Venkatakrishnan, K. Metal-oxide surface-enhanced Raman biosensor template towards point-of-care EGFR detection and cancer diagnostics. Nanoscale Horizons 2020, 5, 294–307. [Google Scholar] [CrossRef]
- Hooshmand, S.; Kargozar, S.; Ghorbani, A.; Darroudi, M.; Keshavarz, M.; Baino, F.; Kim, H.W. Biomedical waste management by using nanophotocatalysts: The need for new options. Materials 2020, 13, 3511. [Google Scholar] [CrossRef] [PubMed]
- Zouzelka, R.; Remzova, M.; Plsek, J.; Brabec, L.; Rathousky, J. Immobilized rGO/TiO2 photocatalyst for decontamination of water. Catalysts 2019, 9, 708. [Google Scholar] [CrossRef] [Green Version]
- Ahmadivand, A.; Gerislioglu, B.; Ramezani, Z. Gated graphene island-enabled tunable charge transfer plasmon terahertz metamodulator. Nanoscale 2019, 11, 8091–8095. [Google Scholar] [CrossRef]
- Ahmadivand, A.; Gerislioglu, B.; Noe, G.T.; Mishra, Y.K. Gated graphene enabled tunable charge-current configurations in hybrid plasmonic metamaterials. ACS Appl. Electron. Mater. 2019, 1, 637–641. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Čizmić, M.; Ljubas, D.; Ćurković, L.; Škorić, I.; Babić, S. Kinetics and degradation pathways of photolytic and photocatalytic oxidation of the anthelmintic drug praziquantel. J. Hazard. Mater. 2017, 323, 500–512. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Photocatalytic degradation of organic pollutant using TiO2/rGO nanocomposites under simulated sunlight. Nanomater. Sci. Eng. 2020, 2, 162–169. [Google Scholar]
- Atout, H.; Álvarez, M.G.; Chebli, D.; Bouguettoucha, A.; Tichit, D.; Llorca, J.; Medina, F. Enhanced photocatalytic degradation of methylene blue: Preparation of TiO2/reduced graphene oxide nanocomposites by direct sol-gel and hydrothermal methods. Mater. Res. Bull. 2017, 95, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Xu, L.; Wang, H.; Wang, W.; Zhang, L. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue. Mater. Des. 2016, 108, 632–639. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, S.; Ma, F.; Xu, Y. Synergistic effects and kinetics of rGO-modified TiO2 nanocomposite on adsorption and photocatalytic degradation of humic acid. J. Environ. Manag. 2019, 235, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Emiru, T.F.; Ayele, D.W. Controlled synthesis, characterization and reduction of graphene oxide: A convenient method for large scale production. Egypt. J. Basic Appl. Sci. 2017, 4, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Ossonon, B.D.; Bélanger, D. Synthesis and characterization of sulfophenyl-functionalized reduced graphene oxide sheets. RSC Adv. 2017, 7, 27224–27234. [Google Scholar] [CrossRef] [Green Version]
- Pan, N.; Guan, D.; He, T.; Wang, R.; Wyman, I.; Jin, Y.; Xia, C. Removal of Th4+ ions from aqueous solutions by graphene oxide. J. Radioanal. Nucl. Chem. 2013, 298, 1999–2008. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Tin oxide/graphene composite fabricated via a hydrothermal method for gas sensors working at room temperature. Sens. Actuators B Chem. 2012, 173, 139–147. [Google Scholar] [CrossRef]
- Harikrishnan, M.M.; Athira, S.; Sykam, N.; Mohan Rao, G.; Mathew, A. Preparation of rGO-TiO2 composite and study of its dye adsorption properties. Mater. Today Proc. 2019, 9, 61–69. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, M.; Rezaee Roknabadi, M.; Behdani, M.; Kompany, A. Enhancement of visible and UV light photocatalytic activity of rGO-TiO2 nanocomposites: The effect of TiO2/Graphene oxide weight ratio. Ceram. Int. 2019, 45, 12625–12634. [Google Scholar] [CrossRef]
- Wanag, A.; Rokicka, P.; Kusiak-Nejman, E.; Kapica-Kozar, J.; Wrobel, R.J.; Markowska-Szczupak, A.; Morawski, A.W. Antibacterial properties of TiO2 modified with reduced graphene oxide. Ecotoxicol. Environ. Saf. 2018, 147, 788–793. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wang, J.; Wang, X.; Yu, H.; Yu, J.; Lei, M.; Wang, Y. One-step synthesis of easy-recycling TiO2-rGO nanocomposite photocatalysts with enhanced photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 452–459. [Google Scholar] [CrossRef]
- Chi, N.; Mai, T.; Thi, T.; Van, T.; Juang, R. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysis over TiO2/ZnO/rGO composites. Sep. Purif. Tech. 2020, 232. [Google Scholar] [CrossRef]
- Pawlyta, M.; Rouzaud, J.N.; Duber, S. Raman microspectroscopy characterization of carbon blacks: Spectral analysis and structural information. Carbon 2015, 84, 479–490. [Google Scholar] [CrossRef]
- Sohail, M.; Xue, H.; Jiao, Q.; Li, H.; Khan, K.; Wang, S.; Zhao, Y. Synthesis of well-dispersed TiO2@ reduced graphene oxide (rGO) nanocomposites and their photocatalytic properties. Mater. Res. Bull. 2017, 90, 125–130. [Google Scholar] [CrossRef]
- Vasilaki, E.; Georgaki, I.; Vernardou, D.; Vamvakaki, M.; Katsarakis, N. Ag-loaded TiO2/reduced graphene oxide nanocomposites for enhanced visible-light photocatalytic activity. Appl. Surf. Sci. 2015, 353, 865–872. [Google Scholar] [CrossRef]
- Hortigüela, M.J.; Machado, D.; Bdikin, I.; Neto, V.; Otero-irurueta, G. Chemical changes of graphene oxide thin films induced by thermal treatment under vacuum conditions. Coatings 2020, 10, 113. [Google Scholar] [CrossRef] [Green Version]
- Tobaldi, D.M.; Seabra, M.P.; Otero-Irurueta, G.; De Miguel, Y.R.; Ball, R.J.; Singh, M.K.; Pullar, R.C.; Labrincha, J.A. Quantitative XRD characterisation and gas-phase photocatalytic activity testing for visible-light (indoor applications) of KRONOClean 7000®. RSC Adv. 2015, 5, 102911–102918. [Google Scholar] [CrossRef] [Green Version]
- Tobaldi, D.M.; Pullar, R.C.; Gualtieri, A.F.; Otero-Irurueta, G.; Singh, M.K.; Seabra, M.P.; Labrincha, J.A. Nitrogen-modified nano-titania: True phase composition, microstructure and visible-light induced photocatalytic NOx abatement. J. Solid State Chem. 2015, 231, 87–100. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Wanag, A.; Kapica-Kozar, J.; Kowalczyk, Ł.; Zgrzebnicki, M.; Tryba, B.; Przepiórski, J.; Morawski, A.W. Methylene blue decomposition on TiO2/reduced graphene oxide hybrid photocatalysts obtained by a two-step hydrothermal and calcination synthesis. Catal. Today 2019. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, X.; Ye, T.; Zheng, G.P.; Zheng, X.C.; Liu, P.; Guan, X.X. Novel assembly of homogeneous reduced graphene oxide-doped mesoporous TiO2 hybrids for elimination of Rhodamine-B dye under visible light irradiation. J. Alloys Compd. 2017, 698, 819–827. [Google Scholar] [CrossRef]







| Samples ID | D-Band, cm−1 | ID | G-Band, cm−1 | IG | ID/IG | La, nm |
|---|---|---|---|---|---|---|
| GO | 1329 | 42 | 1585 | 95 | 0.44 | 87.5 |
| rGO | 1347 | 77 | 1593 | 99 | 0.78 | 49.4 |
| TiO2-rGO (5%) | 1345 | 302 | 1605 | 381 | 0.79 | 48.7 |
| TiO2-rGO (15%) | 1341 | 138 | 1601 | 154 | 0.90 | 42.8 |
| Sample ID | MB Dye | RhB Dye | ||||
|---|---|---|---|---|---|---|
| R2 | k × 10−3, Min−1 | t1/2, Min | R2 | k × 10−3, Min−1 | t1/2, Min | |
| P-25 | 0.9919 | 47.45 | 14.61 | 0.9813 | 52.76 | 13.14 |
| TiO2 | 0.9963 | 10.99 | 63.07 | 0.9958 | 10.19 | 68.02 |
| TiO2@rGO (5 wt.%) | 0.9821 | 26.40 | 26.26 | 0.9688 | 36.15 | 19.17 |
| TiO2@rGO (15 wt.%) | 0.9936 | 32.84 | 21.11 | 0.9951 | 53.05 | 13.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Appl. Sci. 2021, 11, 3966. https://doi.org/10.3390/app11093966
Kocijan M, Ćurković L, Ljubas D, Mužina K, Bačić I, Radošević T, Podlogar M, Bdikin I, Otero-Irurueta G, Hortigüela MJ, et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Applied Sciences. 2021; 11(9):3966. https://doi.org/10.3390/app11093966
Chicago/Turabian StyleKocijan, Martina, Lidija Ćurković, Davor Ljubas, Katarina Mužina, Ivana Bačić, Tina Radošević, Matejka Podlogar, Igor Bdikin, Gonzalo Otero-Irurueta, María J. Hortigüela, and et al. 2021. "Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation" Applied Sciences 11, no. 9: 3966. https://doi.org/10.3390/app11093966