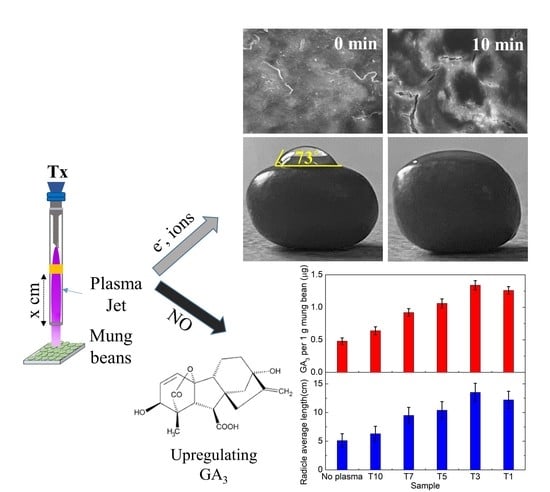
Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean
Abstract
:1. Introduction
2. Materials and Methods
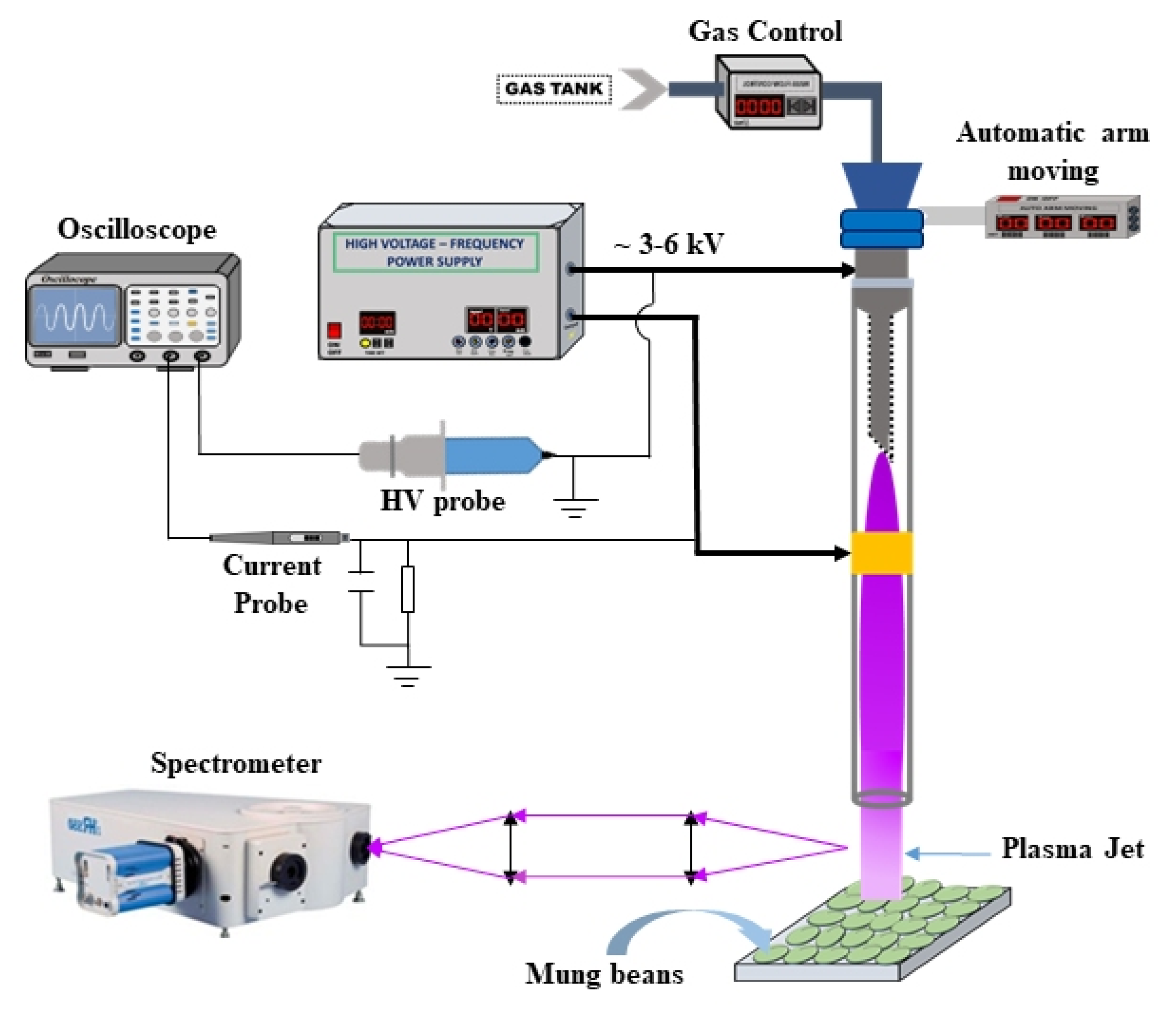
2.1. Non-Thermal Atmospheric Pressure Plasma Jet Apparatus
2.2. Electrical and Optical Spectroscopy Characterization of the Plasma Jet
2.3. Enhancement of Seed Germination and Seedling Growth of Mung Bean
2.4. Scanning Electron Microscopy (SEM)
2.5. Extraction of Gibberellic Acid (GA3) Hormone
- 100 g of bean sprouts after 24 h of incubation (with and without plasma treatment) were dried at 65 °C for 12 h and ground into powder.
- 5 g mung bean sprout powder and 50 mL MeOH were added to a conical beaker, shaken for 25 min, and then sonicated for 15 min.
- After standing for 2.5 h at 4 °C, the supernatant was collected.
- The remaining residue was further extracted with 25 mL MeOH, following the same steps as above.
- The post-extraction solution was evaporated to dryness at 50 °C under a stream of nitrogen and then dissolved in 1 mL of double-distilled water.
- The solution was filtered through a 0.45-mm Millipore filter before using for analysis.
2.6. Separation and Confirmation of Gibberellic Acid (GA3) by LC-MS Analysis
3. Results and Discussion
3.1. Effect of Cold Plasma Treatment on Physical Change of Seed’s Surface
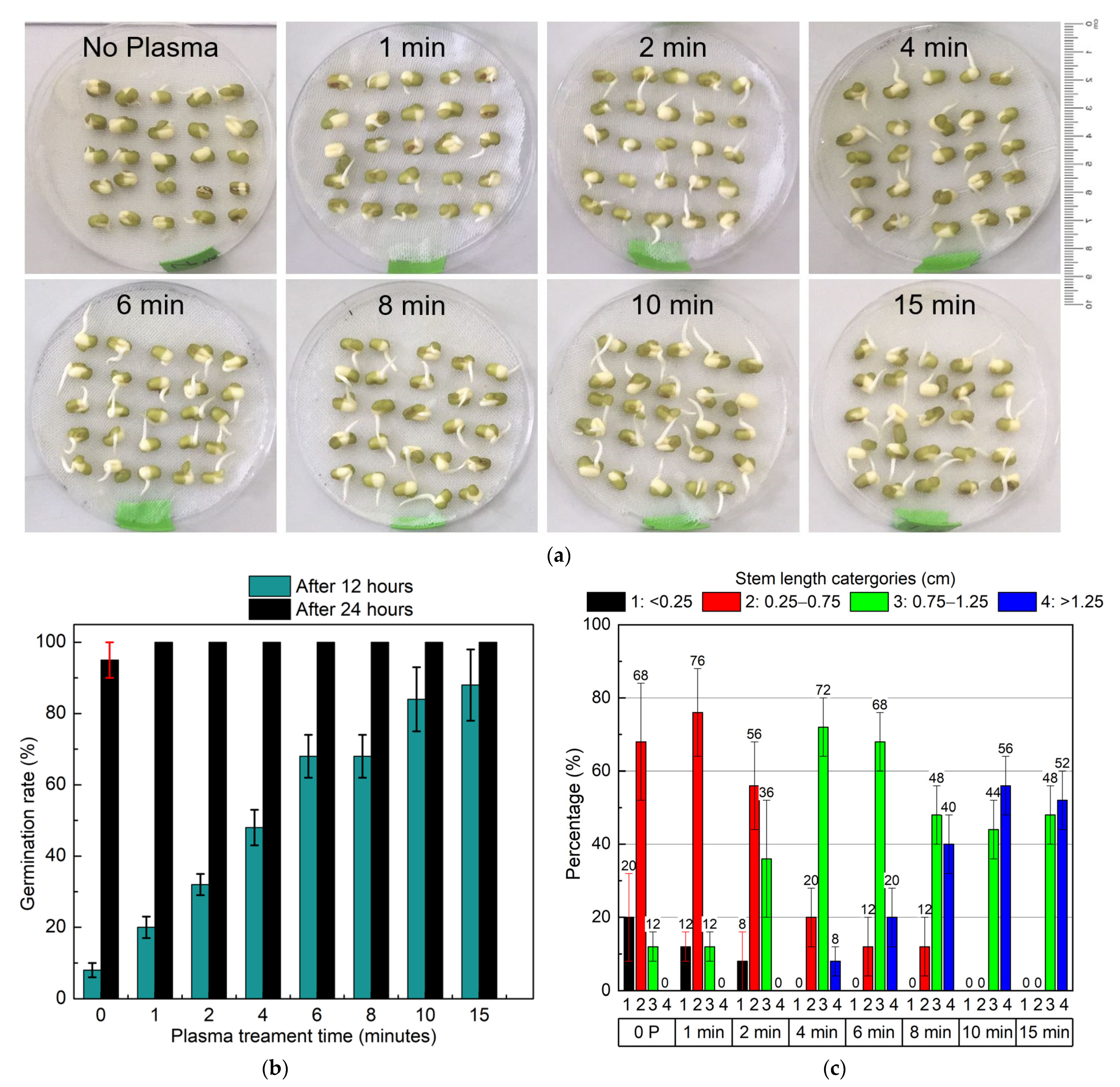
3.1.1. Effect of Cold Plasma Treatment Time on Germination Rate and Root Length
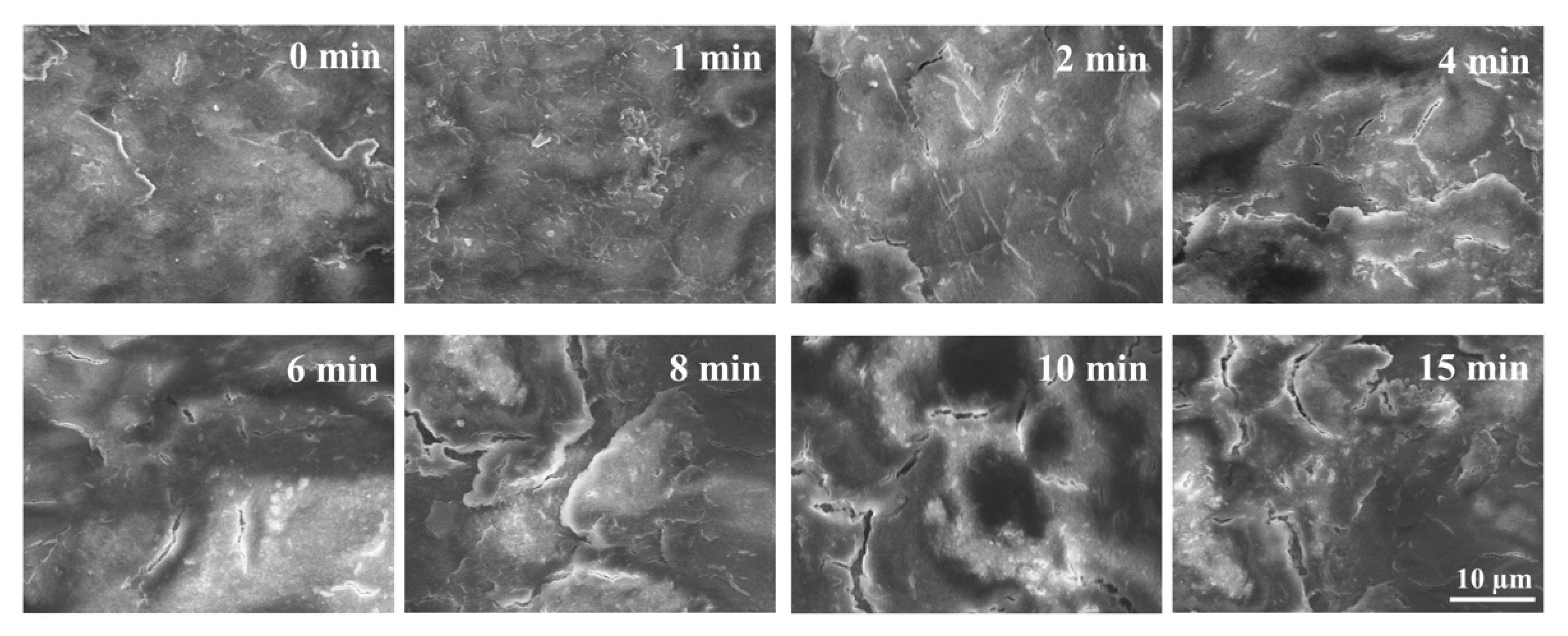
3.1.2. Effect of Cold Plasma Treatment on Seeds’ Surface Morphology
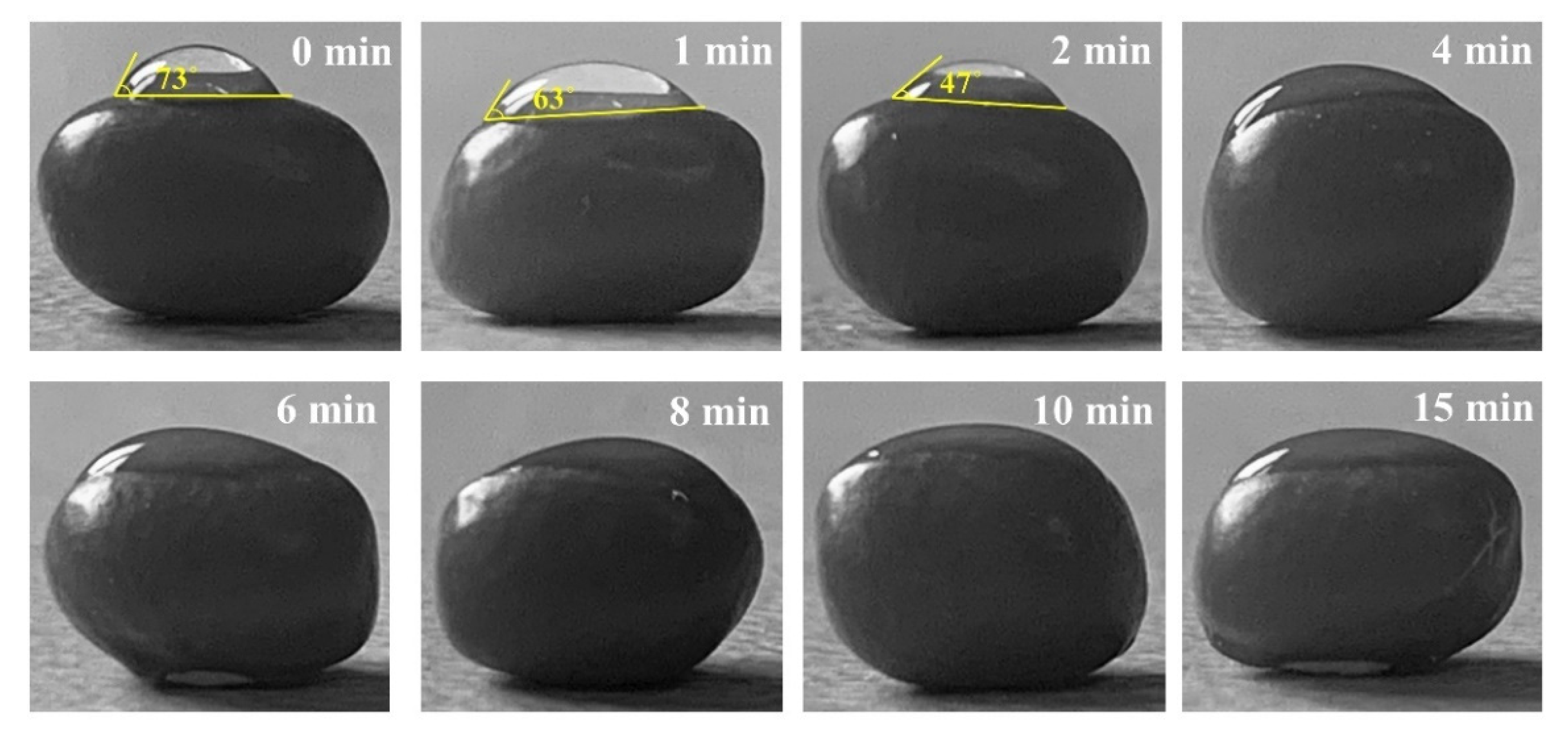
3.1.3. Effect of Cold Plasma Treatment on the Wettability of Seeds
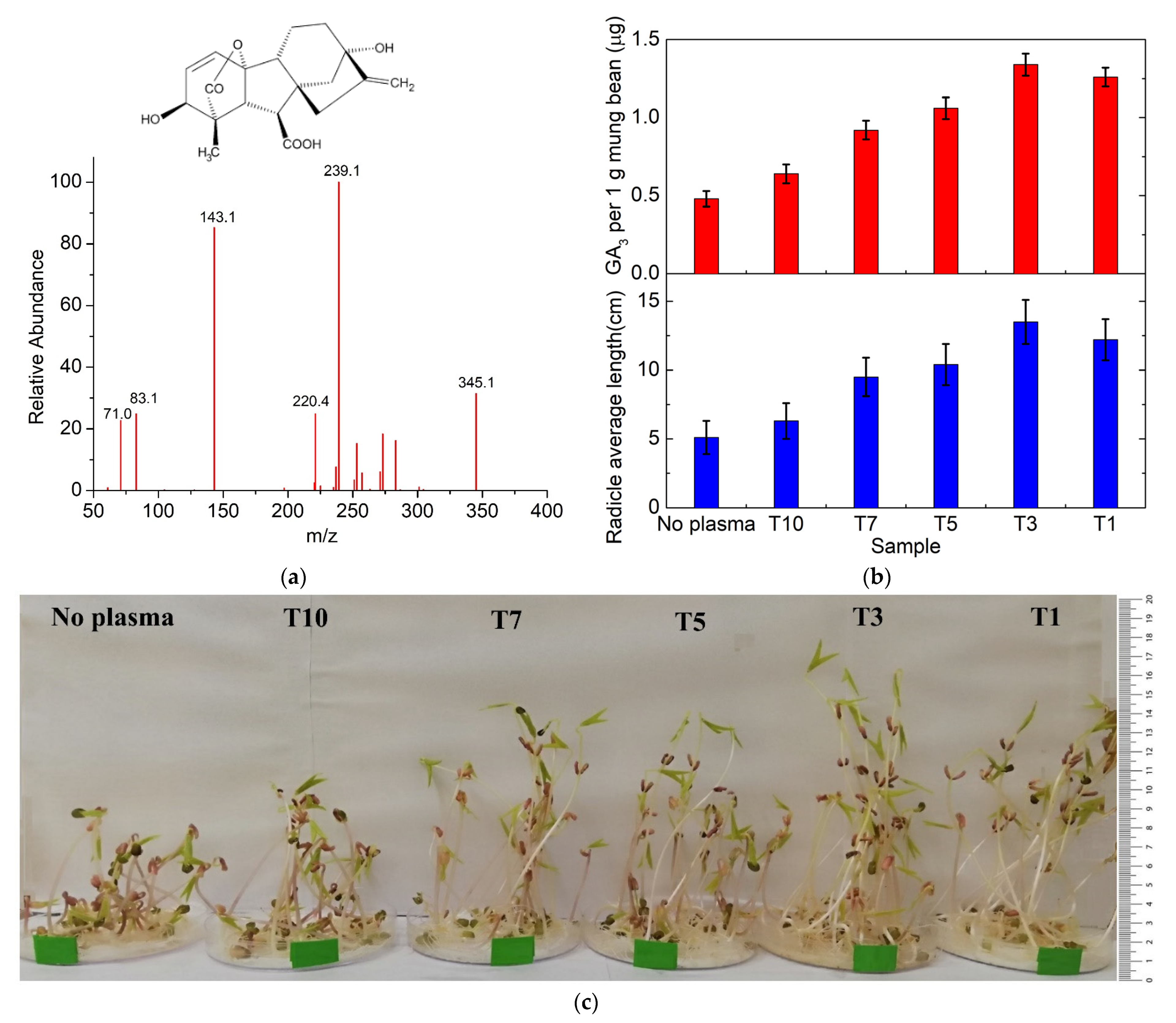
3.2. Effect of Cold Plasma Treatment on Endogenous Hormone Regulation
3.2.1. Regulation of Plasma-Generated Reactive Species (RNS/ROS)
3.2.2. Effect of Cold Plasma Treatment on Endogenous Hormone Regulation and Radicle Growth
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gay-Mimbrera, J.; García, M.C.; Isla-Tejera, B.; Rodero-Serrano, A.; García-Nieto, A.V.; Ruano, J. Clinical and biological principles of cold atmospheric plasma application in skin cancer. Adv. Ther. 2016, 33, 894–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kINPen–A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, D.; Zhou, R.; Song, Y.; Sun, Y.; Zhang, Q.; Niu, J.; Fan, H.; Yang, S.-Z. Atmospheric cold plasma jet for plant disease treatment. Appl. Phys. Lett. 2014, 104, 043702. [Google Scholar] [CrossRef]
- Bourke, P.; Ziuzina, D.; Boehm, D.; Cullen, P.J.; Keener, K. The potential of cold plasma for safe and sustainable food production. Trends Biotechnol. 2018, 36, 615–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef] [Green Version]
- Bafoil, M.; Jemmat, A.; Martinez, Y.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. Effects of low temperature plasmas and plasma activated waters on Arabidopsis thaliana germination and growth. PLoS ONE 2018, 13, e0195512. [Google Scholar] [CrossRef]
- Darmanin, M.; Fröhling, A.; Bußler, S.; Durek, J.; Neugart, S.; Schreiner, M.; Blundell, R.; Gatt, R.; Schlüter, O.; Valdramidis, V.P. Aqueous and gaseous plasma applications for the treatment of mung bean seeds. Sci. Rep. 2021, 11, 19681. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of seed germination performance through cold plasma chemistry technology. Crop Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Waskow, A.; Howling, A.; Furno, I. Mechanisms of plasma-seed treatments as a potential seed processing technology. Front. Phys. 2021, 9, 174. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Wang, G.; Ren, P.; Wu, Y.; He, Q. Effects of typical antimicrobials on growth performance, morphology and antimicrobial residues of mung bean sprouts. Antibiotics 2022, 807, 807. [Google Scholar] [CrossRef] [PubMed]
- Tuan, P.A.; Kumar, R.; Rehal, P.K.; Toora, P.K.; Ayele, B.T. Molecular mechanisms underlying Abscisic acid/Gibberellin balance in the control of seed dormancy and germination in cereals. Front. Plant Sci. 2018, 9, 668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, B.; Adhikari, M.; Park, G. The effects of plasma on plant growth, development, and sustainability. Appl. Sci. 2020, 10, 6045. [Google Scholar] [CrossRef]
- Liu, Y.; Shi, L.; Ye, N.; Liu, R.; Jia, W.; Zhang, J. Nitric oxide-induced rapid decrease of abscisic acid concentration is required in breaking seed dormancy in Arabidopsis. New Phytol 2009, 183, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Kim, T.; Panngom, K.; Hong, Y.J.; Pengkit, A.; Park, D.H.; Kang, M.H.; Lee, S.H.; Im, J.S.; Kim, J.S.; et al. Assessment of the effects of nitrogen plasma and plasma-generated nitric oxide on early development of coriandum sativum. Plasma Process. Polym. 2015, 12, 1164–1173. [Google Scholar] [CrossRef]
- Zhang, S.; Rousseau, A.; Dufour, T. Dufour, Promoting lentil germination and stem growth by plasma activated tap water, demineralized water and liquid fertilizer. RSC Adv. 2017, 7, 31244–31251. [Google Scholar] [CrossRef] [Green Version]
- Attri, P.; Koga, K.; Okumura, T.; Shiratani, M. Impact of atmospheric pressure plasma treated seeds on germination, morphology, gene expression and biochemical responses. Jpn. J. Appl. Phys. 2021, 60, 040502. [Google Scholar] [CrossRef]
- Xuan, L.T.Q.; Nguyen, L.N.; Dao, N.T. Synthesis of stabilizer-free, homogeneous gold nanoparticles by cold atmospheric-pressure plasma jet and their optical sensing property. Nanotechnology 2021, 33, 105603. [Google Scholar] [CrossRef]
- Sun, Y.-N.; Qin, S.-Y.; Lv, Y.-K.; Li, S.-Z.; Wei, C. Simultaneous determination of five phytohormones in mungbean sprouts of China by micellar electrokinetic chromatography. J. Chromatogr. Sci. 2013, 52, 725–729. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Hu, X.; Yin, Y.; Zhu, Y.; Zhuang, J.; Wang, X.; Ma, R.; Jiao, Z. Quality enhancement and microbial reduction of mung bean (Vigna radiata) sprouts by non-thermal plasma pretreatment of seeds. Plasma Sci. Technol. 2022, 24, 045504. [Google Scholar] [CrossRef]
- Zhou, R.; Zhou, R.; Zhang, X.; Zhuang, J.; Yang, S.; Bazaka, K.; Ostrikov, K. Effects of atmospheric-pressure N2, He, Air, and O2 microplasmas on mung bean seed germination and seedling growth. Sci. Rep. 2016, 6, 32603. [Google Scholar] [CrossRef] [Green Version]
- Bormashenko, E.; Grynyov, R.; Bormashenko, Y.; Drori, E. Cold radiofrequency plasma treatment modifies wettability and germination speed of plant seeds. Sci. Rep. 2012, 2, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolárik, T.; Henselová, M.; Martinka, M.; Novák, O.; Zahoranová, A.; Černák, M. Effect of low-temperature plasma on the structure of seeds, growth and metabolism of endogenous phytohormones in pea (Pisum sativum L.). Plasma Chem. Plasma Process. 2015, 35, 659–676. [Google Scholar] [CrossRef]
- Lamichhane, P.; Acharya, T.R.; Kaushik, N.; Nguyen, L.N.; Lim, J.S.; Hessel, V.; Kaushik, N.K.; Choi, E.H. Non-thermal argon plasma jets of various lengths for selective reactive oxygen and nitrogen species production. J. Environ. Chem. Eng. 2022, 10, 107782. [Google Scholar] [CrossRef]
- Xie, W.; Han, C.; Zheng, Z.; Chen, X.; Qian, Y.; Ding, H.; Shi, L.; Lv, C. Determination of Gibberellin A3 residue in fruit samples by liquid chromatography–tandem mass spectrometry. Food Chem. 2011, 127, 890–892. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.; Wahid, S.; Ilyas, M.; Hasanuzzaman, M. Nitric oxide and phytohormones cross-talk during abiotic stresses responses in plants. In Reactive Oxygen, Nitrogen and Sulfur Species in Plants; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 533–554. [Google Scholar]
- Wu, A.P.; Gong, L.; Chen, X.; Wang, J.X. Interactions between nitric oxide, gibberellic acid, and phosphorus regulate primary root growth in Arabidopsis. Biol. Plant 2014, 58, 335–340. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.Q.X.; Nguyen, L.N.; Nguyen, T.T.; Choi, E.H.; Nguyen, Q.L.; Kaushik, N.K.; Dao, N.T. Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean. Appl. Sci. 2022, 12, 10308. https://doi.org/10.3390/app122010308
Le TQX, Nguyen LN, Nguyen TT, Choi EH, Nguyen QL, Kaushik NK, Dao NT. Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean. Applied Sciences. 2022; 12(20):10308. https://doi.org/10.3390/app122010308
Chicago/Turabian StyleLe, Thi Quynh Xuan, Linh Nhat Nguyen, Thanh Tung Nguyen, Eun Ha Choi, Quang Liem Nguyen, Nagendra Kumar Kaushik, and Nguyen Thuan Dao. 2022. "Effects of Cold Plasma Treatment on Physical Modification and Endogenous Hormone Regulation in Enhancing Seed Germination and Radicle Growth of Mung Bean" Applied Sciences 12, no. 20: 10308. https://doi.org/10.3390/app122010308