Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. OLE Extraction
2.3. OLE Characterization
2.4. Scaffold Fabrication
2.5. Phenol Release
2.6. Epidermal Cell Culture and Metabolic Assay
2.7. Evaluation of Immunomodulatory Properties
2.8. Statistical Analysis
3. Results
3.1. OLE Characterization
3.2. Morphological Characterization of PHA Fiber Meshes
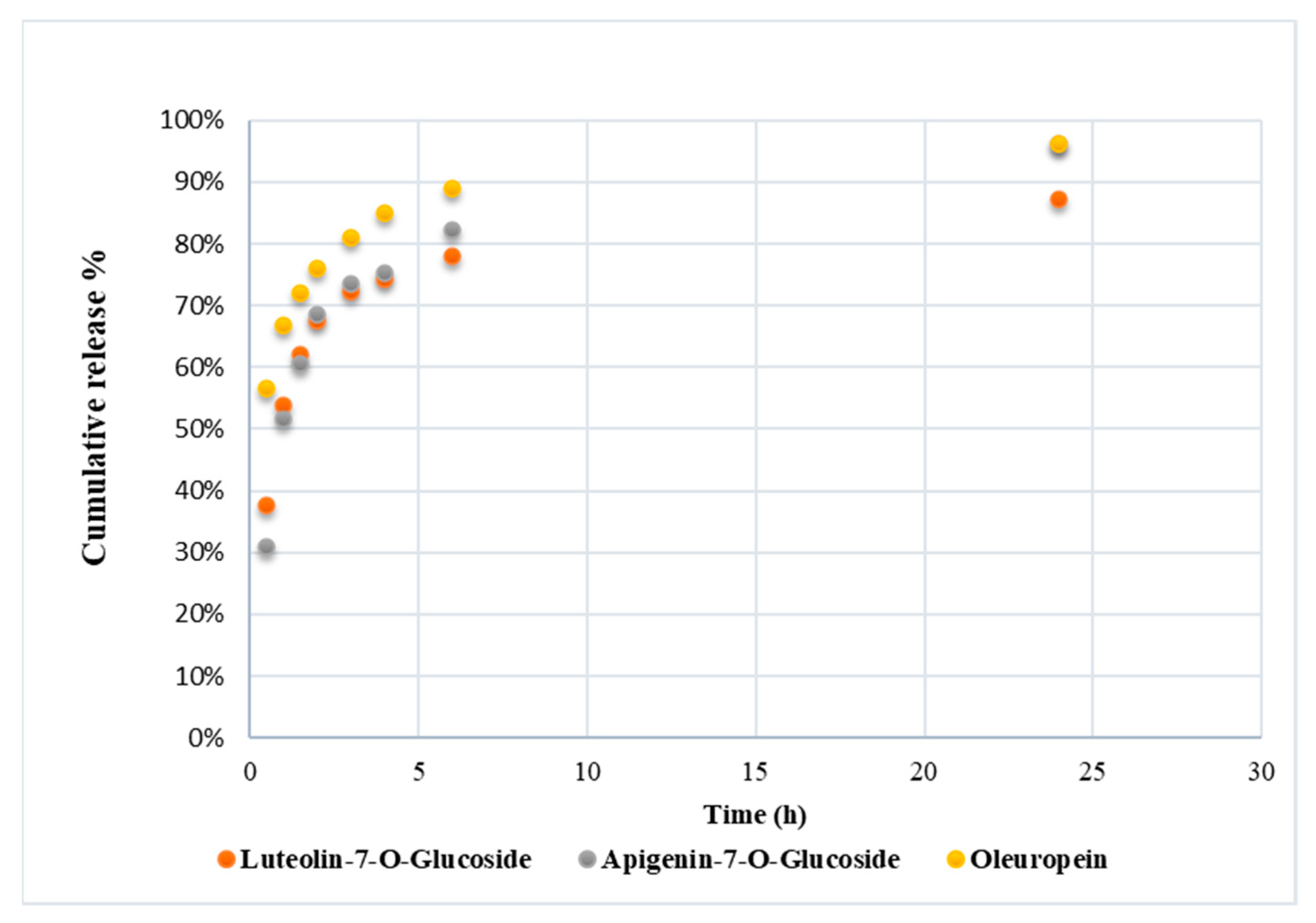
3.3. Polyphenol Release from PHA Fibers
3.4. Cell Metabolic Activity
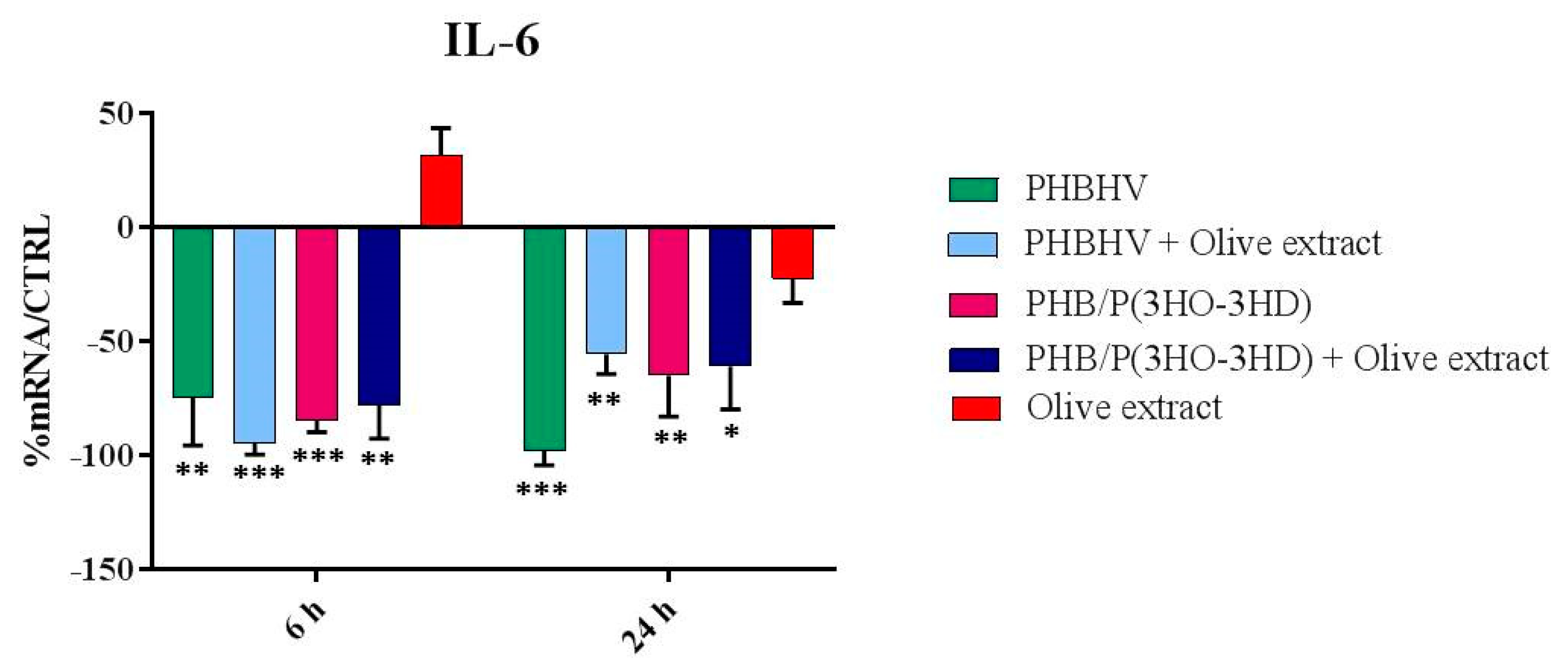
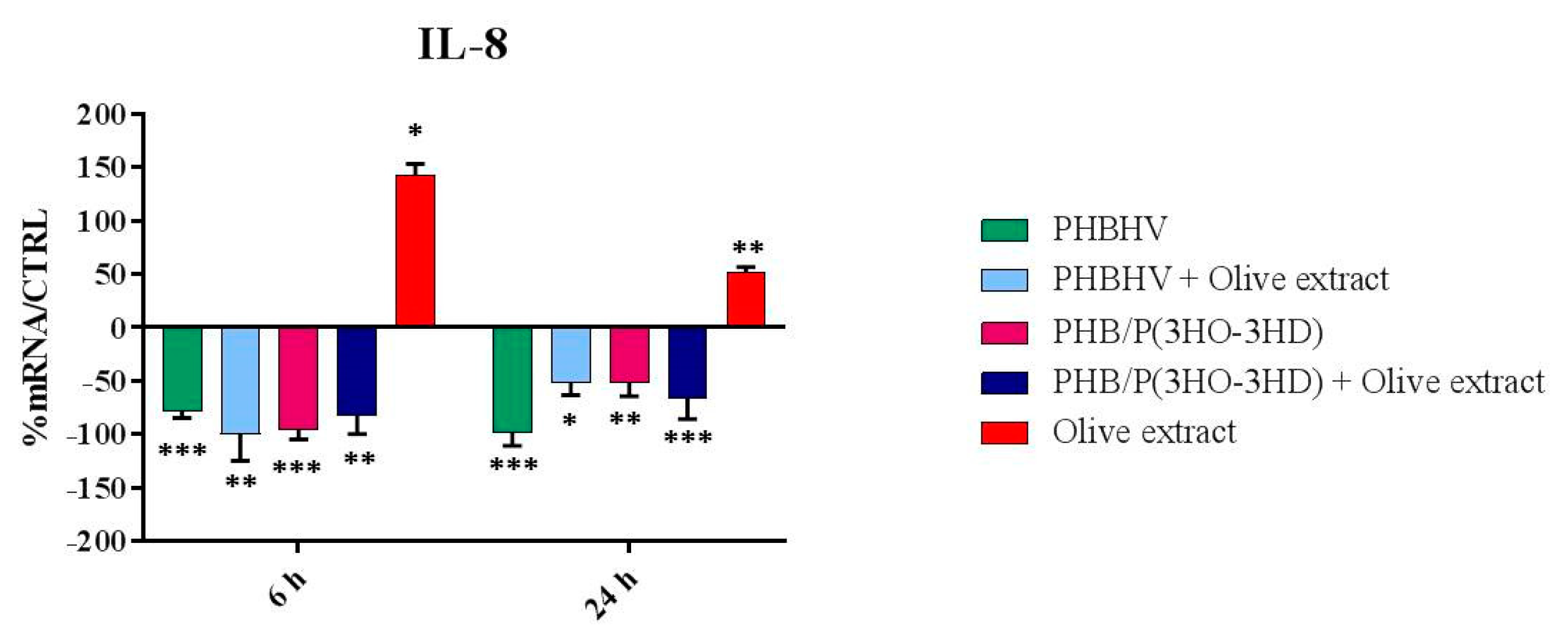
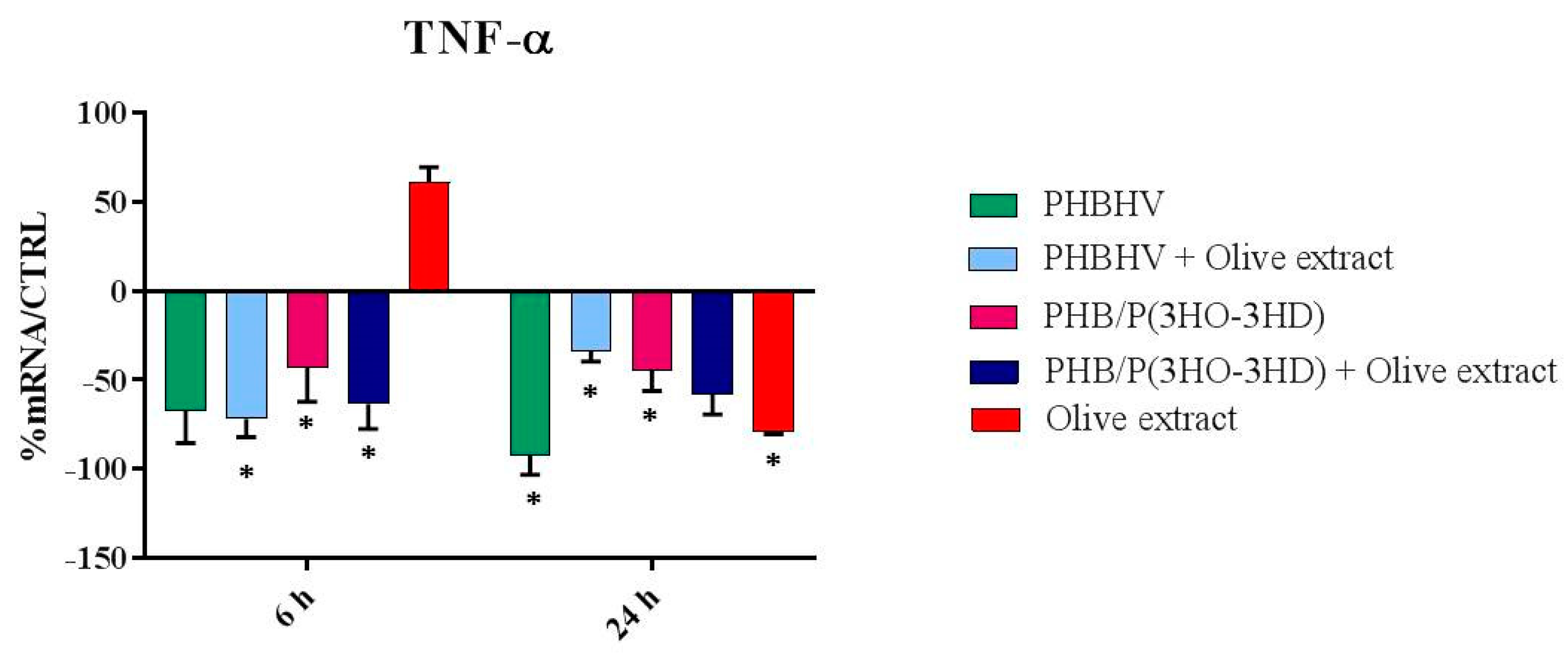
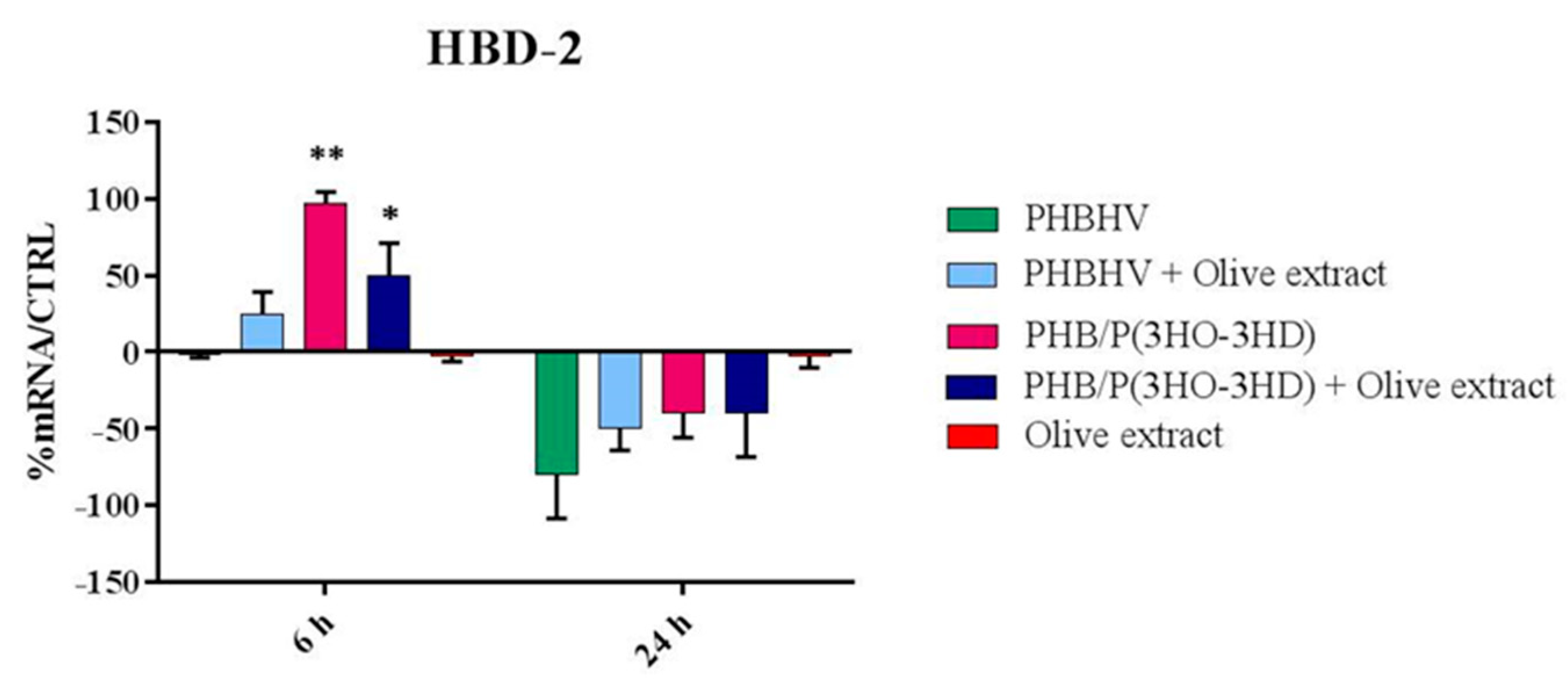
3.5. Cytokine Expression
3.6 Indirect Antimicrobial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef]
- Romero, M.; Toral, M.; Gómez-Guzmán, M.; Jiménez, R.; Galindo, P.; Sánchez, M.; Olivares, M.; Gálvez, J.; Duarte, J. Antihypertensive effects of oleuropein-enriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016, 7, 584–593. [Google Scholar] [CrossRef]
- Susalit, E.; Agus, N.; Effendi, I.; Tjandrawinata, R.R.; Nofiarny, D.; Perrinjaquet-Moccetti, T.; Verbruggen, M. Olive (Olea europaea) leaf extract effective in patients with stage-1 hypertension: Comparison with Captopril. Phytomedicine 2011, 18, 251–258. [Google Scholar] [CrossRef]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [Green Version]
- Koca, U.; Süntar, I.; Akkol, E.K.; Yilmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 Cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- El, S.N.; Karakaya, S. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009, 67, 632–638. [Google Scholar] [CrossRef]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste autochthonous tuscan olive leaves (Olea europaea var. Olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Algieri, F.; Rodríguez-Nogales, A.; Garrido-Mesa, J.; Utrilla, M.P.; Talhaoui, N.; Gómez-Caravaca, A.M.; Segura-Carretero, A.; Rodríguez-Cabezas, M.E.; Monteleone, G.; et al. Immunomodulatory properties of Olea europaea leaf extract in intestinal inflammation. Mol. Nutr. Food Res. 2017, 61, 1601066. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of flavonoids and other polyphenols on inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [CrossRef]
- Wang, K.; Ping, S.; Huang, S.; Hu, L.; Xuan, H.; Zhang, C.; Hu, F. Molecular Mechanisms Underlying the In Vitro Anti-Inflammatory Effects of a Flavonoid-rich Ethanol Extract from Chinese Propolis (Poplar Type). Evid. Based Complement. Altern. Med. 2013, 2013, 11. [Google Scholar] [CrossRef]
- Magrone, T.; Spagnoletta, A.; Salvatore, R.; Magrone, M.; Dentamaro, F.; Russo, M.A.; Difonzo, G.; Summo, C.; Caponio, F.; Jirillo, E. Olive leaf extracts act as modulators of the human immune response. Endocrine. Metab. Immune Disord. Drug Targets 2017, 18, 85–93. [Google Scholar] [CrossRef]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef]
- Azimi, B.; Maleki, H.; Zavagna, L.; De la Ossa, J.G.; Linari, S.; Lazzeri, A.; Danti, S. Bio-based electrospun fibers for wound healing. J. Funct. Biomater. 2020, 11, 67. [Google Scholar] [CrossRef]
- Larsen, S.B.; Cowley, C.J.; Fuchs, E. Epithelial cells: Liaisons of immunity. Curr. Opin. Immunol. 2020, 62, 45–53. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeterior. Biodegrad. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Nigmatullin, R.; Thomas, P.; Lukasiewicz, B.; Puthussery, H.; Roy, I. Polyhydroxyalkanoates, a family of natural polymers, and their applications in drug delivery. J. Chem. Technol. Biotechnol. 2015, 90, 1209–1221. [Google Scholar] [CrossRef]
- Francis, L.; Meng, D.; Knowles, J.; Keshavarz, T.; Boccaccini, A.R.; Roy, I. Controlled Delivery of Gentamicin Using Poly(3-hydroxybutyrate) Microspheres. Int. J. Mol. Sci. 2011, 12, 4294–4314. [Google Scholar] [CrossRef]
- Azimi, B.; Milazzo, M.; Lazzeri, A.; Berrettini, S.; Uddin, M.J.; Qin, Z.; Buehler, M.J.; Danti, S. Electrospinning Piezoelectric Fibers for Biocompatible Devices. Adv. Healthc. Mater. 2020, 9, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Salomone, R.; Ioppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Bioref. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Alessandri, S.; Ieri, F.; Romani, A. Minor polar compounds in extra virgin olive oil: Correlation between HPLC-DAD-MS and the Folin-Ciocalteu spectrophotometric method. J. Agric. Food Chem. 2014, 62, 826–835. [Google Scholar] [CrossRef]
- Doğan, G.; Başal, G.; Bayraktar, O.; Ozyildiz, F.; Uzel, A.; Erdoğan, I. Bioactive Sheath/Core nanofibers containing olive leaf extract. Microsc. Res. Tech. 2016, 79, 38–49. [Google Scholar] [CrossRef] [Green Version]
- Chuysinuan, P.; Thanyacharoen, T.; Techasakul, S.; Ummartyotin, S. Electrospun characteristics of gallic acid-loaded poly vinyl alcohol fibers: Release characteristics and antioxidant properties. J. Sci. Adv. Mater. Devices. 2018, 3, 175–180. [Google Scholar] [CrossRef]
- Frykberg, R.G.; Banks, J. Challenges in the Treatment of Chronic Wounds. Adv. Wound Care 2015, 4, 560–582. [Google Scholar] [CrossRef] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. Biomedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Danti, S.; D’Alessandro, D.; Mota, C.; Bruschini, L.; Berrettini, S. Applications of Bioresorbable Polymers in Skin and Eardrum. In Bioresorbable Polymers for Biomedical Applications: From Fundamentals to Translational Medicine; Elsevier: Amsterdam, The Netherlands, 2017; pp. 423–444. ISBN 9780081002667. [Google Scholar]
- Azimi, B.; Thomas, L.; Fusco, A.; Kalaoglu-Altan, O.I.; Basnett, P.; Cinelli, P.; De Clerck, K.; Roy, I.; Donnarumma, G.; Coltelli, M.-B.; et al. Electrosprayed chitin nanofibril/electrospun polyhydroxyalkanoate fiber mesh as functional nonwoven for skin application. J. Funct. Biomater. 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R.; Taylor, C.S.; Claeyssens, F.; Haycock, J.W.; Knowles, J.C.; Roy, I. Unidirectional neuronal cell growth and differentiation on aligned polyhydroxyalkanoate blend microfibres with varying diameters. J. Tissue Eng. Regen. Med. 2019, 13, 1581–1594. [Google Scholar] [CrossRef]
- Ching, K.Y.; Andriotis, O.G.; Li, S.; Basnett, P.; Su, B.; Roy, I.; Tare, R.S.; Sengers, B.G.; Stolz, M. Nanofibrous poly(3-hydroxybutyrate)/poly(3-hydroxyoctanoate) scaffolds provide a functional microenvironment for cartilage repair. J. Biomater. Appl. 2016, 31, 77–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fong, H.; Chun, I.; Reneker, D.H. Beaded Nanofibers Formed during Electrospinning. Polymer 1999, 40, 4585–4592. [Google Scholar] [CrossRef]
- Liu, Y.-H.; He, J.-H.; Yu, J.Y.; Zeng, H.-M. Controlling numbers and sizes of beads in electrospun nanofibers. Polym. Int. 2008, 57, 632–636. [Google Scholar] [CrossRef]
- Gaharwar, A.K.; Mihaila, S.M.; Kulkarni, A.A.; Patel, A.; Di Luca, A.; Reis, R.L.; Gomes, M.E.; van Blitterswijk, C.; Moroni, L.; Khademhosseini, A. Amphiphilic beads as depots for sustained drug release integrated into fibrillar scaffolds. J. Control. Release 2014, 187, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef] [Green Version]
- Estruch, R. Effects of a Mediterranean-style diet on cardiovascular risk factors: A randomized trial. Ann. Intern. Med. 2006, 145, 1–11. [Google Scholar] [CrossRef]
- Miles, E.A.; Zoubouli, P.; Calder, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef]
- De Cicco, P.; Maisto, M.; Tenore, G.C.; Ianaro, A. Olive Leaf Extract, from Olea europaea L., Reduces Palmitate-Induced Inflammation via Regulation of Murine Macrophages Polarization. Nutrients 2020, 12, 3663. [Google Scholar] [CrossRef]
- Gordon, S.; Martinez, F.O. Alternative activation of macrophages: Mechanism and functions. Immunity 2010, 32, 593–604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 113. [Google Scholar] [CrossRef] [Green Version]
- De La Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined antimicrobial effect of bio-waste olive leaf extract and remote cold atmospheric plasma effluent. Molecules 2021, 26, 1890. [Google Scholar] [CrossRef]
- Chew, S.A.; Danti, S. Biomaterial-based implantable devices for cancer therapy. Adv. Healthc. Mater. 2017, 6, 1600766. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Trombi, L.; Soriga, I.; Piredda, F.; Milazzo, M.; Stefanini, C.; Bruschini, L.; Perale, G.; Pertici, G.; Danti, S. Investigating the microenvironmental effects of scaffold chemistry and topology in human mesenchymal stromal cell/polymeric hollow microfiber constructs. Biomed. Sci. Eng. 2016, 1, 1. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]
- Esposito, E.; Cuzzocrea, S. TNF-alpha as a therapeutic target in inflammatory diseases, ischemia-reperfusion injury and trauma. Curr. Med. Chem. 2009, 16, 3152–3167. [Google Scholar] [CrossRef]
- Coondoo, A. The role of cytokines in the pathomechanism of cutaneous disorders. Indian J. Dermatol. 2012, 57, 90–96. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Masuda, K.; Kishimoto, T. Regulation of IL-6 in Immunity and Diseases. In Regulation of Cytokine Gene Expression in Immunity and Diseases; Ma, X., Ed.; Advances in Experimental Medicine and Biology; Springer: Dordrecht, The Netherlands, 2016; Volume 941. [Google Scholar]
- Harada, A.; Sekido, N.; Akahoshi, T. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J. Leukoc. Biol. 1994, 56, 559–564. [Google Scholar] [CrossRef]
- Maaz Arif, M.; Khan, S.M.; Gull, N.; Tabish, T.A.; Zia, S.; Ullah Khan, R.; Awais, S.M.; Arif Butt, M. Polymer-based biomaterials for chronic wound management: Promises and challenges. Int. J. Pharm. 2021, 598, 120270. [Google Scholar] [CrossRef] [PubMed]
- Wolcott, R.D.; Rhoads, D.D.; Dowd, S.E. Biofilms and chronic wound inflammation. J. Wound Care 2008, 17, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Saino, E.; Focarete, M.L.; Gualandi, C.; Emanuele, E.; Cornaglia, A.I.; Imbriani, M.; Visai, L. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules 2011, 12, 1900–1911. [Google Scholar] [CrossRef] [PubMed]
- Wanke, I.; Steffen, H.; Christ, C.; Krismer, B.; Götz, F.; Peschel, A.; Peschel, A.; Schaller, M.; Schittek, B. Skin commensals amplify the innate immune response to pathogens by activation of distinct signaling pathways. J. Investig. Dermatol. 2011, 131, 382–390. [Google Scholar] [CrossRef] [Green Version]
- Donnarumma, G.; Paoletti, I.; Fusco, A.; Perfetto, B.; Buommino, E.; de Gregorio, V.; Baroni, A. β-Defensins: Work in Progress. Adv. Exp. Med. Bio.I 2016, 901, 59–76. [Google Scholar] [CrossRef]
- Coltelli, M.-B.; Aliotta, L.; Vannozzi, A.; Morganti, P.; Panariello, L.; Danti, S.; Neri, S.; Fernandez-Avila, C.; Fusco, A.; Donnarumma, G.; et al. Properties and skin compatibility of films based on poly(lactic acid) (PLA) bionanocomposites incorporating chitin nanofibrils (CN). J. Funct. Biomater. 2020, 11, 21. [Google Scholar] [CrossRef] [Green Version]
- Danti, S.; Azimi, B.; Candito, M.; Fusco, A.; Sorayani Bafqi, M.S.; Ricci, C.; Milazzo, M.; Cristallini, C.; Latifi, M.; Donnarumma, G.; et al. Lithium niobate nanoparticles as biofunctional interface material for inner ear devices. Biointerphases 2020, 15, 031004. [Google Scholar] [CrossRef]
- Danti, S.; Trombi, L.; Fusco, A.; Azimi, B.; Lazzeri, A.; Morganti, P.; Coltelli, M.-B.; Donnarumma, G. Chitin nanofibrils and nanolignin as functional agents in skin regeneration. Int. J. Mol. Sci. 2019, 20, 2669. [Google Scholar] [CrossRef] [Green Version]
- Gigante, V.; Coltelli, M.-B.; Vannozzi, A.; Panariello, L.; Fusco, A.; Trombi, L.; Donnarumma, G.; Danti, S.; Lazzeri, A. Flat die extruded biocompatible poly(lactic acid) (PLA)/poly(butylene succinate) (PBS) based films. Polymers 2019, 11, 1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teno, J.; Pardo-Rodriguez, M.; Hummel, N.; Bonin, V.; Fusco, A.; Ricci, C.; Donnarumma, G.; Coltelli, M.-B.; Danti, S.; Lagaron, J.M. Preliminary studies on an innovative bioactive skin soluble beauty mask made by combining electrospinning and dry powder impregnation. Cosmetics 2020, 7, 96. [Google Scholar] [CrossRef]



| Gene | Primer Sequence | Conditions | Base Pairs |
|---|---|---|---|
| IL-1α | 5′-CATGTCAAATTTCACTGCTTCATCC-3′ 5′-GTCTCTGAATCAGAAATCCTTCTATC-3′ | 5 s at 95 °C, 8 s at 55 °C, 1 s at 72 °C for 45 cycles | 421 |
| IL-1β | 5′-GCATCCAGCTACGAATCTCC-3′ 5′-CCACATTCAGCACAGGACTC-3′ | 5 s at 95 °C, 14 s at 58 °C, 28 s at 72°C for 40 cycles | 708 |
| TNF-α | 5′-CAGAGGGAAGAGTTCCCCAG-3′ 5’-CCTTGGTCTGGTAGGAGACG-3′ | 5 s at 95 °C, 6 s at 57 °C, 13 s at 72 °C for 40 cycles | 324 |
| IL-6 | 5′-ATGAACTCCTTCTCCACAAGCGC-3′ 5′-GAAGAGCCCTCAGGCTGGACTG-3′ | 5 s at 95 °C, 13 s at 56 °C, 25 s at 72 °C for 40 cycles | 628 |
| IL-8 | 5′-ATGACTTCCAAGCTGGCCGTG-3′ 5′-TGAATTCTCAGCCCTCTTCAAAAACTTCTC-3′ | 5 s at 94 °C, 6 s at 55 °C, 12 s at 72 °C for 40 cycle | 297 |
| TGF-β | 5′-CCGACTACTACGCCAAGGAGGTCAC-3′ 5′-AGGCCGGTTCATGCCATGAATGGTG-3′ | 5 s at 94 °C, 9 s at 60 °C, 18 s at 72 °C for 40 cycles | 439 |
| hBD-2 | 5′-GGATCCATGGGTATAGGCGATCCTGTTA-3′ 5′-AAGCTTCTCTGATGAGGGAGCCCTTTCT-3′ | 5 s at 94 °C, 6 s at 63 °C, 10 s at 72 °C for 50 cycles | 198 |
| Sample | PHBHV Fibers | OLE/PHBHV Fibers | PHB/PHOHD Fibers | OLE/(PHB/PHOHD) Fibers | Plain OLE |
|---|---|---|---|---|---|
| %ABred | 74% | 77% | 76% | 75% | 94% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Ossa, J.G.; Fusco, A.; Azimi, B.; Esposito Salsano, J.; Digiacomo, M.; Coltelli, M.-B.; De Clerck, K.; Roy, I.; Macchia, M.; Lazzeri, A.; et al. Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract. Appl. Sci. 2021, 11, 4006. https://doi.org/10.3390/app11094006
De la Ossa JG, Fusco A, Azimi B, Esposito Salsano J, Digiacomo M, Coltelli M-B, De Clerck K, Roy I, Macchia M, Lazzeri A, et al. Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract. Applied Sciences. 2021; 11(9):4006. https://doi.org/10.3390/app11094006
Chicago/Turabian StyleDe la Ossa, Jose Gustavo, Alessandra Fusco, Bahareh Azimi, Jasmine Esposito Salsano, Maria Digiacomo, Maria-Beatrice Coltelli, Karen De Clerck, Ipsita Roy, Marco Macchia, Andrea Lazzeri, and et al. 2021. "Immunomodulatory Activity of Electrospun Polyhydroxyalkanoate Fiber Scaffolds Incorporating Olive Leaf Extract" Applied Sciences 11, no. 9: 4006. https://doi.org/10.3390/app11094006








