Medical Gas Plasma—A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications
Abstract
:1. Introduction
2. Platelets in Cellular Blood Hemostasis
2.1. Platelet Functional Morphology
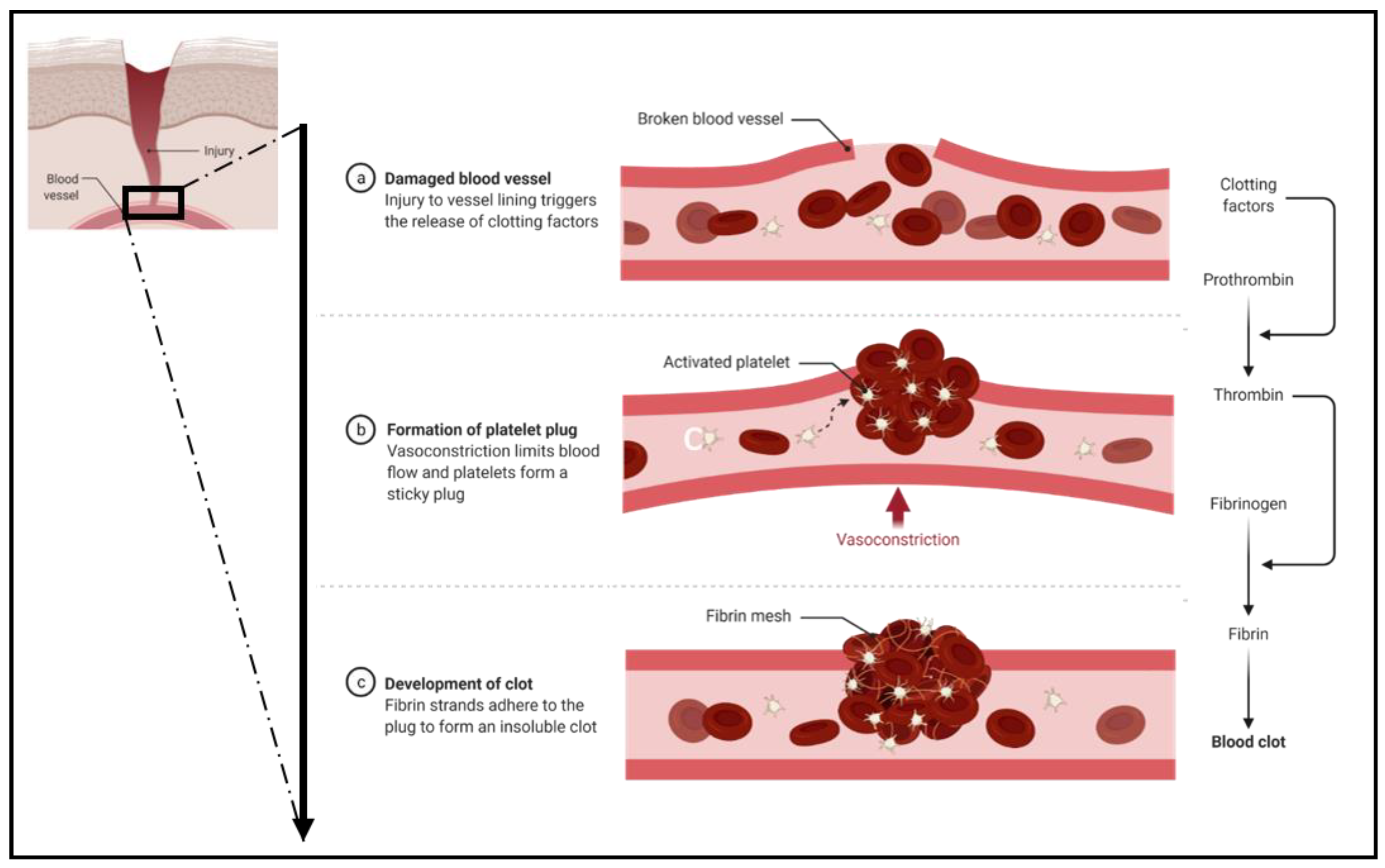
2.2. Primary Hemostasis
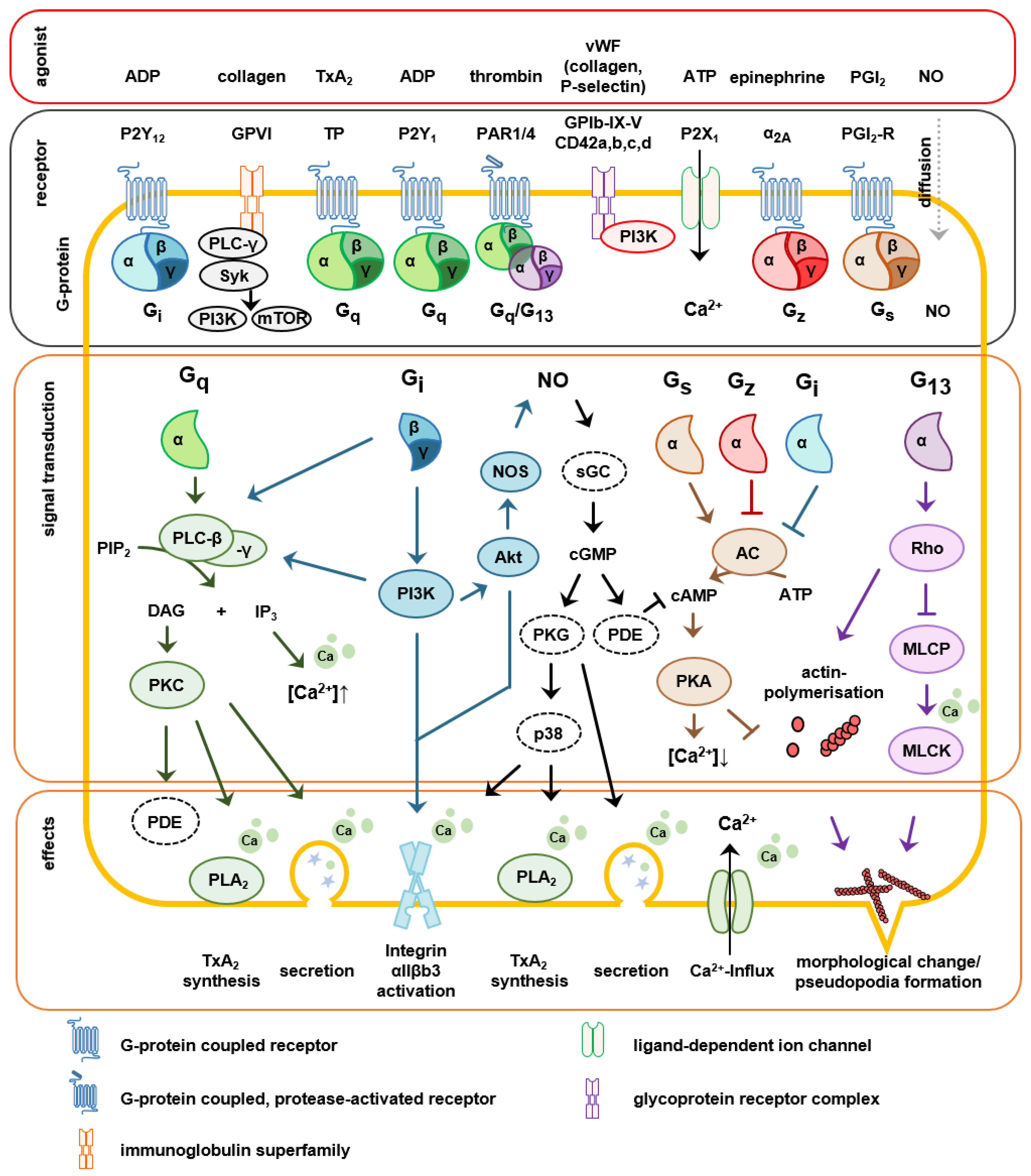
2.3. Signaling Involved in Platelet Activation
2.4. Secondary Hemostasis
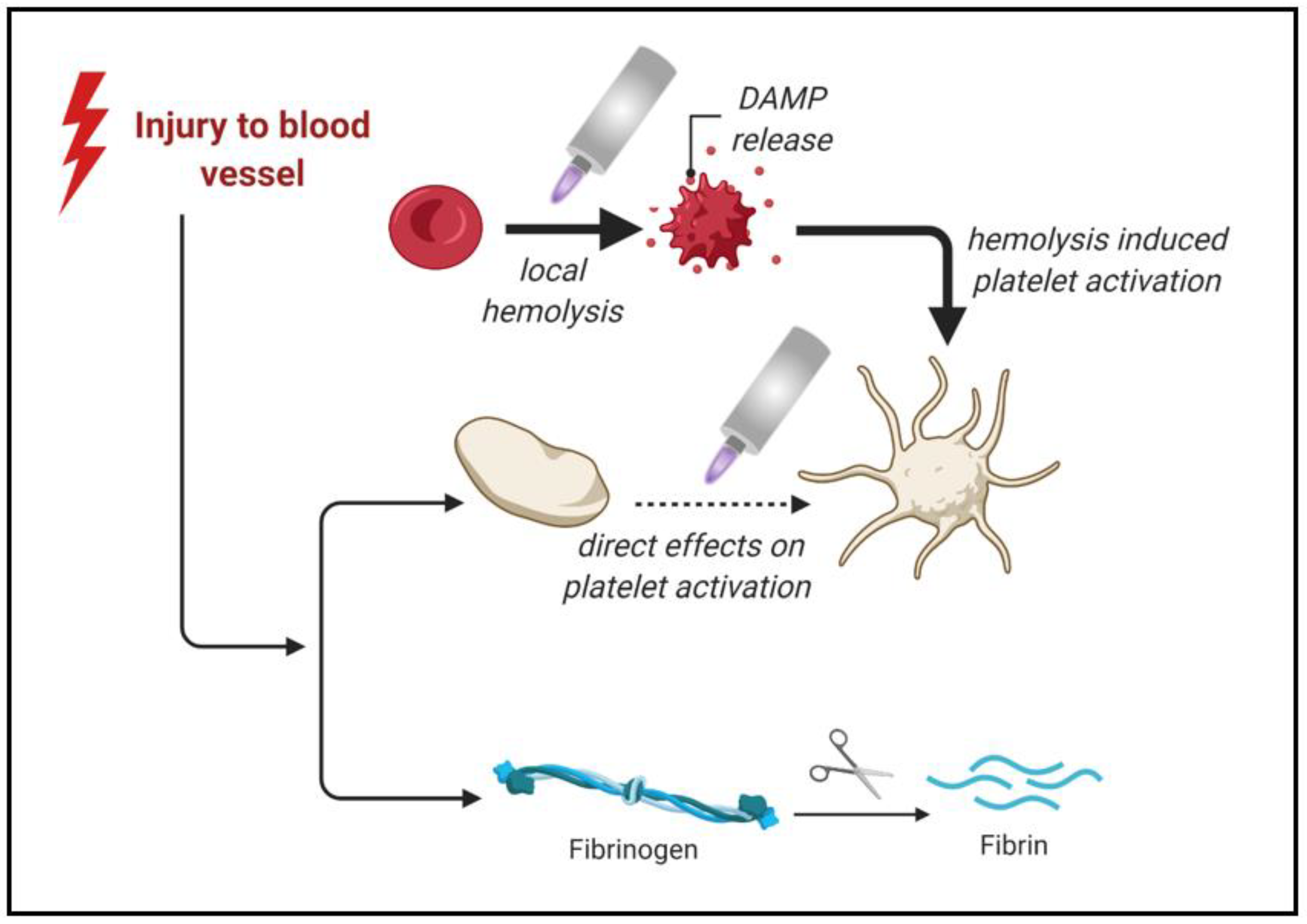
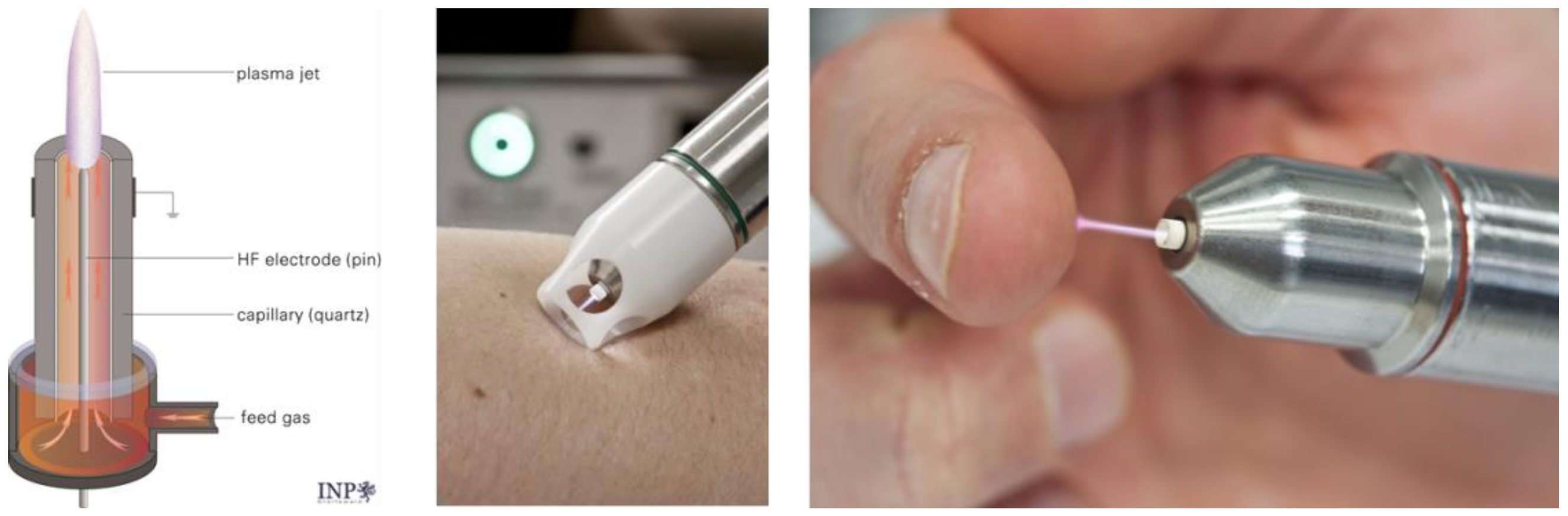
3. Medical Gas Plasma as a Hemostatic Agent
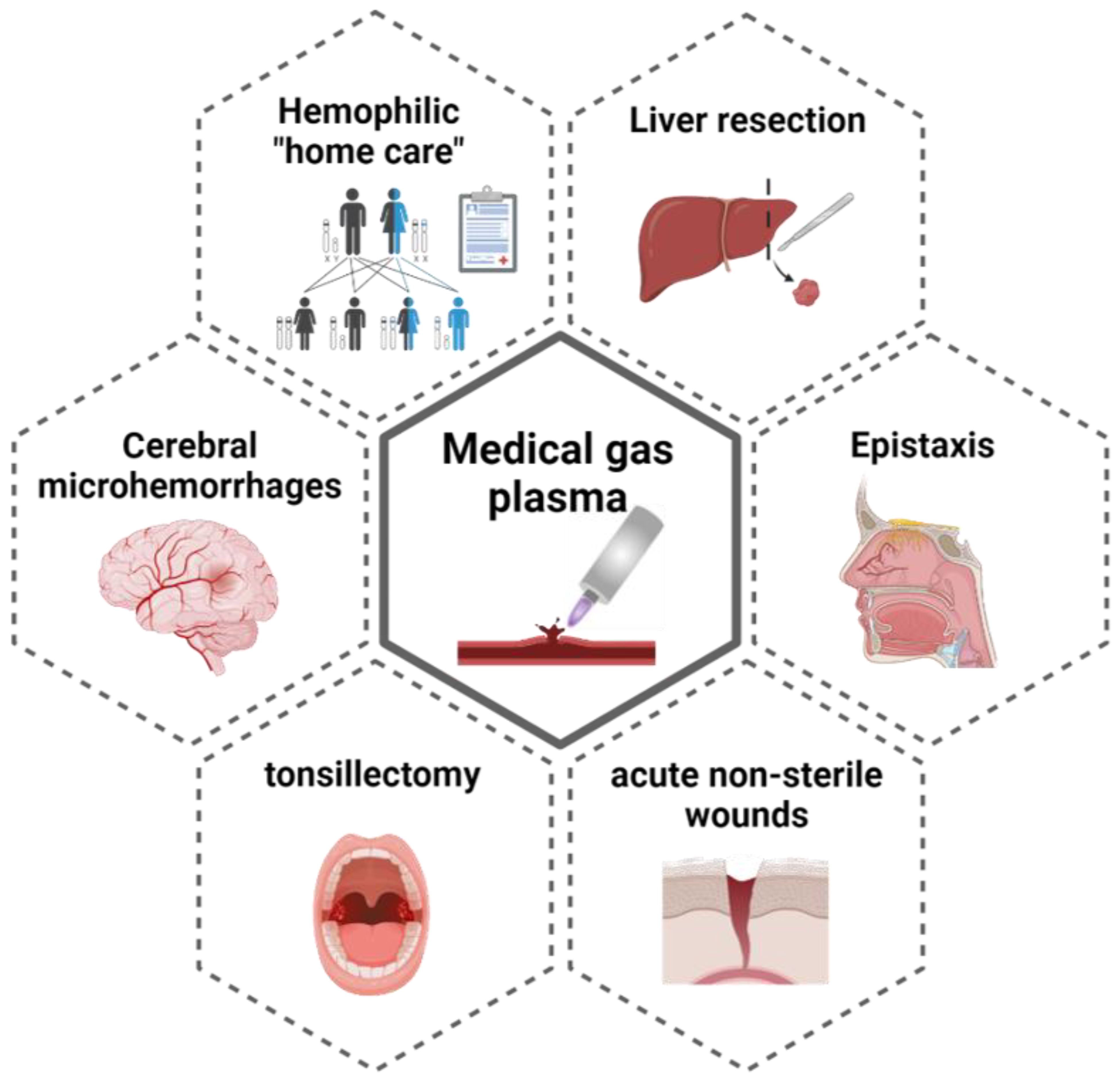
4. Application Fields and Clinical Obstacles
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Ref. | Plasma Source | Sample | Species | Main Findings |
|---|---|---|---|---|
| Ex Vivo | ||||
| Fridman et al. (2005) [17] | DBD | - | H |
|
| Fridman et al. (2006) [70] | FE-DBD | PRP, spleen | H | suggested mechanism:
|
| Kalghatgi et al. (2007) [72] | FE-DBD | WB, fibrinogen solution | H |
|
| Chen-Yen et al. [105] (2009) | Plasma jet | WB, PPP, PRP | H |
|
| Dobrynin et al. (2011) [69] | FE-DBD, pin-to-hole spark discharge | skin wound | P |
|
| Baik et al. [83] (2012) | Plasma jet | WB | D |
|
| Miyamoto et al. (2016) [84] | Plasma-Jet (He), BPC-HP1, PN-110/120TPG | WB, isolated RBCs | H |
|
| Bekeschus et al. (2018) [71] | kINPen and kINPen MED (various feed gas admixtures) | WB | H |
suggested mechanism:
|
| Bekeschus et al. (2021) [24] | kINPen and kINPen MED | PRP, WB, BL | H | suggested mechanism:
|
| Jia et al. (2021) [106] | microsecond-pulsed helium plasma jet | WB, PRP | R | suggested mechanism:
|
| in vivo | ||||
| Fridman et al. (2008) [64] | FE-DBD | vena saphena/WB (anticoagulated) | M/H |
|
| Ikehara et al. (2013 [65]) | Plasma jet | femoral artery | M |
|
| Ikehara et al. (2015) | Plasma jet | proteins in buffer solution, skin wound | M |
|
| Aleinik et al. (2017) [66] | DBD | hepatectomy | R |
|
| Nomura et al. (2017) [107] | plasma jet, various gas admixtures | WB, gastric, and liver incision | H/P |
|
| Bekeschus et al. (2017) [8] | kINPen MED | WB, liver incision model | M |
|
| Aleinik et al. (2018) [67] | DBD | splenic surgery | Rb |
|
| Yan et al. (2018) [108] | microsecond-pulsed helium plasma jet | WB, hepatectomy | R |
|
| Rad et al. (2018) [109] | helium plasma jet | liver incision model | M |
|
References
- Porte, R. The risk of bleeding during liver surgery and liver transplantation and effect on outcome. Bloodline Rev. 2001, 1, 14–15. [Google Scholar]
- Nesbakken, A.; Nygaard, K.; Westerheim, O.; Lunde, O.C.; Mala, T. Audit of intraoperative and early postoperative complications after introduction of mesorectal excision for rectal cancer. Eur. J. Surg. 2002, 168, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Copeland, G.P.; Jones, D.; Walters, M. Possum: A scoring system for surgical audit. Br. J. Surg. 1991, 78, 355–360. [Google Scholar] [CrossRef]
- Boyd, C.R.; Tolson, M.A.; Copes, W.S. Evaluating trauma care: The triss method. Trauma score and the injury severity score. J. Trauma 1987, 27, 370–378. [Google Scholar] [PubMed]
- Marietta, M.; Facchini, L.; Pedrazzi, P.; Busani, S.; Torelli, G. Pathophysiology of bleeding in surgery. Transplant. Proc. 2006, 38, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Lynn, M.; Jeroukhimov, I.; Klein, Y.; Martinowitz, U. Updates in the management of severe coagulopathy in trauma patients. Intensive Care Med. 2002, 28 (Suppl. S2), S241–S247. [Google Scholar] [CrossRef] [PubMed]
- Raiser, J.; Zenker, M. Argon plasma coagulation for open surgical and endoscopic applications: State of the art. J. Phys. D Appl. Phys. 2006, 39, 3520–3523. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. Platelets are key in cold physical plasma-facilitated blood coagulation in mice. Clin. Plas. Med. 2017, 7–8, 58–65. [Google Scholar] [CrossRef]
- Carus, T.; Rackebrandt, K. Collateral tissue damage by several types of coagulation (monopolar, bipolar, cold plasma and ultrasonic) in a minimally invasive, perfused liver model. ISRN Surg. 2011, 2011, 518924. [Google Scholar] [CrossRef] [Green Version]
- Castell, D.O. Consensus statement on therapeutic endoscopy and bleeding ulcers. Gastrointest. Endosc. 1990, 36, S62–S65. [Google Scholar]
- Lawson, J.H.; Murphy, M.P. Challenges for providing effective hemostasis in surgery and trauma. Semin. Hematol. 2004, 41, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mussbacher, M.; Kral-Pointner, J.B.; Salzmann, M.; Schrottmaier, W.C.; Assinger, A. Mechanisms of hemostasis: Contributions of platelets, coagulation factors, and the vessel wall. In Fundamentals of Vascular Biology; Geiger, M., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 145–169. [Google Scholar]
- Valsami, S.; Asmis, L.M. A brief review of 50 years of perioperative thrombosis and hemostasis management. Semin. Hematol. 2013, 50, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.H.; Sim, E.H.; Goh, R.Y.; Park, J.I.; Han, J.Y. Platelet activation: The mechanisms and potential biomarkers. Biomed. Res. Int. 2016, 2016, 9060143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzzetta, N.A.; Miller, B.E. Principles of hemostasis in children: Models and maturation. Paediatr. Anaesth. 2011, 21, 3–9. [Google Scholar] [CrossRef]
- Mor-Cohen, R. Disulfide bonds as regulators of integrin function in thrombosis and hemostasis. Antioxid. Redox Signal. 2016, 24, 16–31. [Google Scholar] [CrossRef]
- Fridman, G.; Peddinghaus, M.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Friedman, G. Use of Non-Thermal Atmospheric Pressure Plasma Discharge for Coagulation and Sterilization of Surface Wounds. In Proceedings of the 32nd IEEE International Conference on Plasma Science, Traverse City, MI, USA, 4–8 June 2005; p. 257. [Google Scholar]
- Langmuir, I. Oscillations in ionized gases. Proc. Natl. Acad. Sci. USA 1928, 14, 627–637. [Google Scholar] [CrossRef] [Green Version]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kinpen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]
- Privat-Maldonado, A.; Schmidt, A.; Lin, A.; Weltmann, K.D.; Wende, K.; Bogaerts, A.; Bekeschus, S. Ros from physical plasmas: Redox chemistry for biomedical therapy. Oxid. Med. Cell. Longev. 2019, 2019, 9062098. [Google Scholar] [CrossRef] [Green Version]
- Von Woedtke, T.; Schmidt, A.; Bekeschus, S.; Wende, K.; Weltmann, K.D. Plasma medicine: A field of applied redox biology. In Vivo 2019, 33, 1011–1026. [Google Scholar] [CrossRef] [Green Version]
- Graves, D.B. Low temperature plasma biomedicine: A tutorial review. Phys. Plasmas 2014, 21, 080901. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Poschkamp, B.; van der Linde, J. Medical gas plasma promotes blood coagulation via platelet activation. Biomaterials 2021, 278, 120433. [Google Scholar] [CrossRef] [PubMed]
- Scurfield, G.; Radley, J.M. Aspects of platelet formation and release. Am. J. Hematol. 1981, 10, 285–296. [Google Scholar] [CrossRef]
- Lefrancais, E.; Ortiz-Munoz, G.; Caudrillier, A.; Mallavia, B.; Liu, F.; Sayah, D.M.; Thornton, E.E.; Headley, M.B.; David, T.; Coughlin, S.R.; et al. The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature 2017, 544, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Lebois, M.; Josefsson, E.C. Regulation of platelet lifespan by apoptosis. Platelets 2016, 27, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vieira-de-Abreu, A.; Campbell, R.A.; Weyrich, A.S.; Zimmerman, G.A. Platelets: Versatile effector cells in hemostasis, inflammation, and the immune continuum. Semin. Immunopathol. 2012, 34, 5–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.G.; Krumwiede, M.; Burris, S.M.; Heagan, B. Isolation of microtubule coils from platelets after exposure to aggregating agents. Am. J. Pathol. 1986, 125, 319–326. [Google Scholar]
- White, J.G.; Clawson, C.C. The surface-connected canalicular system of blood platelets—A fenestrated membrane system. Am. J. Pathol. 1980, 101, 353–364. [Google Scholar]
- White, J.G. Platelets are covercytes, not phagocytes: Uptake of bacteria involves channels of the open canalicular system. Platelets 2005, 16, 121–131. [Google Scholar] [CrossRef]
- Rosado, J.A. Acidic ca(2+) stores in platelets. Cell Calcium 2011, 50, 168–174. [Google Scholar] [CrossRef]
- Farndale, R.W.; Sixma, J.J.; Barnes, M.J.; de Groot, P.G. The role of collagen in thrombosis and hemostasis. J. Thromb. Haemost. 2004, 2, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Di Stasio, E.; De Cristofaro, R. The effect of shear stress on protein conformation: Physical forces operating on biochemical systems: The case of von willebrand factor. Biophys. Chem. 2010, 153, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, C.A.; Lestini, B.J.; Kottke-Marchant, K.K.; Eppell, S.J.; Wilson, D.L.; Marchant, R.E. Shear-dependent changes in the three-dimensional structure of human von willebrand factor. Blood 1996, 88, 2939–2950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reininger, A.J.; Heijnen, H.F.; Schumann, H.; Specht, H.M.; Schramm, W.; Ruggeri, Z.M. Mechanism of platelet adhesion to von willebrand factor and microparticle formation under high shear stress. Blood 2006, 107, 3537–3545. [Google Scholar] [CrossRef]
- Tomaiuolo, M.; Brass, L.F.; Stalker, T.J. Regulation of platelet activation and coagulation and its role in vascular injury and arterial thrombosis. Interv. Cardiol. Clin. 2017, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Clemetson, K.J.; Clemetson, J.M. Platelet receptor signalling. Hematol. J. 2004, 5 (Suppl. S3), S159–S163. [Google Scholar] [CrossRef]
- Hollopeter, G.; Jantzen, H.M.; Vincent, D.; Li, G.; England, L.; Ramakrishnan, V.; Yang, R.B.; Nurden, P.; Nurden, A.; Julius, D.; et al. Identification of the platelet adp receptor targeted by antithrombotic drugs. Nature 2001, 409, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Ayyanathan, K.; Webbs, T.E.; Sandhu, A.K.; Athwal, R.S.; Barnard, E.A.; Kunapuli, S.P. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem. Biophys. Res. Commun. 1996, 218, 783–788. [Google Scholar] [CrossRef]
- Hirata, M.; Hayashi, Y.; Ushikubi, F.; Yokota, Y.; Kageyama, R.; Nakanishi, S.; Narumiya, S. Cloning and expression of cdna for a human thromboxane A2 receptor. Nature 1991, 349, 617–620. [Google Scholar] [CrossRef]
- Xu, W.F.; Andersen, H.; Whitmore, T.E.; Presnell, S.R.; Yee, D.P.; Ching, A.; Gilbert, T.; Davie, E.W.; Foster, D.C. Cloning and characterization of human protease-activated receptor 4. Proc. Natl. Acad. Sci. USA 1998, 95, 6642–6646. [Google Scholar] [CrossRef] [Green Version]
- Stalker, T.J.; Newman, D.K.; Ma, P.; Wannemacher, K.M.; Brass, L.F. Platelet signaling. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; pp. 59–85. [Google Scholar] [CrossRef] [Green Version]
- Harper, M.T.; Poole, A.W. Diverse functions of protein kinase c isoforms in platelet activation and thrombus formation. J. Thromb. Haemost. 2010, 8, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Bergmeier, W.; Stefanini, L. Novel molecules in calcium signaling in platelets. J. Thromb. Haemost. 2009, 7 (Suppl. S1), 187–190. [Google Scholar] [CrossRef] [PubMed]
- Shattil, S.J.; Kim, C.; Ginsberg, M.H. The final steps of integrin activation: The end game. Nat. Rev. Mol. Cell Biol. 2010, 11, 288–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Delaney, M.K.; O’Brien, K.A.; Du, X. Signaling during platelet adhesion and activation. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 2341–2349. [Google Scholar] [CrossRef] [Green Version]
- Guidetti, G.F.; Canobbio, I.; Torti, M. Pi3k/akt in platelet integrin signaling and implications in thrombosis. Adv. Biol. Regul. 2015, 59, 36–52. [Google Scholar] [CrossRef]
- Pasquet, J.M.; Bobe, R.; Gross, B.; Gratacap, M.P.; Tomlinson, M.G.; Payrastre, B.; Watson, S.P. A collagen-related peptide regulates phospholipase cγ2 via phosphatidylinositol 3-kinase in human platelets. Biochem. J. 1999, 342, 171–177. [Google Scholar] [CrossRef]
- Hirsch, E.; Bosco, O.; Tropel, P.; Laffargue, M.; Calvez, R.; Altruda, F.; Wymann, M.; Montrucchio, G. Resistance to thromboembolism in pi3kγ-deficient mice. FASEB J. 2001, 15, 2019–2021. [Google Scholar] [CrossRef]
- Watanabe, N.; Nakajima, H.; Suzuki, H.; Oda, A.; Matsubara, Y.; Moroi, M.; Terauchi, Y.; Kadowaki, T.; Suzuki, H.; Koyasu, S.; et al. Functional phenotype of phosphoinositide 3-kinase p85α-null platelets characterized by an impaired response to gp vi stimulation. Blood 2003, 102, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Clemetson, K.J.; McGregor, J.L.; James, E.; Dechavanne, M.; Luscher, E.F. Characterization of the platelet membrane glycoprotein abnormalities in bernard-soulier syndrome and comparison with normal by surface-labeling techniques and high-resolution two-dimensional gel electrophoresis. J. Clin. Investig. 1982, 70, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Berndt, M.C.; Gregory, C.; Kabral, A.; Zola, H.; Fournier, D.; Castaldi, P.A. Purification and preliminary characterization of the glycoprotein ib complex in the human platelet membrane. Eur. J. Biochem. 1985, 151, 637–649. [Google Scholar] [CrossRef]
- Woulfe, D.S. Akt signaling in platelets and thrombosis. Expert Rev. Hematol. 2010, 3, 81–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, J.R., Jr. A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide 1997, 1, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Dangel, O.; Mergia, E.; Karlisch, K.; Groneberg, D.; Koesling, D.; Friebe, A. Nitric oxide-sensitive guanylyl cyclase is the only nitric oxide receptor mediating platelet inhibition. J. Thromb. Haemost. 2010, 8, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Xi, X.; Gu, M.; Feil, R.; Ye, R.D.; Eigenthaler, M.; Hofmann, F.; Du, X. A stimulatory role for cgmp-dependent protein kinase in platelet activation. Cell 2003, 112, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Smolenski, A. Novel roles of camp/cgmp-dependent signaling in platelets. J. Thromb. Haemost. 2012, 10, 167–176. [Google Scholar] [CrossRef] [Green Version]
- El-Daher, S.S.; Patel, Y.; Siddiqua, A.; Hassock, S.; Edmunds, S.; Maddison, B.; Patel, G.; Goulding, D.; Lupu, F.; Wojcikiewicz, R.J.; et al. Distinct localization and function of (1,4,5)ip(3) receptor subtypes and the (1,3,4,5)ip(4) receptor gap1(ip4bp) in highly purified human platelet membranes. Blood 2000, 95, 3412–3422. [Google Scholar] [CrossRef]
- Jantzen, H.M.; Milstone, D.S.; Gousset, L.; Conley, P.B.; Mortensen, R.M. Impaired activation of murine platelets lacking gα(i2). J. Clin. Investig. 2001, 108, 477–483. [Google Scholar] [CrossRef]
- Katsuyamaa, M.; Sugimotoa, Y.; Nambab, T.; Irie, A.; Negishi, M.; Narumiyab, S.; Ichikawa, A. Cloning and expression of a cdna for the human prostacyclin receptor. FEBS J. 1994, 344, 74–78. [Google Scholar] [CrossRef] [Green Version]
- Stassen, J.M.; Arnout, J.; Deckmyn, H. The hemostatic system. Curr. Med. Chem. 2004, 11, 2245–2260. [Google Scholar] [CrossRef]
- Revel-Vilk, S.; Rand, M.L.; Israels, S.J. Primary and secondary hemostasis, regulators of coagulation, and fibrinolysis: Understanding the basics. In SickKids Handbook of Pediatric Thrombosis and Hemostasis; Karger Publishers: Basel, Switzerland, 2013; pp. 5–13. [Google Scholar]
- Fridman, G.; Friedman, G.; Gutsol, A.; Shekhter, A.B.; Vasilets, V.N.; Fridman, A. Applied plasma medicine. Plasma Process. Polym. 2008, 5, 503–533. [Google Scholar] [CrossRef]
- Ikehara, Y.; Sakakita, H.; Shimizu, N.; Ikehara, S.; Nakanishi, H. Formation of membrane-like structures in clotted blood by mild plasma treatment during hemostasis. J. Photopolym. Sci. Technol. 2013, 26, 555–557. [Google Scholar] [CrossRef] [Green Version]
- Aleinik, A.; Baikov, A.; Dambaev, G.; Semichev, E.; Bushlanov, P. Liver hemostasis by using cold plasma. Surg. Innov. 2017, 24, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Aleinik, A.; Baikov, A.; Shevtsova, N.; Semichev, E.; Bushlanov, P.; Turgunova, N. Application of cold plasma for performing a typical resection of the spleen. Biomed. Phys. Eng. Express 2018, 4, 055026. [Google Scholar] [CrossRef]
- Ikehara, S.; Sakakita, H.; Ishikawa, K.; Akimoto, Y.; Yamaguchi, T.; Yamagishi, M.; Kim, J.; Ueda, M.; Ikeda, J.; Nakanishi, H.; et al. Plasma blood coagulation without involving the activation of platelets and coagulation factors. Plasma Process. Polym. 2015, 12, 1348–1353. [Google Scholar] [CrossRef]
- Dobrynin, D.; Wu, A.; Kalghatgi, S.; Park, S.; Shainsky, N.; Wasko, K.; Dumani, E.; Ownbey, R.; Joshi, S.; Sensenig, R.; et al. Live pig skin tissue and wound toxicity of cold plasma treatment. Plasma Med. 2011, 1, 93–108. [Google Scholar] [CrossRef] [Green Version]
- Fridman, G.; Peddinghaus, M.; Ayan, H.; Fridman, A.; Balasubramanian, M.; Gutsol, A.; Brooks, A.; Friedman, G. Blood coagulation and living tissue sterilization by floating-electrode dielectric barrier discharge in air. Plasma Chem. Plasma Process. 2006, 26, 425–442. [Google Scholar] [CrossRef]
- Bekeschus, S.; Brüggemeier, J.; Hackbarth, C.; Weltmann, K.-D.; von Woedtke, T.; Partecke, L.-I.; van der Linde, J. The feed gas composition determines the degree of physical plasma-induced platelet activation for blood coagulation. Plasma Sources Sci. Technol. 2018, 27, 034001. [Google Scholar] [CrossRef]
- Kalghatgi, S.U.; Fridman, G.; Cooper, M.; Nagaraj, G.; Peddinghaus, M.; Balasubramanian, M.; Vasilets, V.N.; Gutsol, A.F.; Fridman, A.; Friedman, G. Mechanism of blood coagulation by nonthermal atmospheric pressure dielectric barrier discharge plasma. IEEE Trans. Plasma Sci. 2007, 35, 1559–1566. [Google Scholar] [CrossRef]
- Winter, J.; Wende, K.; Masur, K.; Iseni, S.; Dunnbier, M.; Hammer, M.U.; Tresp, H.; Weltmann, K.D.; Reuter, S. Feed gas humidity: A vital parameter affecting a cold atmospheric-pressure plasma jet and plasma-treated human skin cells. J. Phys. D Appl. Phys. 2013, 46, 295401. [Google Scholar] [CrossRef]
- Wende, K.; Williams, P.; Dalluge, J.; Gaens, W.V.; Aboubakr, H.; Bischof, J.; von Woedtke, T.; Goyal, S.M.; Weltmann, K.D.; Bogaerts, A.; et al. Identification of the biologically active liquid chemistry induced by a nonthermal atmospheric pressure plasma jet. Biointerphases 2015, 10, 029518. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Schmidt, A.; Niessner, F.; Gerling, T.; Weltmann, K.D.; Wende, K. Basic research in plasma medicine—A throughput approach from liquids to cells. J. Vis. Exp. 2017, e56331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvemini, D.; de Nucci, G.; Sneddon, J.M.; Vane, J.R. Superoxide anions enhance platelet adhesion and aggregation. Br. J. Pharmacol. 1989, 97, 1145–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bocci, V.; Valacchi, G.; Rossi, R.; Giustarini, D.; Paccagnini, E.; Pucci, A.M.; Simplicio, P.D. Studies on the biological effects of ozone: 9. Effects of ozone on human platelets. Platelets 2010, 10, 110–116. [Google Scholar] [CrossRef]
- Iseni, S.; Zhang, S.; van Gessel, A.F.H.; Hofmann, S.; van Ham, B.T.J.; Reuter, S.; Weltmann, K.D.; Bruggeman, P.J. Nitric oxide density distributions in the effluent of an RF argon APPJ: Effect of gas flow rate and substrate. New J. Phys. 2014, 16, 123011. [Google Scholar] [CrossRef] [Green Version]
- Leo, F.; Hutzler, B.; Ruddiman, C.A.; Isakson, B.E.; Cortese-Krott, M.M. Cellular microdomains for nitric oxide signaling in endothelium and red blood cells. Nitric Oxide 2020, 96, 44–53. [Google Scholar] [CrossRef]
- Lackmann, J.W.; Bruno, G.; Jablonowski, H.; Kogelheide, F.; Offerhaus, B.; Held, J.; Schulz-von der Gathen, V.; Stapelmann, K.; von Woedtke, T.; Wende, K. Nitrosylation vs. Oxidation—How to modulate cold physical plasmas for biological applications. PLoS ONE 2019, 14, e0216606. [Google Scholar] [CrossRef] [Green Version]
- Gow, A.J.; Luchsinger, B.P.; Pawloski, J.R.; Singel, D.J.; Stamler, J.S. The oxyhemoglobin reaction of nitric oxide. Proc. Natl. Acad. Sci. USA 1999, 96, 9027–9032. [Google Scholar] [CrossRef] [Green Version]
- Ki, S.H.; Sin, S.; Shin, J.H.; Kwon, Y.W.; Chae, M.W.; Uhm, H.S.; Baik, K.Y.; Choi, E.H. Hemoglobin as a diagnosing molecule for biological effects of atmospheric-pressure plasma. Plasma Chem. Plasma Process. 2018, 38, 937–952. [Google Scholar] [CrossRef]
- Baik, K.Y.; Kim, Y.H.; Hur, E.-H. Selective toxicity on canine blood cells by using atmospheric-pressure plasma jets. J. Korean Phys. Soc. 2012, 60, 965–969. [Google Scholar] [CrossRef]
- Miyamoto, K.; Ikehara, S.; Takei, H.; Akimoto, Y.; Sakakita, H.; Ishikawa, K.; Ueda, M.; Ikeda, J.; Yamagishi, M.; Kim, J.; et al. Red blood cell coagulation induced by low-temperature plasma treatment. Arch. Biochem. Biophys. 2016, 605, 95–101. [Google Scholar] [CrossRef]
- Helms, C.C.; Marvel, M.; Zhao, W.; Stahle, M.; Vest, R.; Kato, G.J.; Lee, J.S.; Christ, G.; Gladwin, M.T.; Hantgan, R.R.; et al. Mechanisms of hemolysis-associated platelet activation. J. Thromb. Haemost. 2013, 11, 2148–2154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonca, R.; Silveira, A.A.; Conran, N. Red cell damps and inflammation. Inflamm. Res. 2016, 65, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kunapuli, S.P. P2y12 receptor in platelet activation. Platelets 2011, 22, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Savi, P.; Herbert, J.M. Clopidogrel and ticlopidine: P2y12 adenosine diphosphate-receptor antagonists for the prevention of atherothrombosis. Semin. Thromb. Hemost. 2005, 31, 174–183. [Google Scholar] [CrossRef]
- Sapey, E.; Greenwood, H.; Walton, G.; Mann, E.; Love, A.; Aaronson, N.; Insall, R.H.; Stockley, R.A.; Lord, J.M. Phosphoinositide 3-kinase inhibition restores neutrophil accuracy in the elderly: Toward targeted treatments for immunosenescence. Blood 2014, 123, 239–248. [Google Scholar] [CrossRef] [Green Version]
- Cardenes, N.; Corey, C.; Geary, L.; Jain, S.; Zharikov, S.; Barge, S.; Novelli, E.M.; Shiva, S. Platelet bioenergetic screen in sickle cell patients reveals mitochondrial complex v inhibition, which contributes to platelet activation. Blood 2014, 123, 2864–2872. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, C.S.; Limm, W.M.; Lurie, F.; Wong, L.L. Factors affecting outcome in liver resection. HPB 2005, 7, 226–230. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.C.; Dworkin-Valenti, J.; Chiodo, L.; Haupert, M. Postoperative tonsillectomy bleeding complications in children: A comparison of three surgical techniques. Int. J. Pediatr. Otorhinolaryngol. 2016, 88, 184–188. [Google Scholar] [CrossRef]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kinpen—A powerful tool for wound healing. Clin. Plas. Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Isbary, G.; Morfill, G.; Schmidt, H.U.; Georgi, M.; Ramrath, K.; Heinlin, J.; Karrer, S.; Landthaler, M.; Shimizu, T.; Steffes, B.; et al. A first prospective randomized controlled trial to decrease bacterial load using cold atmospheric argon plasma on chronic wounds in patients. Br. J. Dermatol. 2010, 163, 78–82. [Google Scholar] [CrossRef]
- Metelmann, H.-R.; Vu, T.T.; Do, H.T.; Le, T.N.B.; Hoang, T.H.A.; Phi, T.T.T.; Luong, T.M.L.; Doan, V.T.; Nguyen, T.T.H.; Nguyen, T.H.M.; et al. Scar formation of laser skin lesions after cold atmospheric pressure plasma (cap) treatment: A clinical long term observation. Clin. Plas. Med. 2013, 1, 30–35. [Google Scholar] [CrossRef]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One year follow-up risk assessment in skh-1 mice and wounds treated with an argon plasma jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Van der Linde, J.; Liedtke, K.R.; Matthes, R.; Kramer, A.; Heidecke, C.-D.; Partecke, L.I. Repeated cold atmospheric plasma application to intact skin does not cause sensitization in a standardized murine model. Plasma Med. 2017, 7, 383–393. [Google Scholar] [CrossRef]
- Bekeschus, S.; Rödder, K.; Schmidt, A.; Stope, M.B.; von Woedtke, T.; Miller, V.; Fridman, A.; Weltmann, K.-D.; Masur, K.; Metelmann, H.-R.; et al. Cold physical plasma selects for specific t helper cell subsets with distinct cells surface markers in a caspase-dependent and nf-κb-independent manner. Plasma Process. Polym. 2016, 13, 1144–1150. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Kramer, A.; Weltmann, K.-D.; Masur, K. Cold physical plasma treatment alters redox balance in human immune cells. Plasma Med. 2013, 3, 267–278. [Google Scholar] [CrossRef]
- Arndt, S.; Schmidt, A.; Karrer, S.; von Woedtke, T. Comparing two different plasma devices kinpen and adtec steriplas regarding their molecular and cellular effects on wound healing. Clin. Plas. Med. 2018, 9, 24–33. [Google Scholar] [CrossRef]
- Bastin, O.; Thulliez, M.; Servais, J.; Nonclercq, A.; Delchambre, A.; Hadefi, A.; Deviere, J.; Reniers, F. Optical and electrical characteristics of an endoscopic dbd plasma jet. Plasma Med. 2020, 10, 71–90. [Google Scholar] [CrossRef]
- Winter, J.; Nishime, T.M.C.; Glitsch, S.; Luhder, H.; Weltmann, K.D. On the development of a deployable cold plasma endoscope. Contrib. Plasma Phys. 2018, 58, 404–414. [Google Scholar] [CrossRef]
- Zuo, X.; Wei, Y.; Chen, L.W.; Meng, Y.D.; Team, P.M. Non-equilibrium atmospheric pressure microplasma jet: An approach to endoscopic therapies. Phys. Plasmas 2013, 20, 083507. [Google Scholar] [CrossRef]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ros and rns delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef]
- Cheng-Yen, C.; Hsin-Wen, F.; Kuo, S.P.; Jenghwa, C.; Pedersen, T.; Mills, T.J.; Cheng-Chiu, H. Blood clotting by low-temperature air plasma. IEEE Trans. Plasma Sci. 2009, 37, 993–999. [Google Scholar] [CrossRef]
- Jia, B.; Liu, J.; Yin, S.; Liu, Z.; Zheng, S.; Yan, K. Low temperature plasma treatment of rat blood is accompanied by platelet aggregation. Plasma Chem. Plasma Process. 2021, 41, 955–972. [Google Scholar] [CrossRef]
- Nomura, Y.; Takamatsu, T.; Kawano, H.; Miyahara, H.; Okino, A.; Yoshida, M.; Azuma, T. Investigation of blood coagulation effect of nonthermal multigas plasma jet in vitro and in vivo. J. Surg. Res. 2017, 219, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.P.; Jin, Q.K.; Zheng, C.; Deng, G.L.; Yin, S.Y.; Liu, Z. Pulsed cold plasma-induced blood coagulation and its pilot application in stanching bleeding during rat hepatectomy. Plasma Sci. Technol. 2018, 20, 044005. [Google Scholar] [CrossRef] [Green Version]
- Shahbazi Rad, Z.; Abbasi Davani, F.; Etaati, G. Determination of proper treatment time for in vivo blood coagulation and wound healing application by non-thermal helium plasma jet. Australas. Phys. Eng. Sci. Med. 2018, 41, 905–917. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miebach, L.; Poschkamp, B.; van der Linde, J.; Bekeschus, S. Medical Gas Plasma—A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications. Appl. Sci. 2022, 12, 3800. https://doi.org/10.3390/app12083800
Miebach L, Poschkamp B, van der Linde J, Bekeschus S. Medical Gas Plasma—A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications. Applied Sciences. 2022; 12(8):3800. https://doi.org/10.3390/app12083800
Chicago/Turabian StyleMiebach, Lea, Broder Poschkamp, Julia van der Linde, and Sander Bekeschus. 2022. "Medical Gas Plasma—A Potent ROS-Generating Technology for Managing Intraoperative Bleeding Complications" Applied Sciences 12, no. 8: 3800. https://doi.org/10.3390/app12083800






