Performance of Artificial Intelligence (AI) Models Designed for Application in Pediatric Dentistry—A Systematic Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
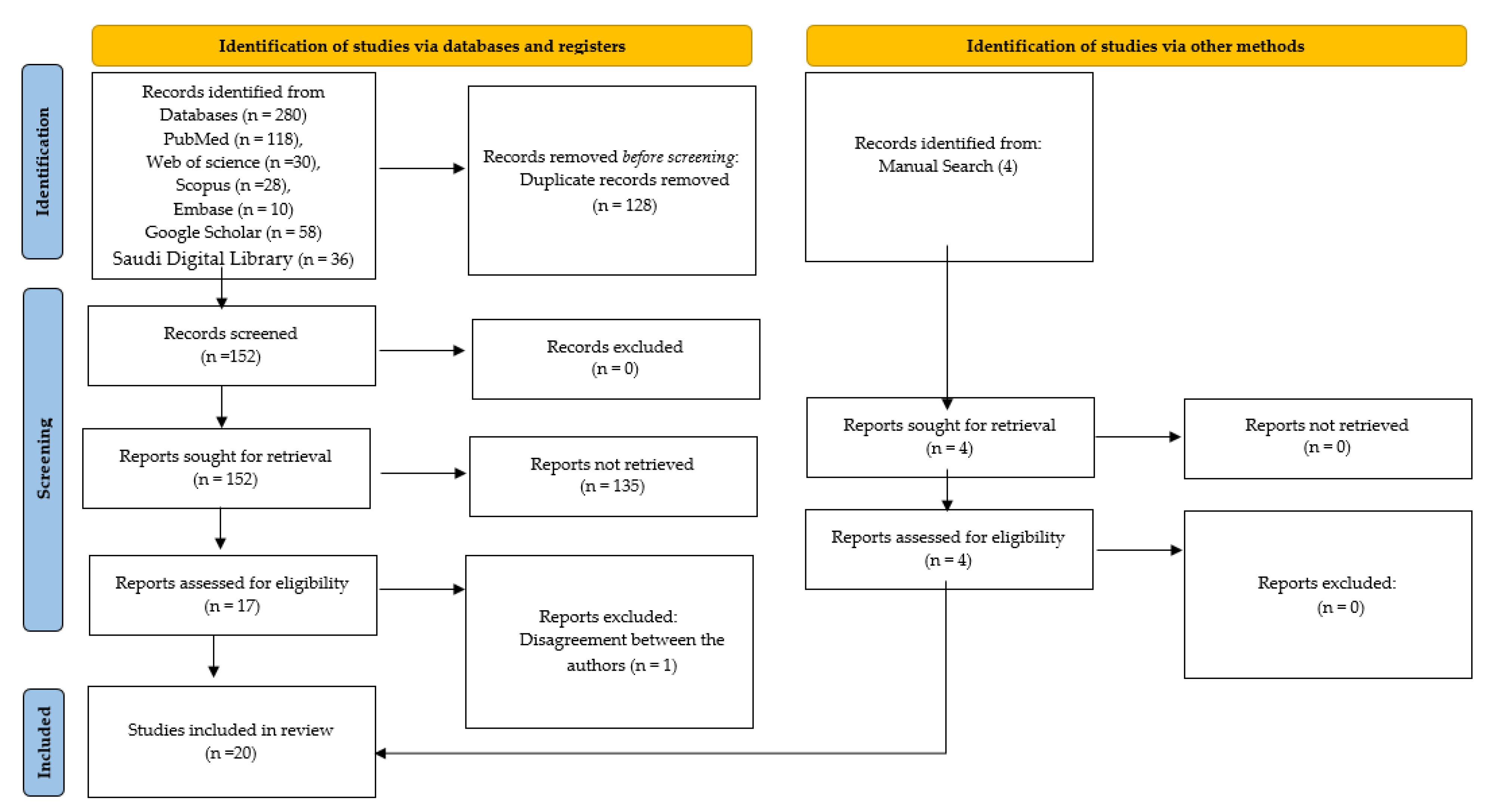
2.2. Study Selection
2.3. Eligibility Criteria
2.4. Data Extraction
3. Results
3.1. Qualitative Synthesis of the Included Studies
3.2. Study Characteristics
3.3. Outcome Measures
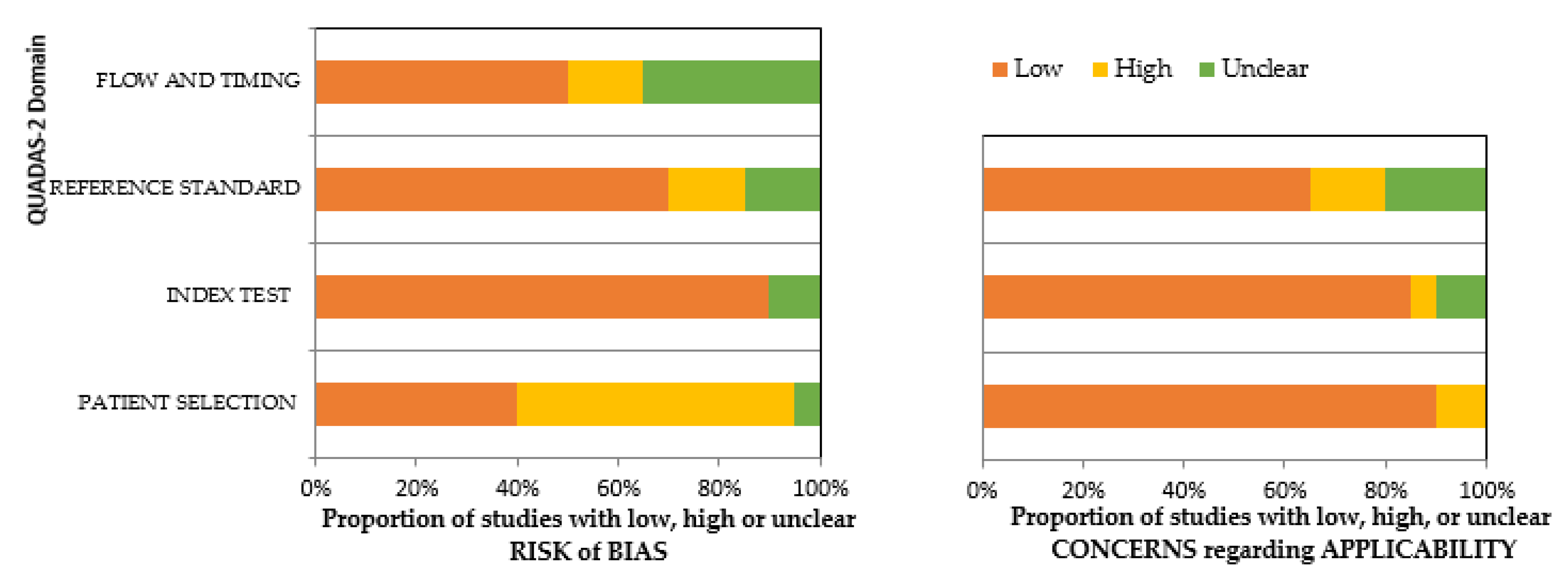
3.4. Risk of Bias Assessment and Applicability Concerns
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Search/Filters | Topic and Terms |
|---|---|
| “English” Language | “artificial intelligence” OR “neural networks” OR “deep learning” OR “machine learning” OR “supervised machine learning” OR “automated learning” OR “unsupervised machine learning” OR “computational intellegence” OR ” machine intellegence” OR ”expert systems” OR ” fuzzy networks” OR ” AI networks” OR “ AI models” OR “ computational systems” OR ”dental plaque” OR “plaque detection” OR “dental caries” OR ”caries prediction” OR ” preventive dentistry” OR ” supernumerary teeth” OR” fissure sealants” OR ” fluorides” OR “pediatric dentistry” OR “pedodontics”OR “caries detection” OR “prediction” OR “diagnosis” OR “age estimation” |
| “English” Language | “artificial intelligence” AND “deep learning” AND “machine learning” AND “supervised machine learning” “computational intellegence” AND ” machine intellegence” AND ”expert systems” AND ” fuzzy networks” AND ” AI networks” AND “ AI models” AND “ computational systems” AND ”dental plaque” AND “dental caries” AND ”caries prediction” AND ” preventive dentistry” AND ” supernumerary teeth” AND” fissure sealants” AND ” fluorides” AND ”plaque detection“ AND “automated learning” AND “unsupervised machine learning” AND “pediatric dentistry” AND “pedodontics” AND “caries detection” AND “prediction” AND “diagnosis” AND “prognosis” AND “age estimation” |
| Risk of Bias | Applicability Concerns | ||||||
|---|---|---|---|---|---|---|---|
| Author | Patient Selection | Index Test | Reference Standard | Flow and Timing | Patient Selection | Index Test | Reference Standard |
| You, W., et al. [19] |  |  |  |  |  |  |  |
| Wang, Y., et al. [20] |  |  |  |  |  |  |  |
| Karhade, D.S., et al. [21] |  |  |  |  |  |  |  |
| Ramos-Gomez, F., et al. [22] |  |  |  |  |  |  |  |
| Schlickenrieder, A. [23] |  |  |  |  |  |  |  |
| Zaborowicz, K. [24] |  |  |  |  |  |  |  |
| Zaorska, K., et al. [25] |  |  |  |  |  |  |  |
| Pang, L., et al. [26] |  |  |  |  |  |  |  |
| Park, Y.H., et al. [27] |  |  |  |  |  |  |  |
| Koopaie, M., et al. [28] |  |  |  |  |  |  |  |
| Gajic, M., et al. [29] |  |  |  |  |  |  |  |
| Kılıc, M.C., et al. [30] |  |  |  |  |  |  |  |
| Ruff, R.R., et al. [31] |  |  |  |  |  |  |  |
| Ahn, Y., et al. [32] |  |  |  |  |  |  |  |
| Mine, Y., et al. [33] |  |  |  |  |  |  |  |
| Li, R.Z., et al. [34] |  |  |  |  |  |  |  |
| Zaborowicz, M., et al. [35] |  |  |  |  |  |  |  |
| Bunyarit, S.S., et al. [36] |  |  |  |  |  |  |  |
| Galibourg, A., et al. [37] |  |  |  |  |  |  |  |
| Shen, S., et al. [38] |  |  |  |  |  |  |  |
 = High Risk,
= High Risk,  = Low Risk,
= Low Risk,  = Unclear.
= Unclear.References
- Doméjean, S.; Banerjee, A.; Featherstone, J.D.B. Caries Risk/Susceptibility Assessment: Its Value in Minimum Intervention Oral Healthcare. Br. Dent. J. 2017, 223, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Petersson, G.H.; Twetman, S. Caries Risk Assessment in Young Adults: A 3 Year Validation of the Cariogram Model. BMC Oral Health 2015, 15, 17. [Google Scholar] [CrossRef] [PubMed]
- Cagetti, M.G.; Bontà, G.; Cocco, F.; Lingstrom, P.; Strohmenger, L.; Campus, G. Are Standardized Caries Risk Assessment Models Effective in Assessing Actual Caries Status and Future Caries Increment? A Systematic Review. BMC Oral Health 2018, 18, 123. [Google Scholar] [CrossRef] [PubMed]
- Shibly, O.; Rifai, S.; Zambon, J.J. Supragingival Dental Plaque in the Etiology of Oral Diseases. Periodontol. 2000 1995, 8, 42–59. [Google Scholar] [CrossRef]
- Axelsson, P.; Lindhe, J. The Effect of a Preventive Programme on Dental Plaque, Gingivitis and Caries in Schoolchildren. Results after One and Two Years. J. Clin. Periodontol. 1974, 1, 126–138. [Google Scholar] [CrossRef]
- Bashirian, S.; Shirahmadi, S.; Seyedzadeh-Sabounchi, S.; Soltanian, A.R.; Karimi-shahanjarini, A.; Vahdatinia, F. Association of Caries Experience and Dental Plaque with Sociodemographic Characteristics in Elementary School-Aged Children: A Cross-Sectional Study. BMC Oral Health 2018, 18, 7. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Gillings, B.R. Recent Developments in Dental Plaque Disclosants. Aust. Dent. J. 1977, 22, 260–266. [Google Scholar] [CrossRef]
- Joseph, B.; Prasanth, C.S.; Jayanthi, J.L.; Presanthila, J.; Subhash, N. Detection and Quantification of Dental Plaque Based on Laser-Induced Autofluorescence Intensity Ratio Values. J. Biomed. Opt. 2015, 20, 048001. [Google Scholar] [CrossRef]
- Volgenant, C.M.C.; Fernandez y Mostajo, M.; Rosema, N.A.M.; van der Weijden, F.A.; ten Cate, J.M.; van der Veen, M.H. Comparison of Red Autofluorescing Plaque and Disclosed Plaque—a Cross-Sectional Study. Clin. Oral Investig. 2016, 20, 2551–2558. [Google Scholar] [CrossRef] [Green Version]
- Carter, K.; Landini, G.; Walmsley, A.D. Automated Quantification of Dental Plaque Accumulation Using Digital Imaging. J. Dent. 2004, 32, 623–628. [Google Scholar] [CrossRef]
- Kumar, Y.; Koul, A.; Singla, R.; Ijaz, M.F. Artificial Intelligence in Disease Diagnosis: A Systematic Literature Review, Synthesizing Framework and Future Research Agenda. J. Ambient Intell. Humaniz. Comput. 2022, 1–28. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, Y.; Wang, D.; Tong, X.; Liu, T.; Zhang, S.; Huang, J.; Zhang, L.; Chen, L.; Fan, H.; et al. Artificial Intelligence for COVID-19: A Systematic Review. Front. Med. 2021, 8, 704256. [Google Scholar] [CrossRef]
- Bichu, Y.M.; Hansa, I.; Bichu, A.Y.; Premjani, P.; Flores-Mir, C.; Vaid, N.R. Applications of Artificial Intelligence and Machine Learning in Orthodontics: A Scoping Review. Prog. Orthod. 2021, 22, 18. [Google Scholar] [CrossRef]
- Bouletreau, P.; Makaremi, M.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Artificial Intelligence: Applications in Orthognathic Surgery. J. Stomatol. Oral Maxillofac. Surg. 2019, 120, 347–354. [Google Scholar] [CrossRef]
- Al-Rawi, N.; Sultan, A.; Rajai, B.; Shuaeeb, H.; Alnajjar, M.; Alketbi, M.; Mohammad, Y.; Shetty, S.R.; Mashrah, M.A. The Effectiveness of Artificial Intelligence in Detection of Oral Cancer. Int. Dent. J. 2022, 72, 436–447. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Br. Med. J. 2021, 372, n71. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- You, W.; Hao, A.; Li, S.; Wang, Y.; Xia, B. Deep Learning-Based Dental Plaque Detection on Primary Teeth: A Comparison with Clinical Assessments. BMC Oral Health 2020, 20, 141. [Google Scholar] [CrossRef]
- Wang, Y.; Hays, R.D.; Marcus, M.; Maida, C.A.; Shen, J.; Xiong, D.; Coulter, I.D.; Lee, S.Y.; Spolsky, V.; Crall, J.; et al. Developing Children’s Oral Health Assessment Toolkits Using Machine Learning Algorithm. JDR Clin. Transl. Res. 2020, 5, 233–243. [Google Scholar] [CrossRef]
- Karhade, D.S.; Roach, J.; Shrestha, P.; Simancas-Pallares, M.A.; Ginnis, J.; Burk, Z.J.S.; Ribeiro, A.A.; Cho, H.; Wu, D.; Divaris, K. An Automated Machine Learning Classifier for Early Childhood Caries. Pediatric Dent. 2021, 43, 191–197. [Google Scholar]
- Ramos-Gomez, F.; Marcus, M.; Maida, C.A.; Wang, Y.; Kinsler, J.J.; Xiong, D.; Lee, S.Y.; Hays, R.D.; Shen, J.; Crall, J.J.; et al. Using a Machine Learning Algorithm to Predict the Likelihood of Presence of Dental Caries among Children Aged 2 to 7. Dent. J. 2021, 9, 141. [Google Scholar] [CrossRef]
- Schlickenrieder, A.; Meyer, O.; Schönewolf, J.; Engels, P.; Hickel, R.; Gruhn, V.; Hesenius, M.; Kühnisch, J. Automatized Detection and Categorization of Fissure Sealants from Intraoral Digital Photographs Using Artificial Intelligence. Diagnostics 2021, 11, 1608. [Google Scholar] [CrossRef]
- Zaborowicz, K.; Biedziak, B.; Olszewska, A.; Zaborowicz, M. Tooth and Bone Parameters in the Assessment of the Chronological Age of Children and Adolescents Using Neural Modelling Methods. Sensors 2021, 21, 6008. [Google Scholar] [CrossRef]
- Zaorska, K.; Szczapa, T.; Borysewicz-Lewicka, M.; Nowicki, M.; Gerreth, K. Prediction of Early Childhood Caries Based on Single Nucleotide Polymorphisms Using Neural Networks. Genes 2021, 12, 462. [Google Scholar] [CrossRef]
- Pang, L.; Wang, K.; Tao, Y.; Zhi, Q.; Zhang, J.; Lin, H. A New Model for Caries Risk Prediction in Teenagers Using a Machine Learning Algorithm Based on Environmental and Genetic Factors. Front. Genet. 2021, 12, 636867. [Google Scholar] [CrossRef]
- Park, Y.-H.; Kim, S.-H.; Choi, Y.-Y. Prediction Models of Early Childhood Caries Based on Machine Learning Algorithms. Int. J. Environ. Res. Public Health 2021, 18, 8613. [Google Scholar] [CrossRef]
- Koopaie, M.; Salamati, M.; Montazeri, R.; Davoudi, M.; Kolahdooz, S. Salivary Cystatin S Levels in Children with Early Childhood Caries in Comparison with Caries-Free Children; Statistical Analysis and Machine Learning. BMC Oral Health 2021, 21, 650. [Google Scholar] [CrossRef] [PubMed]
- Gajic, M.; Vojinovic, J.; Kalevski, K.; Pavlovic, M.; Kolak, V.; Vukovic, B.; Mladenovic, R.; Aleksic, E. Analysis of the Impact of Oral Health on Adolescent Quality of Life Using Standard Statistical Methods and Artificial Intelligence Algorithms. Children 2021, 8, 1156. [Google Scholar] [CrossRef] [PubMed]
- Kılıc, M.C.; Bayrakdar, I.S.; Çelik, Ö.; Bilgir, E.; Orhan, K.; Aydın, O.B.; Kaplan, F.A.; Sağlam, H.; Odabaş, A.; Aslan, A.F.; et al. Artificial Intelligence System for Automatic Deciduous Tooth Detection and Numbering in Panoramic Radiographs. Dentomaxillofacial Radiol. 2021, 50, 20200172. [Google Scholar] [CrossRef] [PubMed]
- Ruff, R.R.; Paul, B.; Sierra, M.A.; Xu, F.; Li, X.; Crystal, Y.O.; Saxena, D. Predicting Treatment Nonresponse in Hispanic/Latino Children Receiving Silver Diamine Fluoride for Caries Arrest: A Pilot Study Using Machine Learning. Front. Oral Health 2021, 2, 695759. [Google Scholar] [CrossRef]
- Ahn, Y.; Hwang, J.J.; Jung, Y.-H.; Jeong, T.; Shin, J. Automated Mesiodens Classification System Using Deep Learning on Panoramic Radiographs of Children. Diagnostics 2021, 11, 1477. [Google Scholar] [CrossRef]
- Mine, Y.; Iwamoto, Y.; Okazaki, S.; Nakamura, K.; Takeda, S.; Peng, T.-Y.; Mitsuhata, C.; Kakimoto, N.; Kozai, K.; Murayama, T. Detecting the Presence of Supernumerary Teeth during the Early Mixed Dentition Stage Using Deep Learning Algorithms: A Pilot Study. Int. J. Paediatr. Dent. 2022, 32, 678–685. [Google Scholar] [CrossRef]
- Li, R.Z.; Zhu, J.X.; Wang, Y.Y.; Zhao, S.Y.; Peng, C.F.; Zhou, Q.; Sun, R.Q.; Hao, A.M. Development of a Deep Learning Based Prototype Artificial Intelligence System for the Detection of Dental Caries in Children. Chin. J. Stomatol. 2021, 56, 1253–1260. [Google Scholar] [CrossRef]
- Zaborowicz, M.; Zaborowicz, K.; Biedziak, B.; Garbowski, T. Deep Learning Neural Modelling as a Precise Method in the Assessment of the Chronological Age of Children and Adolescents Using Tooth and Bone Parameters. Sensors 2022, 22, 637. [Google Scholar] [CrossRef]
- Bunyarit, S.S.; Nambiar, P.; Naidu, M.K.; Ying, R.P.Y.; Asif, M.K. Dental Age Estimation of Malay Children and Adolescents: Chaillet and Demirjian’s Data Improved Using Artificial Multilayer Perceptron Neural Network. Pediatr. Dent. J. 2021, 31, 176–185. [Google Scholar] [CrossRef]
- Galibourg, A.; Cussat-Blanc, S.; Dumoncel, J.; Telmon, N.; Monsarrat, P.; Maret, D. Comparison of Different Machine Learning Approaches to Predict Dental Age Using Demirjian’s Staging Approach. Int. J. Leg. Med. 2021, 135, 665–675. [Google Scholar] [CrossRef]
- Shen, S.; Liu, Z.; Wang, J.; Fan, L.; Ji, F.; Tao, J. Machine Learning Assisted Cameriere Method for Dental Age Estimation. BMC Oral Health 2021, 21, 641. [Google Scholar] [CrossRef]
- Gilchrist, F.; Marshman, Z.; Deery, C.; Rodd, H.D. The Impact of Dental Caries on Children and Young People: What They Have to Say? Int. J. Paediatr. Dent. 2015, 25, 327–338. [Google Scholar] [CrossRef]
- Poikela, A.; Kantomaa, T.; Pirttiniemi, P. Craniofacial Growth after a Period of Unilateral Masticatory Function in Young Rabbits. Eur. J. Oral Sci. 1997, 105, 331–337. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, J.; Li, X. Effect of Unilateral Mastication on the Remodeling of the Glenoid Fossae in Wistar Rats. West China J. Stomatol. 2003, 21, 155–157. [Google Scholar]
- Jordan, A.R.; Becker, N.; Jöhren, H.-P.; Zimmer, S. Early Childhood Caries and Caries Experience in Permanent Dentition: A 15-Year Cohort Study. Swiss Dent. J. 2016, 126, 114–119. [Google Scholar]
- Li, Y.; Wang, W. Predicting Caries in Permanent Teeth from Caries in Primary Teeth: An Eight-Year Cohort Study. J. Dent. Res. 2002, 81, 561–566. [Google Scholar] [CrossRef]
- Chi, D.L.; Rossitch, K.C.; Beeles, E.M. Developmental Delays and Dental Caries in Low-Income Preschoolers in the USA: A Pilot Cross-Sectional Study and Preliminary Explanatory Model. BMC Oral Health 2013, 13, 53. [Google Scholar] [CrossRef]
- Richards, D. Oral Diseases Affect Some 3.9 Billion People. Evid.-Based Dent. 2013, 14, 35. [Google Scholar] [CrossRef]
- Shah, P.; Velani, P.; Lakade, L.; Dukle, S. Teeth in Forensics: A Review. Indian J. Dent. Res. 2019, 30, 291–299. [Google Scholar] [CrossRef]
- Tóth, Z.O.; Udvar, O.; Angyal, J. Chronological Age Estimation Based on Dental Panoramic Radiography. Fogorv. Szle. 2014, 107, 93–98. [Google Scholar]
- Demirjian, A.; Goldstein, H.; Tanner, J.M. A New System of Dental Age Assessment. Hum. Biol. 1973, 45, 211–227. [Google Scholar]
- Demirjian, A.; Goldstein, H. New Systems for Dental Maturity Based on Seven and Four Teeth. Ann. Hum. Biol. 1976, 3, 411–421. [Google Scholar] [CrossRef]
- Mughal, A.M.; Hassan, N.; Ahmed, A. Bone Age Assessment Methods: A Critical Review. Pak. J. Med. Sci. 2014, 30, 211–215. [Google Scholar] [CrossRef]
- AlQahtani, S.J.; Hector, M.P.; Liversidge, H.M. Accuracy of Dental Age Estimation Charts: Schour and Massler, Ubelaker and the London Atlas. Am. J. Phys. Anthropol. 2014, 154, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Panchbhai, A. Dental Radiographic Indicators, a Key to Age Estimation. Dentomaxillofacial Radiol. 2011, 40, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Moorrees, C.F.A.; Fanning, E.A.; Hunt, E.E. Age Variation of Formation Stages for Ten Permanent Teeth. J. Dent. Res. 1963, 42, 1490–1502. [Google Scholar] [CrossRef]
- Gg, U.H.; Matsson, L. Dental Maturity as an Indicator of Chronological Age: The Accuracy and Precision of Three Methods. Eur. J. Orthod. 1985, 7, 25–34. [Google Scholar] [CrossRef]
- Bagherian, A.; Sadeghi, M. Assessment of Dental Maturity of Children Aged 3.5 to 13.5 Years Using the Demirjian Method in an Iranian Population. J. Oral Sci. 2011, 53, 37–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, A.B. Comparisons between Dental and Skeletal Ages. Angle Orthod. 1991, 61, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Cameriere, R.; De Angelis, D.; Ferrante, L.; Scarpino, F.; Cingolani, M. Age Estimation in Children by Measurement of Open Apices in Teeth: A European Formula. Int. J. Leg. Med. 2007, 121, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Meighani, G.; Pakdaman, A. Diagnosis and Management of Supernumerary (Mesiodens): A Review of the Literature. J. Dent. 2010, 7, 41–49. [Google Scholar]
| Research question | What is the performance of AI-based models designed for pediatric patients? |
| Population | Pediatric patients who underwent investigation for oral disease |
| Intervention | AI applications designed for detection, diagnosis, prediction of oral diseases in pediatric patients |
| Comparison | Expert/Specialist opinions, Reference standards/models |
| Outcome | Measurable or predictive outcomes, such as Accuracy, Sensitivity, Specificity, ROC = Receiver Operating Characteristic curve, AUC = Area Under the Curve, Area Under the Receiver Operating Characteristic = AUROC, ICC = Intraclass Correlation Coefficient, IOU = intersection-over-union, PRC = precision recall curve, Statistical Significance, F1 Scores, vDSC: Volumetric Dice Similarity Coefficient, sDSC: Surface Dice Similarity Coefficient, PPV = Positive Predictive Value, NPV = Negative Predictive Value, Mean Decreased Gini (MDG), Mean Decreased Accuracy (MDA) coefficients, Intersection over Union (IoU), Dice coefficient |
| Serial No. | Authors | Year of Publcation | Study Design | Algorithm Architecture | Objective of the Study | No. of Patients/Images/Photographs for Testing | Study Factor | Modality | Comparison If Any | Evaluation Accuracy/Average Accuracy/Statistical Significance | Results (+) Effective, (−) Non effective (N) Neutral | Outcomes | Authors Suggestions/Conclusions |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | You, W., et al. [19] | 2020 | Comparative study | CNNs | To evaluate the accuracy of AI-based model for detecting plaque on primary teeth | 886 samples for training, 98 for validation | Dental Plaque | Intra oral photographs | Experienced pediatric dentist | MIoU of 0.726 ± 0.165. There was no difference between the AI model and specialist (p > 0.05) | (+) Effective | CNNs-based model demonstrated high accuracy in detecting plaque, in comparison with the pediatric dentist | This model can help children to improve their oral health |
| 2 | Wang, Y., et al. [20] | 2020 | Comparative study | ANNs | To assess the performance of ML model (XGBoost) for predicting children’s oral health status (OHS) and treatment needs (TN) | 545 subjects (70% for training and 30% for validation) | Oral health status and treatment needs | Data sets | Dentist | Sensitivity of 93% and specificity of 49% for predicting referral for treatment needs (RFTN) | (+) Effective | These models were efficient in predicting OHS and TN | This model can be of great use in school oral health programs |
| 3 | Karhade, D.S., et al. [21] | 2021 | Retrospective cohort | ANNs | To evaluate the accuracy of an automated ML algorithm for classification of early childhood caries (ECC) | 6040 (5123 subjects for training 1281 subjects for testing) | Dental caries | Data sets | External National Health and Nutrition Examination Survey (NHANES) dataset/ 10 trained and calibrated clinical examiners | AUC of (0.74), Sensitivity of (0.67) and PPV of (0.64) | (+) Effective | This ML model’s performance was similar to the reference model | This model is valuable for ECC screening |
| 4 | Ramos-Gomez, F., et al. [22] | 2021 | Retrospective cohort | ANNs | ML algorithm (Random forest) for identifying survey items for predicting dental caries (DC) | 182 subjects | Dental caries | Data sets | 2 Trained dentists | For classifying active caries parent’s age mean decreased Gini MDG = 0.84; mean decreased accuracy MDA =1.97, unmet needs (MDG = 0.71; MDA = 2.06). Predictors of caries with parent’s age (MDG = 2.97; MDA = 4.74), with oral health problems in past 12 months (MDG = 2.20; MDA = 4.04 | (+) Effective | This model has potential for screening DC | This model is potential for screening for DC for children |
| 5 | Schlickenrieder, A. [23] | 2021 | Comparative study | CNNs | To assess the performance of convolutional neural network (CNN) for detecting and categorizing fissure sealants | 2352 permanent posterior teeth | Fissure sealants | Digital photographs | Experienced examiner | 98.7% accuracy in detecting sealants with an AUC of 0.996. The diagnostic accuracy and AUC were 89.6% and 0.951 for Intact sealant; 83.2% and 0.888 for Sufficient sealant; 92.4 and 0.942 for insufficient sealant. | (+) Effective | CNN detected sealant intraoral photographs with an agreement of 98.7%, in comparison with reference decisions | Additional training of AI-based is required before clinical use |
| 6 | Zaborowicz, K. [24] | 2021 | Comparative study | ANNs | Three Radial Basis Function neural models RBF 22:22-15-1:1 RBF 13:13-1-1:1 RBF 18:18-1-1:1 for determining the chronological age | 619 subjects (296 girls and 323 boys) | Age assessment | Digital pantomographic images | PNN (probabilistic neural network), GRNN (generalized regression neural network), and three- and four-layer MLP (multilayer perceptron) networks | This model demonstrated an accuracy of 99.7% for chronological age assessment | (+) Effective | RBF networks were characterized by the best quality indicators | This is an effective and innovative tool for the assessment of the chronological age |
| 7 | Zaorska, K., et al. [25] | 2021 | Prospective cohort | CNNs | AI model for predicting DC based on chosen polymorphisms | 95 patients | DC lesions | Data sets | Logistic regression model | Sensitivity of 90, specificity of 96% overall accuracy of 93% (p < 0.0001), AUC was 0.970 (p < 0.0001). Prediction accuracy of 90.9–98.4% | (+) Effective | This model displayed high accuracy in predicting DC | The knowledge of potential risk status could be useful in designing oral hygiene and adopting eating habits for patients |
| 8 | Pang, L., et al. [26] | 2021 | Prospective cohort | ANNs | AI-based ML model for caries risk prediction based on environmental and genetic factors | 953 patients (633 for training and 320 for testing) | DC lesions | Data sets | Logistic regression model | AUC of 0.73 | (+) Effective | This model could accurately identify individuals at high and very high caries risk | This is a powerful tool for identifying individuals at high caries risk at community level |
| 9 | Park, Y.H., et al. [27] | 2021 | Prospective cohort | ANNs | ML-based AI models (XGBoost, random forest, LightGBM algorithms and Final model) for predicting early childhood caries | 4195 (2936 for training and 1259 for testing) | DC lesions | Data sets | Traditional regression model | AUROC = 0.774–0.785 | (+) Effective | ML-based models showed favorable performance in predicting DC | Can be useful in identifying high risk groups and implementing preventive treatments |
| 10 | Koopaie, M., et al. [28] | 2021 | Comparative study Case-control study | ANNs | ML-based AI models feed-forward neural network (S1 and S2), for comparing the salivary level of cystatin S in ECC patients and caries-free (CF) children | 20 cases of ECC and 20 caries free children as control | ECC prediction | Data sets | XGBoost, random forest and support vector machine | S1 model demonstrated an accuracy of 88.1%, sensitivity of 100% and specificity of 71.3%. S1 model demonstrated an accuracy of 90.9%, sensitivity of 100% and specificity of 72.1% | (+) Effective | The logistic regression model based on salivary cystatin S levels and birth weight had the most acceptable potential for discriminating early childhood caries from caries-free controls. | Considering clinical examination, demographic and socioeconomic factors, along with the salivary cystatin S levels, could be useful for early diagnosis of ECC |
| 11 | Gajic, M., et al. [29] | 2021 | Comparative study | ANNs | Determining the impact of oral health on adolescents’ quality of life and comparison between standard statistical methods and AI algorithms | 374 (128 male and 246 female) | Adolescent quality of life | Data sets | Standard statistical methods | Not clear | (+) Effective | Using artificial intelligence algorithms, the respondents can be clustered into characteristic groups | Dental education will need to accompany the introduction of clinical AI solutions by fostering digital literacy in the future dental workforce. |
| 12 | Kılıc, M.C., et al. [30] | 2021 | Observational study | CNNs | A deep-learning model for automated detection and enumeration of the deciduous teeth on panoramic radiographs | 421 | Tooth | Panoramic images | Not clear | Sensitivity of 0.9804, precision of 0.9571 and F1 score was 0.9686 | (+) Effective | A promising tool for the automated charting of panoramic dental radiographs | It will aid clinicians by serving as a time-saving measure |
| 13 | Ruff, R.R., et al. [31] | 2021 | Observational study | ANNs | An MI-based predictive model for treatment non-response to Silver diamine fluoride (SDF) therapy | 20 | Microbial analysis | Plaque samples and data sets | Lasso regression | Not clear | (+) Effective | These are the only possible models that could be useful in predicting non-response | There is a need of making predictions in larger, independent datasets |
| 14 | Ahn, Y., et al. [32] | 2021 | Comparative study | CNNs | Deep-learning models SqueezeNet, ResNet-18, ResNet-101 and Inception-ResNet-V2 for automatically classify mesiodens in primary or mixed dentition | 1100 Images (1000 images for validating and 100 images for testing) | Mesiodens | Panoramic radiographs | Six pediatric dentists and six general dentists | The AUC values were 0.862 for SqueezeNet, 0.955 for ResNet-18, 0.941 for ResNet-101 and 0.932 for Inception-ResNet-V2 | (+) Effective | These models delivered high accuracy in classifying the presence of mesiodens in the mixed dentition panoramic radiographs | Deep-learning technologies may help clinicians with insufficient clinical experience in more accurate and faster diagnosis |
| 15 | Mine, Y., et al. [33] | 2021 | Comparative study | CNNs | Deep-learning Models AlexNet, VGG16-TL and InceptionV3-TL for detecting the presence of supernumerary teeth during the early mixed dentition stage | 220 | Supernumerary teeth | Panoramic radiographs | Two experienced pediatric dentists | VGG16 model demonstrated high performance with AUC of 0.89, accuracy of 82.3%, sensitivity of 85.0% and specificity of 79.0%. AlexNet, VGG16-TL and InceptionV3-TL models achieved sensitivity values of 82.5%, 85.0% and 83.3%, respectively | (+) Effective | VGG16-TL model had the highest performance, in comparison with others. | CNN-based deep learning is a promising approach for detecting the presence of supernumerary teeth during the early mixed dentition stage. |
| 16 | Li, R.Z., et al. [34] | 2021 | Comparative study | CNNs | Deep learning-based image recognition system for detecting dental caries | 712 | Dental Caries | Intraoral photographs | Pediatric dentists | Sensitivity of 96.0% and specificity of 97.0% for caries with cavities, 95.8% and 99.0% for pit and fissure caries and 88.1% and 97.1% for approximal caries | (+) Effective | Demonstrated the ability to detect dental caries | AI system could accurately verify different types of dental caries. |
| 17 | Zaborowicz, M., et al. [35] | 2021 | Comparative study | CNNs | Deep learning-based model for estimating the age | 619 (296 male and 323 female) | Tooth and bone parameters | Digital pantomographs | Statistical 7.1 simulator | The MAE (mean squared error) error of the produced models, depending on the learning set used, is between 2.34 and 4.61 months, while the RMSE (root mean squared error) error is between 5.58 and 7.49 months. The correlation coefficient R2 ranges from 0.92 to 0.96. | (+) Effective | Deep neural models have higher quality already in the first iteration of learning the network using all the developed metrics | It is recommended to prepare deep neural networks based on the set of indicators used in the first stage of the research. |
| 18 | Bunyarit, S.S., et al. [36] | 2021 | Comparative study | ANNs | To develop reliable teeth maturity scores for age estimation based on artificial neural networks | 1569 | Dental age and chronological age | Panoramic radiographs | Demirjian’s eight developmental stages—trained observers | Significant correlation was observed between chronological age and new dental maturity scores after ANN in both girls and boys (p < 0.001); R2 of 0.951 with predicting accuracy of 95.1% for boys (ANOVA, F ¼ 5096.6, p < 0.001); an adjusted R2 of 0.938 was found for girls, with an accuracy of 93.8% for predicting the actual age | (+) Effective | Demonstrated greater accuracy in age estimation | Can be applied for clinical and forensic cases. |
| 19. | Galibourg, A., et al. [37] | 2021 | Comparative study | ANNs | To develop machine learning algorithms to predict dental age in children | 3605 (1734 females and 1871 males) | Dental age | Panoramic radiographs | Demirjian’s reference method | Mean absolute error (MAE) under 0.811 years | (+) Effective | The machine learning methods were significantly more accurate than the two reference methods. | These results support the use of ML algorithms instead of using standard population tables. |
| 20. | Shen, S., et al. [38] | 2021 | Comparative study | ANNs | Random forest (RF), support vector machine (SVM) and linear regression (LR) based on the Cameriere method to predict children’s dental age | 748 children (356 females and 392 males) | Dental age | Panoramic radiographs | Cameriere age estimation | ML models have better accuracy than the traditional Cameriere formula. The mean error (ME), mean absolute error (MAE), mean square error (MSE) and root mean square error (RMSE) values of the SVM model (0.004, 0.489, 0.392 and 0.625, respectively). In contrast, the ME, MAE, MSE and RMSE of the European Cameriere formula were 0.592, 0.846, 0.755 and 0.869, respectively, and those of the Chinese Cameriere formula were 0.748, 0.812, 0.890 and 0.943, respectively | (+) Effective | Compared to the Cameriere formula, ML methods based on the Cameriere’s maturation stages were more accurate in estimating dental age | ML models have better accuracy than the traditional Cameriere formula |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanagar, S.B.; Alfouzan, K.; Alkadi, L.; Albalawi, F.; Iyer, K.; Awawdeh, M. Performance of Artificial Intelligence (AI) Models Designed for Application in Pediatric Dentistry—A Systematic Review. Appl. Sci. 2022, 12, 9819. https://doi.org/10.3390/app12199819
Khanagar SB, Alfouzan K, Alkadi L, Albalawi F, Iyer K, Awawdeh M. Performance of Artificial Intelligence (AI) Models Designed for Application in Pediatric Dentistry—A Systematic Review. Applied Sciences. 2022; 12(19):9819. https://doi.org/10.3390/app12199819
Chicago/Turabian StyleKhanagar, Sanjeev Balappa, Khalid Alfouzan, Lubna Alkadi, Farraj Albalawi, Kiran Iyer, and Mohammed Awawdeh. 2022. "Performance of Artificial Intelligence (AI) Models Designed for Application in Pediatric Dentistry—A Systematic Review" Applied Sciences 12, no. 19: 9819. https://doi.org/10.3390/app12199819







