The Statistical Error Optimization of Dye Sorption Equilibria for the Precise Prediction of Adsorption Isotherms on Activated Graphene
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Batch Adsorption
2.3. Isotherm Model Fitting
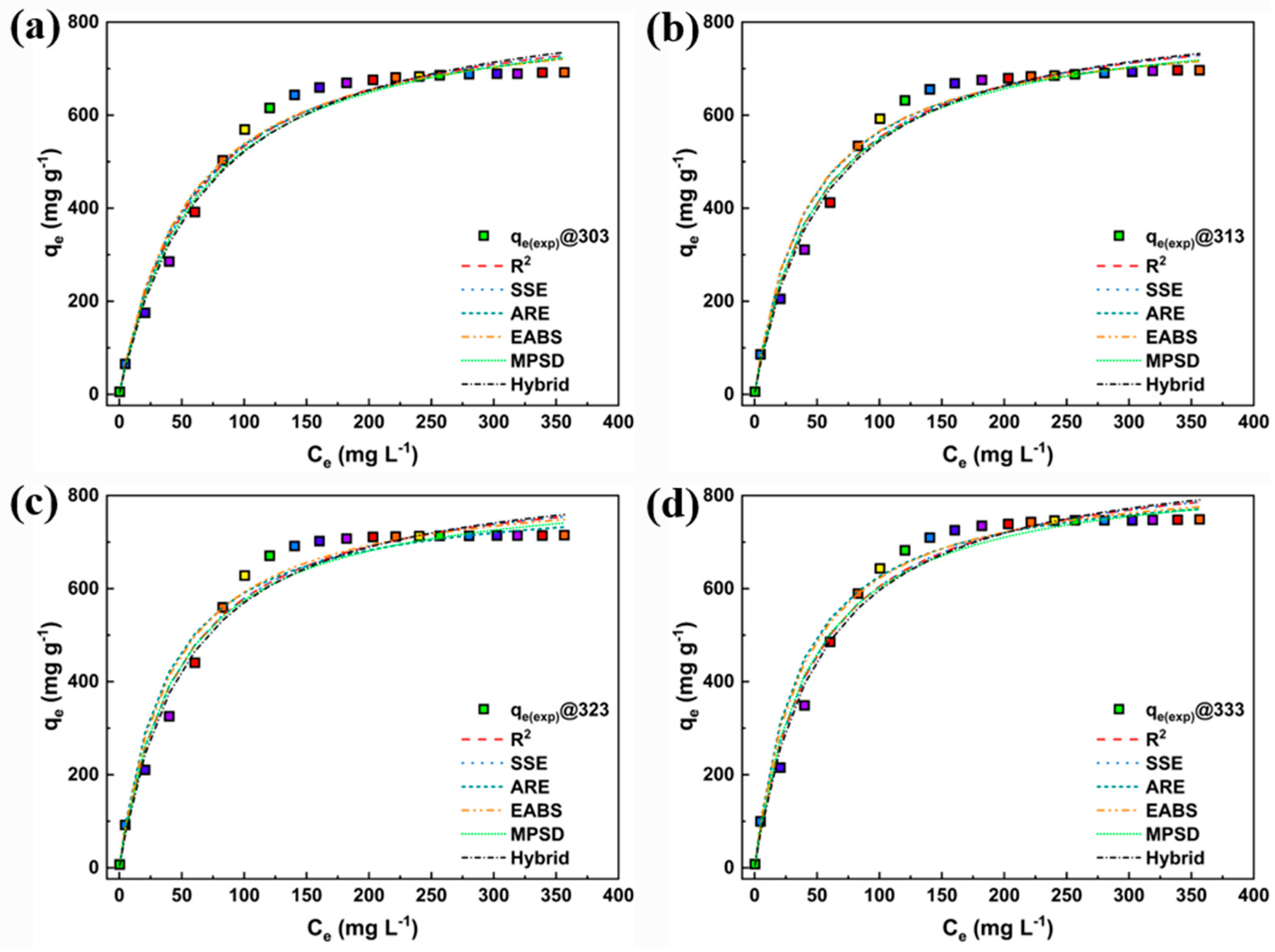
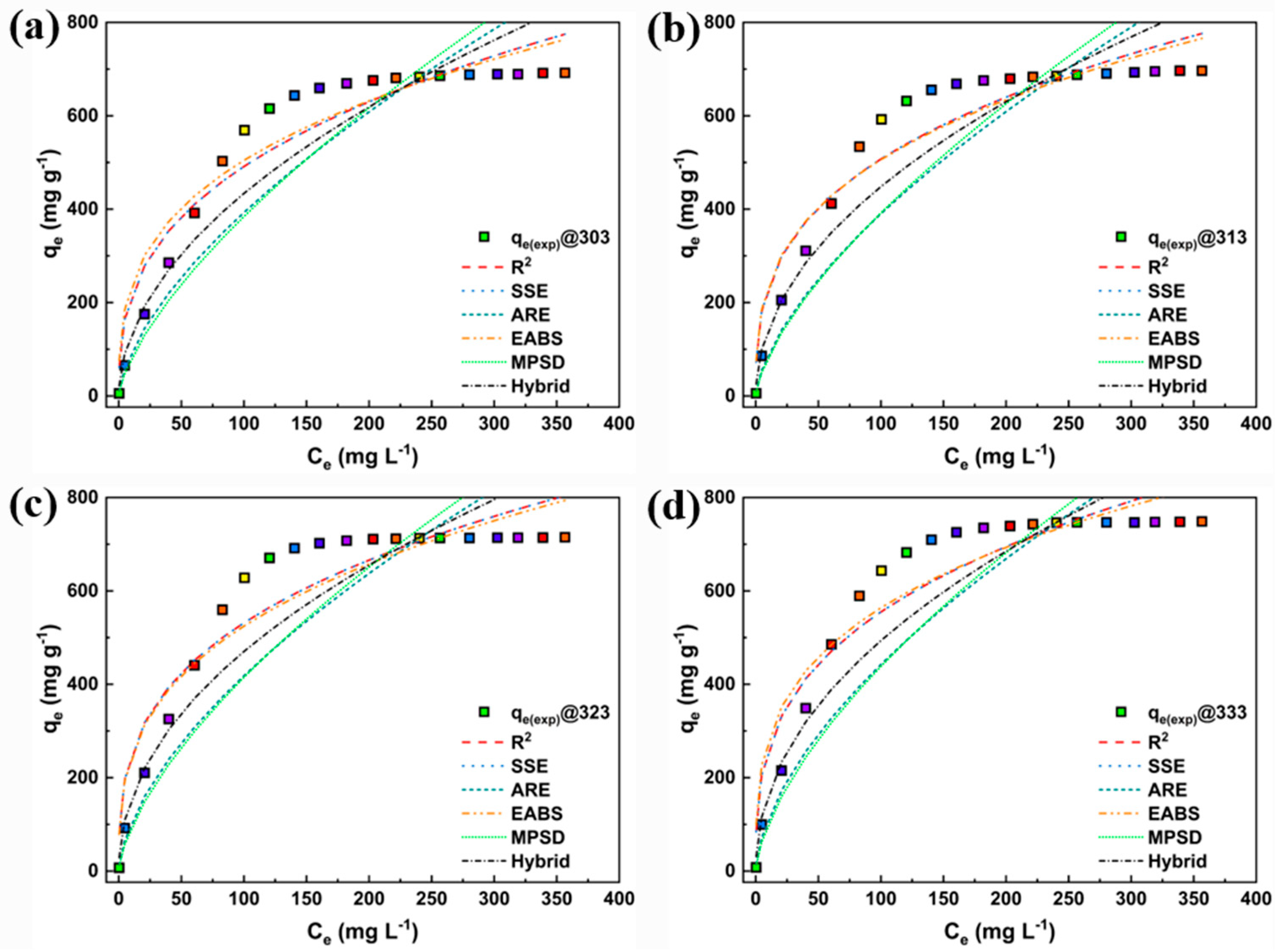
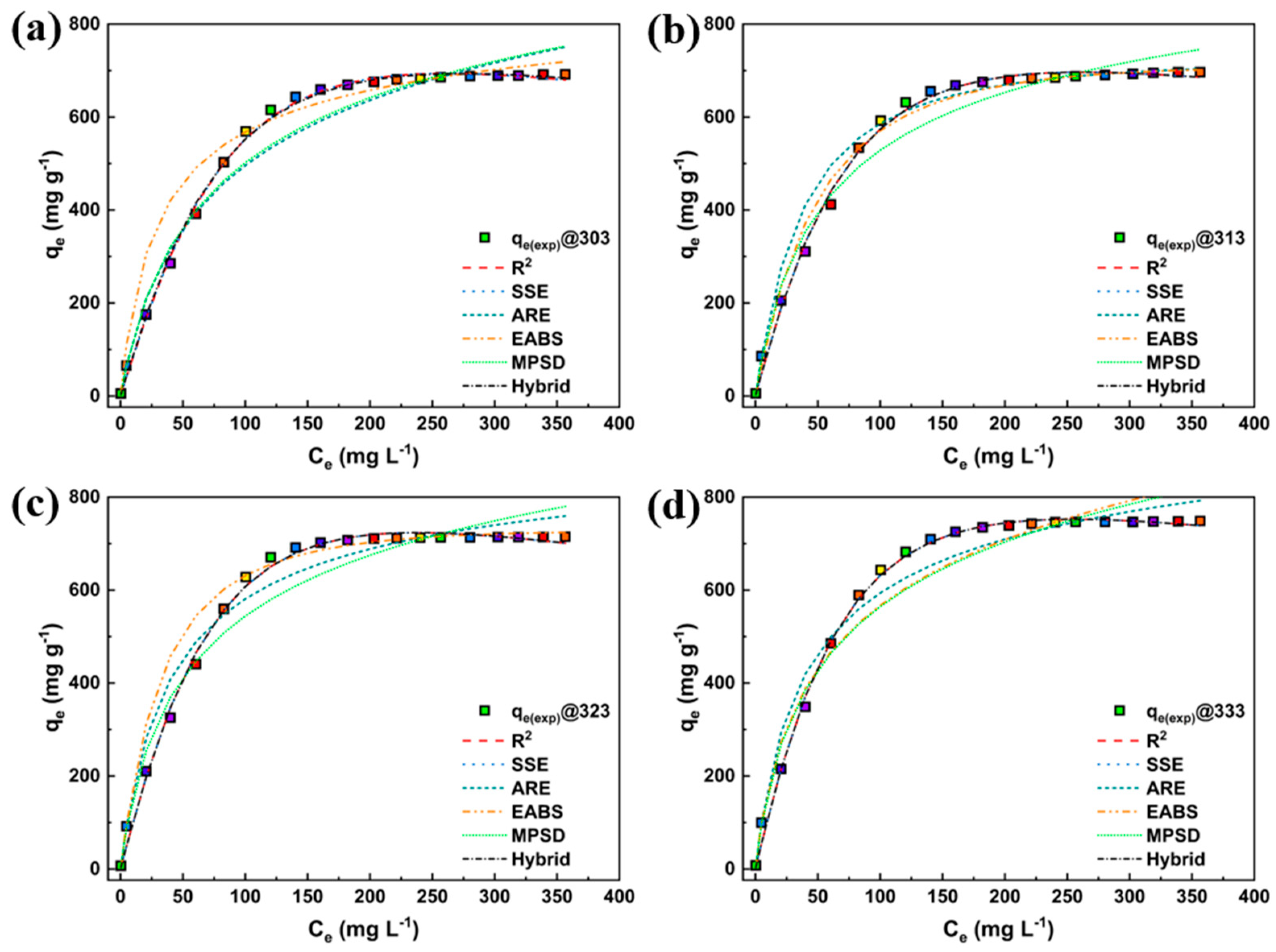
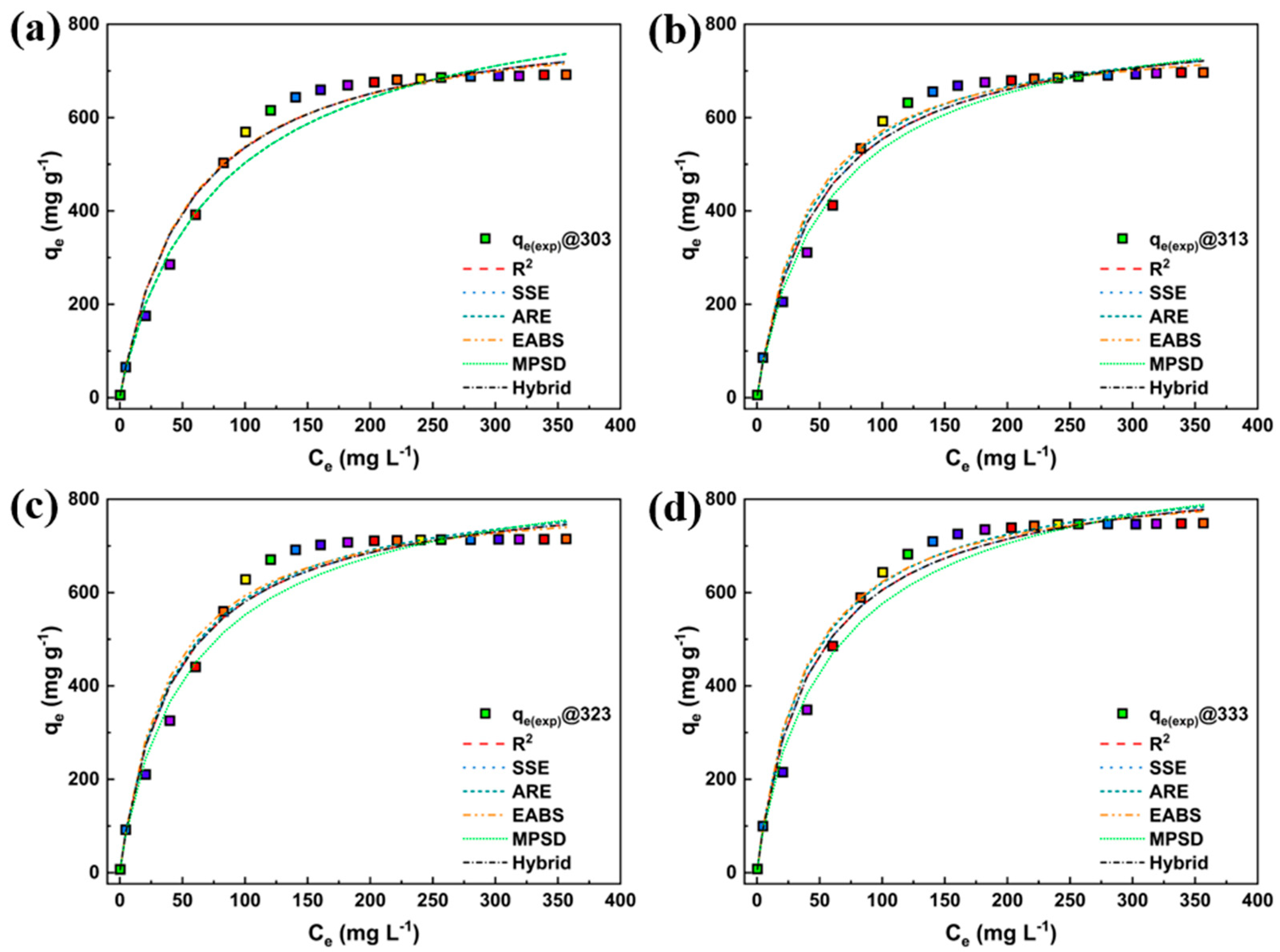
3. Results and Discussion
3.1. Material Characterization
3.2. Adsorption Study
3.3. Linear Fitting
3.4. Nonlinear Fitting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AG | Activated graphene |
| ARE | Average relative error |
| AT | Temkin isotherm equilibrium binding constant |
| Ce | Adsorbate concentration at equilibrium |
| CND | Coefficient of non-determination |
| EABS | Sum of absolute errors |
| FESEM | Field emission scanning electron microscopy |
| FTIR | Fourier transform infrared spectroscopy |
| HYBRID | Hybrid fractional error function |
| KF | Freundlich isotherm constant |
| KL | Langmuir affinity constant |
| KT | Toth isotherm constant |
| MPSD | Marquardt’s percent standard deviation |
| n | Freundlich isotherm adsorption intensity |
| qe | Sorption capacity at equilibrium |
| qm | Maximum adsorption capacity |
| SNE | Sum of normalized errors |
| SSE | Sum of squared errors |
| T | Temperature in Kelvin |
| UV-vis | Ultraviolet–visible spectroscopy |
| XRD | X-ray diffraction |
| nT | Toth isotherm heterogeneity factor |
References
- Chen, C.; Zhang, X.; Deb, H.; Liang, F.; Zhu, H.; Cui, K.; Zhang, Y.; Yao, J. Synthesis of an Eco-friendly Bamboo Cellulose-grafted-ployacrylamide Flocculant and Its Flocculation Performance on Papermaking Wastewater. Fibers Polym. 2021, 22, 1518–1525. [Google Scholar] [CrossRef]
- Ranau, R.; Steinhart, H. Identification and evaluation of volatile odor-active pollutants from different odor emission sources in the food industry. Eur. Food Res. Technol. 2005, 220, 226–231. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jadhav, A.J.; Pinjari, D.V.; Mahamuni, N.M.; Pandit, A.B. A critical review on textile wastewater treatments: Possible approaches. J. Environ. Manag. 2016, 182, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Paluri, P.; Ahmad, K.A.; Durbha, K.S. Importance of estimation of optimum isotherm model parameters for adsorption of methylene blue onto biomass derived activated carbons: Comparison between linear and non-linear methods. Biomass Convers. Biorefinery 2022, 12, 4031–4048. [Google Scholar] [CrossRef]
- Xie, H.; Xiong, X. A porous molybdenum disulfide and reduced graphene oxide nanocomposite (MoS2-rGO) with high adsorption capacity for fast and preferential adsorption towards Congo red. J. Environ. Chem. Eng. 2017, 5, 1150–1158. [Google Scholar] [CrossRef]
- Tanzifi, M.; Tavakkoli Yaraki, M.; Karami, M.; Karimi, S.; Dehghani Kiadehi, A.; Karimipour, K.; Wang, S. Modelling of dye adsorption from aqueous solution on polyaniline/carboxymethyl cellulose/TiO2 nanocomposites. J. Colloid Interface Sci. 2018, 519, 154–173. [Google Scholar] [CrossRef]
- Deb, H.; Xiao, S.; Morshed, M.N.; Al Azad, S. Immobilization of Cationic Titanium Dioxide (TiO2+) on Electrospun Nanofibrous Mat: Synthesis, Characterization, and Potential Environmental Application. Fibers Polym. 2018, 19, 1715–1725. [Google Scholar] [CrossRef]
- Raffi, M.; Batool, Z.; Ahmad, M.; Zakria, M.; Shakoor, R.I.; Mirza, M.A.; Mahmood, A. Synthesis of Ag-Loaded TiO2 Electrospun Nanofibers for Photocatalytic Decolorization of Methylene Blue. Fibers Polym. 2018, 19, 1930–1939. [Google Scholar] [CrossRef]
- Deb, H.; Islam, M.Z.; Ahmed, A.; Hasan, M.K.; Hossain, M.K.; Hu, H.; Chen, C.; Yang, S.; Zhang, Y.; Yao, J. Kinetics & dynamic studies of dye adsorption by porous graphene nano-adsorbent for facile toxic wastewater remediation. J. Water Process Eng. 2022, 47, 102818. [Google Scholar] [CrossRef]
- Deb, H.; Morshed, M.N.; Xiao, S.; Azad, S.A.; Cai, Z.; Ahmed, A. Design and development of TiO2-Fe0 nanoparticle-immobilized nanofibrous mat for photocatalytic degradation of hazardous water pollutants. J. Mater. Sci. Mater. Electron. 2019, 30, 4842–4854. [Google Scholar] [CrossRef]
- Morshed, M.N.; Al Azad, S.; Deb, H.; Shaun, B.B.; Shen, X.L. Titania-loaded cellulose-based functional hybrid nanomaterial for photocatalytic degradation of toxic aromatic dye in water. J. Water Process Eng. 2020, 33, 101062. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, P.; Chen, J.; Zhang, R.; Huang, J.; Xu, W.; Xiao, S. Immobilization of nZVI particles on cotton fibers for rapid decolorization of organic dyes. Cellulose 2021, 28, 7925–7940. [Google Scholar] [CrossRef]
- Mia, M.S.; Yao, P.; Zhu, X.; Lei, X.; Xing, T.; Chen, G. Degradation of textile dyes from aqueous solution using tea-polyphenol/Fe loaded waste silk fabrics as Fenton-like catalysts. RSC Adv. 2021, 11, 8290–8305. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Misra, R.; Kushwaha, P.; Das, P. Optimum Sorption Isotherm by Linear and Nonlinear Methods for Safranin onto Alkali-Treated Rice Husk. Bioremediation J. 2011, 15, 77–89. [Google Scholar] [CrossRef]
- Saad, M.; Tahir, H.; Mustafa, S.; Attala, O.A.; El-Saoud, W.A.; Attia, K.A.; Filfilan, W.M.; Zeb, J. Polyvinyl Alcohol Assisted Iron–Zinc Nanocomposite for Enhanced Optimized Rapid Removal of Malachite Green Dye. Nanomaterials 2023, 13, 1747. [Google Scholar]
- Tahir, H.; Saad, M.; Attala, O.A.; El-Saoud, W.A.; Attia, K.A.; Jabeen, S.; Zeb, J. Sustainable Synthesis of Iron—Zinc Nanocomposites by Azadirachta indica Leaves Extract for RSM-Optimized Sono-Adsorptive Removal of Crystal Violet Dye. Materials 2023, 16, 1023. [Google Scholar] [CrossRef]
- Gaber, D.; Abu Haija, M.; Eskhan, A.; Banat, F. Graphene as an Efficient and Reusable Adsorbent Compared to Activated Carbons for the Removal of Phenol from Aqueous Solutions. Water Air Soil Pollut. 2017, 228, 320. [Google Scholar] [CrossRef]
- Wang, J.; Chen, Z.; Chen, B. Adsorption of Polycyclic Aromatic Hydrocarbons by Graphene and Graphene Oxide Nanosheets. Environ. Sci. Technol. 2014, 48, 4817–4825. [Google Scholar] [CrossRef]
- Jiao, X.; Zhang, L.; Qiu, Y.; Guan, J. Comparison of the adsorption of cationic blue onto graphene oxides prepared from natural graphites with different graphitization degrees. Colloids Surf. A Physicochem. Eng. Asp. 2017, 529, 292–301. [Google Scholar] [CrossRef]
- Kong, Q.; Preis, S.; Li, L.; Luo, P.; Wei, C.; Li, Z.; Hu, Y.; Wei, C. Relations between metal ion characteristics and adsorption performance of graphene oxide: A comprehensive experimental and theoretical study. Sep. Purif. Technol. 2020, 232, 115956. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Osmari, T.A.; Gallon, R.; Schwaab, M.; Barbosa-Coutinho, E.; Severo, J.B.; Pinto, J.C. Statistical Analysis of Linear and Non-Linear Regression for the Estimation of Adsorption Isotherm Parameters. Adsorpt. Sci. Technol. 2013, 31, 433–458. [Google Scholar] [CrossRef]
- El-Khaiary, M.I. Least-squares regression of adsorption equilibrium data: Comparing the options. J. Hazard. Mater. 2008, 158, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Brdar, M.; Takaci, A.; Šćiban, M.; Rakić, D. Isotherms for the adsorption of Cu(II) onto lignin—Comparison of linear and non-linear methods. Hem. Ind. 2012, 66, 497–503. [Google Scholar] [CrossRef] [Green Version]
- Sahin, R.; Tapadia, K. Comparison of linear and non-linear models for the adsorption of fluoride onto geo-material: Limonite. Water Sci. Technol. 2015, 72, 2262–2269. [Google Scholar] [CrossRef]
- Watwe, V.; Bandal, G.; Kulkarni, P. Source-normalized error analysis method for accurate prediction of adsorption isotherm: Application to Cu(II) adsorption on PVA-blended alginate beads. J. Iran. Chem. Soc. 2023, 20, 949–959. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Comparison of Optimized Isotherm Models and Error Functions for Carbon Dioxide Adsorption on Activated Carbon. J. Chem. Eng. Data 2015, 60, 3148–3158. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017, 3039817. [Google Scholar] [CrossRef] [Green Version]
- Foo, K.Y.; Hameed, B.H. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium Studies of the Sorption of Cu(II) Ions onto Chitosan. J. Colloid Interface Sci. 2002, 255, 64–74. [Google Scholar] [CrossRef]
- Ng, J.C.Y.; Cheung, W.H.; McKay, G. Equilibrium studies for the sorption of lead from effluents using chitosan. Chemosphere 2003, 52, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Al-Bahri, T.A.; Al-Haddad, A. Adsorption of phenol based organic pollutants on activated carbon from multi-component dilute aqueous solutions. Water Res. 1997, 31, 2102–2112. [Google Scholar] [CrossRef]
- Chan, L.S.; Cheung, W.H.; Allen, S.J.; McKay, G. Error Analysis of Adsorption Isotherm Models for Acid Dyes onto Bamboo Derived Activated Carbon. Chin. J. Chem. Eng. 2012, 20, 535–542. [Google Scholar] [CrossRef]
- Marquardt, D.W. An Algorithm for Least-Squares Estimation of Nonlinear Parameters. J. Soc. Ind. Appl. Math. 1963, 11, 431–441. [Google Scholar] [CrossRef]
- Porter, J.F.; McKay, G.; Choy, K.H. The prediction of sorption from a binary mixture of acidic dyes using single- and mixed-isotherm variants of the ideal adsorbed solute theory. Chem. Eng. Sci. 1999, 54, 5863–5885. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Yang, T.C.-K.; Pan, G.-T.; Thangalazhy-Gopakumar, S.; Gan, S. Utilisation of eco-friendly and low cost 3D graphene-based composite for treatment of aqueous Reactive Black 5 dye: Characterisation, adsorption mechanism and recyclability studies. J. Taiwan Inst. Chem. Eng. 2020, 114, 57–66. [Google Scholar] [CrossRef]
- Nazar de Souza, A.P.; Licea, Y.E.; Colaço, M.V.; Senra, J.D.; Carvalho, N.M.F. Green iron oxides/amino-functionalized MCM-41 composites as adsorbent for anionic azo dye: Kinetic and isotherm studies. J. Environ. Chem. Eng. 2021, 9, 105062. [Google Scholar] [CrossRef]
- Lai, K.C.; Hiew, B.Y.Z.; Lee, L.Y.; Gan, S.; Thangalazhy-Gopakumar, S.; Chiu, W.S.; Khiew, P.S. Ice-templated graphene oxide/chitosan aerogel as an effective adsorbent for sequestration of metanil yellow dye. Bioresour. Technol. 2019, 274, 134–144. [Google Scholar] [CrossRef]
- Mallakpour, S.; Tabesh, F. Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int. J. Biol. Macromol. 2019, 133, 754–766. [Google Scholar] [CrossRef]
- Nebaghe, K.C.; El Boundati, Y.; Ziat, K.; Naji, A.; Rghioui, L.; Saidi, M. Comparison of linear and non-linear method for determination of optimum equilibrium isotherm for adsorption of copper(II) onto treated Martil sand. Fluid Phase Equilibria 2016, 430, 188–194. [Google Scholar] [CrossRef]
- Vilardi, G.; Di Palma, L.; Verdone, N. Heavy metals adsorption by banana peels micro-powder: Equilibrium modeling by non-linear models. Chin. J. Chem. Eng. 2018, 26, 455–464. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Isotherms and thermodynamics by linear and non-linear regression analysis for the sorption of methylene blue onto activated carbon: Comparison of various error functions. J. Hazard. Mater. 2008, 151, 794–804. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Din, A.T.M.; Yahaya, N.K.E.M.; Khasri, A.; Ahmad, M.A. Adsorption of basic green 4 onto gasified Glyricidia sepium woodchip based activated carbon: Optimization, characterization, batch and column study. Arab. J. Chem. 2020, 13, 6887–6903. [Google Scholar] [CrossRef]
- Pacakova, B.; Verhagen, T.; Bousa, M.; Hübner, U.; Vejpravova, J.; Kalbac, M.; Frank, O. Mastering the Wrinkling of Self-supported Graphene. Sci. Rep. 2017, 7, 10003. [Google Scholar] [CrossRef] [PubMed]
- Rethinasabapathy, M.; Kang, S.-M.; Jang, S.-C.; Huh, Y.S. Three-dimensional porous graphene materials for environmental applications. Carbon Lett. 2017, 22, 1–13. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef]
- Shin, H.-J.; Kim, K.K.; Benayad, A.; Yoon, S.-M.; Park, H.K.; Jung, I.-S.; Jin, M.H.; Jeong, H.-K.; Kim, J.M.; Choi, J.-Y.; et al. Efficient Reduction of Graphite Oxide by Sodium Borohydride and Its Effect on Electrical Conductance. Adv. Funct. Mater. 2009, 19, 1987–1992. [Google Scholar] [CrossRef]
- Wang, G.; Yang, J.; Park, J.; Gou, X.; Wang, B.; Liu, H.; Yao, J. Facile Synthesis and Characterization of Graphene Nanosheets. J. Phys. Chem. C 2008, 112, 8192–8195. [Google Scholar] [CrossRef]
- Jeong, H.K.; Noh, H.J.; Kim, J.Y.; Jin, M.H.; Park, C.Y.; Lee, Y.H. X-ray absorption spectroscopy of graphite oxide. Europhys. Lett. 2008, 82, 67004. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malard, L.M.; Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S. Raman spectroscopy in graphene. Phys. Rep. 2009, 473, 51–87. [Google Scholar] [CrossRef]
- Reina, A.; Jia, X.; Ho, J.; Nezich, D.; Son, H.; Bulovic, V.; Dresselhaus, M.S.; Kong, J. Large Area, Few-Layer Graphene Films on Arbitrary Substrates by Chemical Vapor Deposition. Nano Lett. 2009, 9, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bradder, P.; Ling, S.K.; Wang, S.; Liu, S. Dye Adsorption on Layered Graphite Oxide. J. Chem. Eng. Data 2011, 56, 138–141. [Google Scholar] [CrossRef]
- Mao, B.; Sidhureddy, B.; Thiruppathi, A.R.; Wood, P.C.; Chen, A. Efficient dye removal and separation based on graphene oxide nanomaterials. New J. Chem. 2020, 44, 4519–4528. [Google Scholar] [CrossRef]
- Li, Y.; Du, Q.; Liu, T.; Peng, X.; Wang, J.; Sun, J.; Wang, Y.; Wu, S.; Wang, Z.; Xia, Y.; et al. Comparative study of methylene blue dye adsorption onto activated carbon, graphene oxide, and carbon nanotubes. Chem. Eng. Res. Des. 2013, 91, 361–368. [Google Scholar] [CrossRef]
- Tu, T.H.; Cam, P.T.N.; Huy, L.V.T.; Phong, M.T.; Nam, H.M.; Hieu, N.H. Synthesis and application of graphene oxide aerogel as an adsorbent for removal of dyes from water. Mater. Lett. 2019, 238, 134–137. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Water-Soluble Dye Removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Mahesh, K.; Le, N.H.; Kemp, K.C.; Timilsina, R.; Tiwari, R.N.; Kim, K.S. Reduced graphene oxide-based hydrogels for the efficient capture of dye pollutants from aqueous solutions. Carbon 2013, 56, 173–182. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Du, Q.; Sun, J.; Jiao, Y.; Yang, G.; Wang, Z.; Xia, Y.; Zhang, W.; Wang, K.; et al. Adsorption of methylene blue from aqueous solution by graphene. Colloids Surf. B Biointerfaces 2012, 90, 197–203. [Google Scholar] [CrossRef]
- Gupta, K.; Khatri, O.P. Reduced graphene oxide as an effective adsorbent for removal of malachite green dye: Plausible adsorption pathways. J. Colloid Interface Sci. 2017, 501, 11–21. [Google Scholar] [CrossRef]
- Yang, S.-T.; Chen, S.; Chang, Y.; Cao, A.; Liu, Y.; Wang, H. Removal of methylene blue from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2011, 359, 24–29. [Google Scholar] [CrossRef]
- Tabish, T.A.; Memon, F.A.; Gomez, D.E.; Horsell, D.W.; Zhang, S. A facile synthesis of porous graphene for efficient water and wastewater treatment. Sci. Rep. 2018, 8, 1817. [Google Scholar] [CrossRef] [Green Version]
- Allen, S.J.; Gan, Q.; Matthews, R.; Johnson, P.A. Comparison of optimised isotherm models for basic dye adsorption by kudzu. Bioresour. Technol. 2003, 88, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Wong, Y.C.; Szeto, Y.S.; Cheung, W.H.; McKay, G. Adsorption of acid dyes on chitosan—Equilibrium isotherm analyses. Process Biochem. 2004, 39, 695–704. [Google Scholar] [CrossRef]
- Rahbari, M.; Goharrizi, A.S. Adsorption of Lead(II) from Water by Carbon Nanotubes: Equilibrium, Kinetics, and Thermodynamics. Water Environ. Res. 2009, 81, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef] [PubMed]
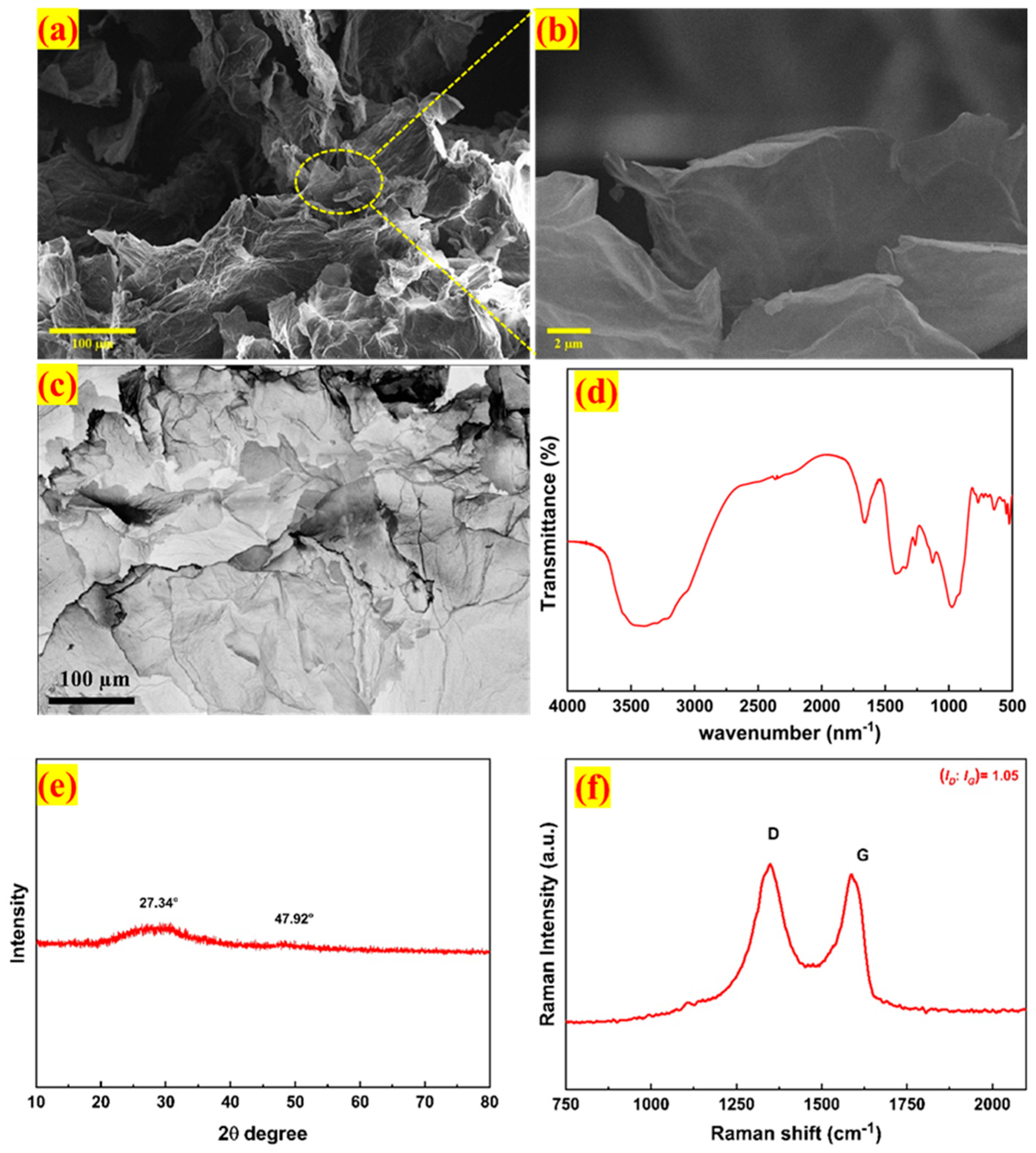
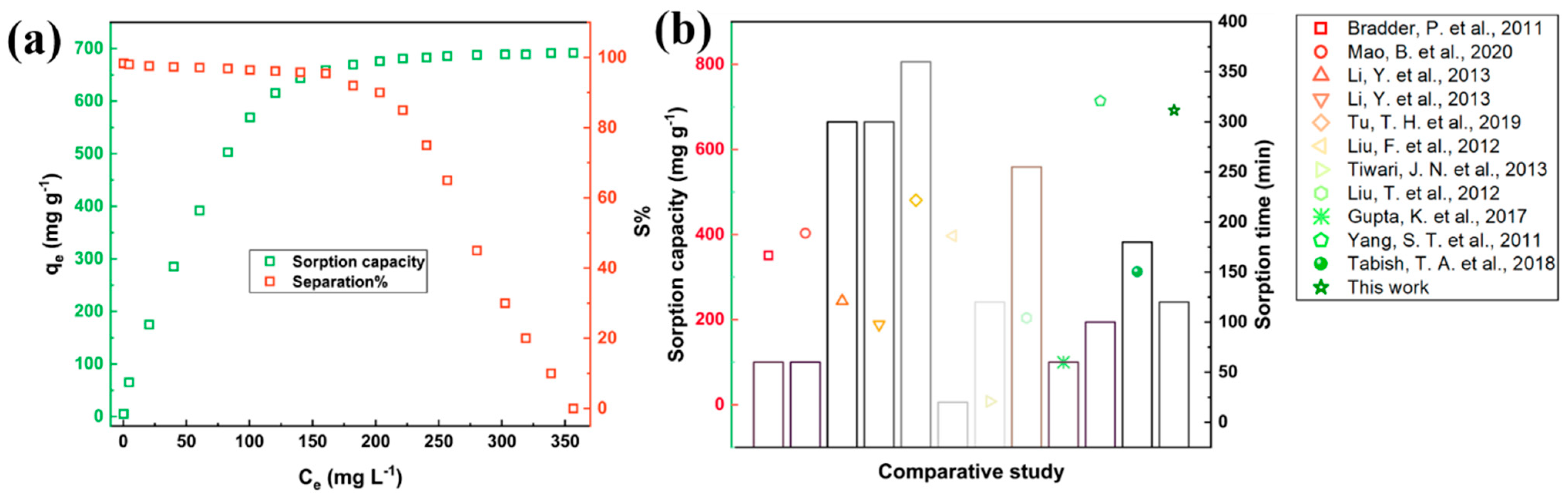
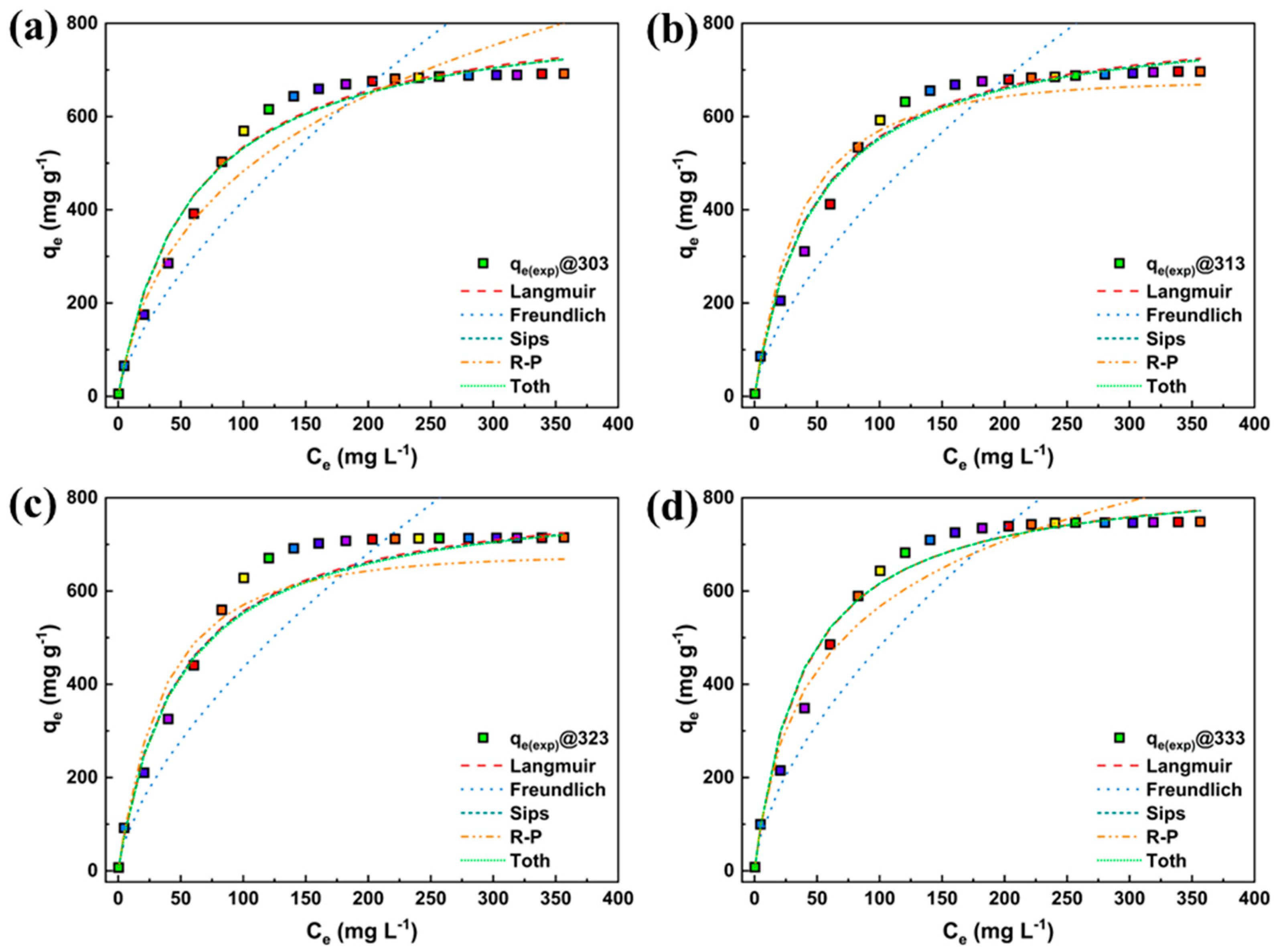
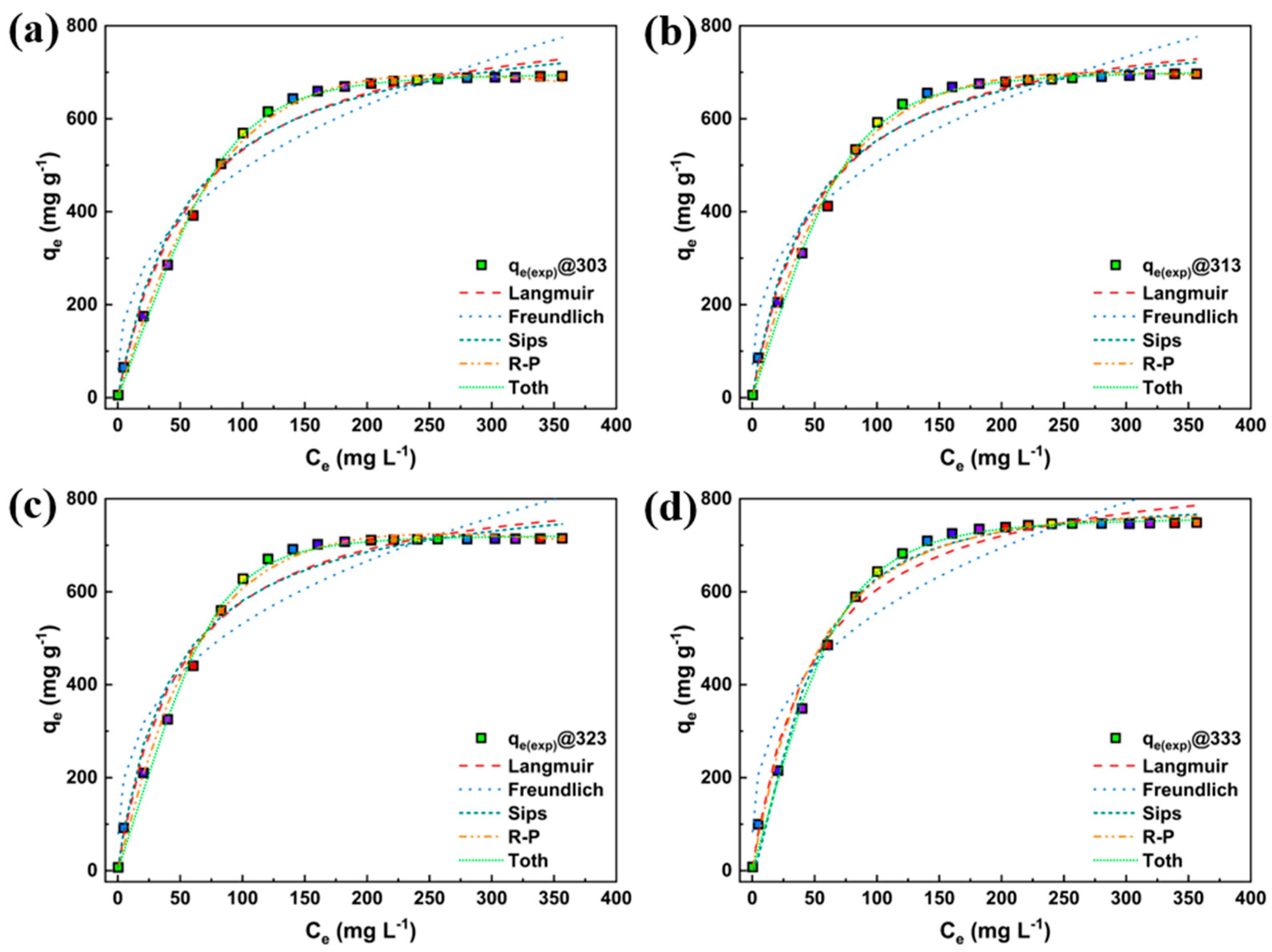
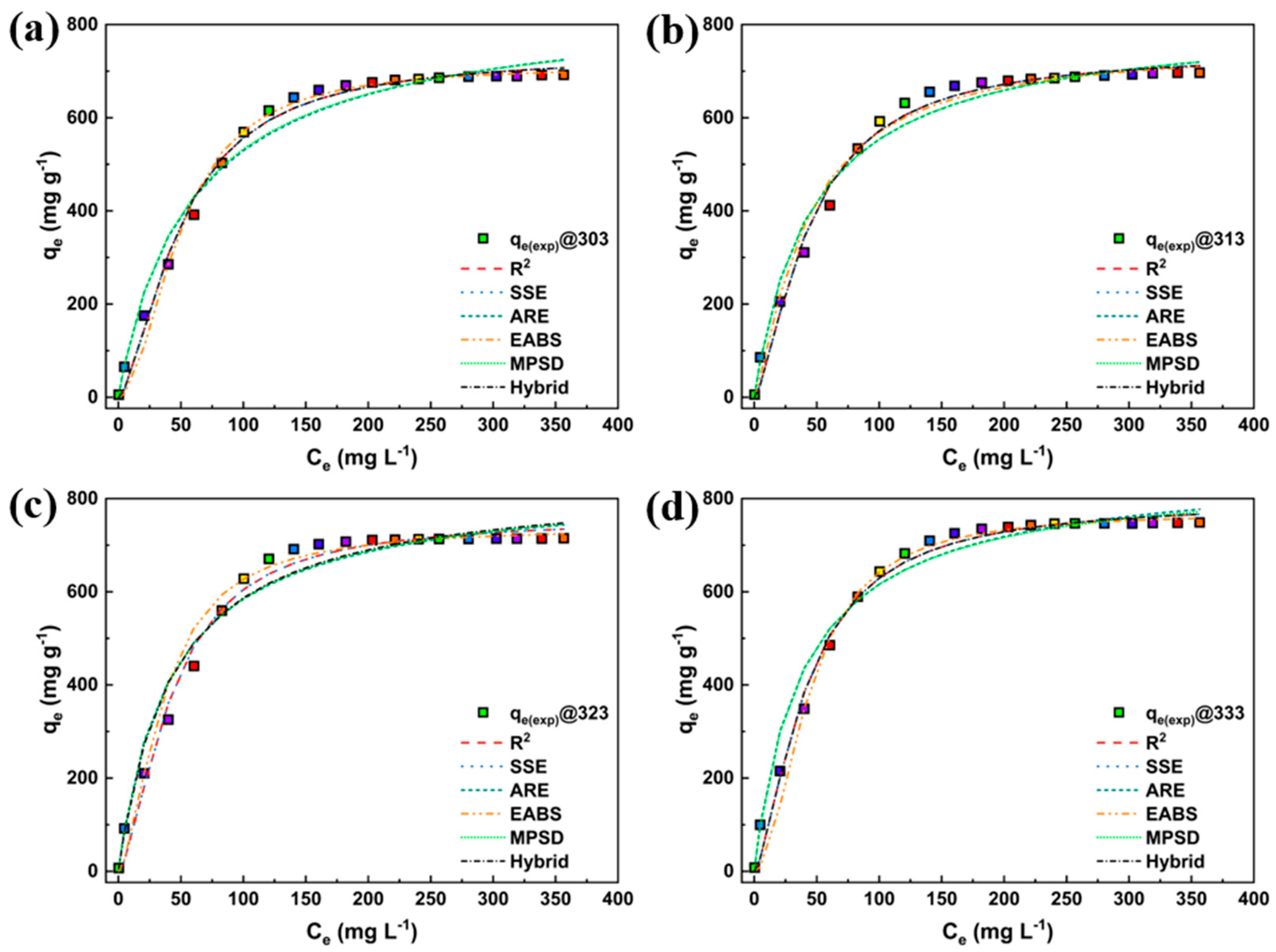
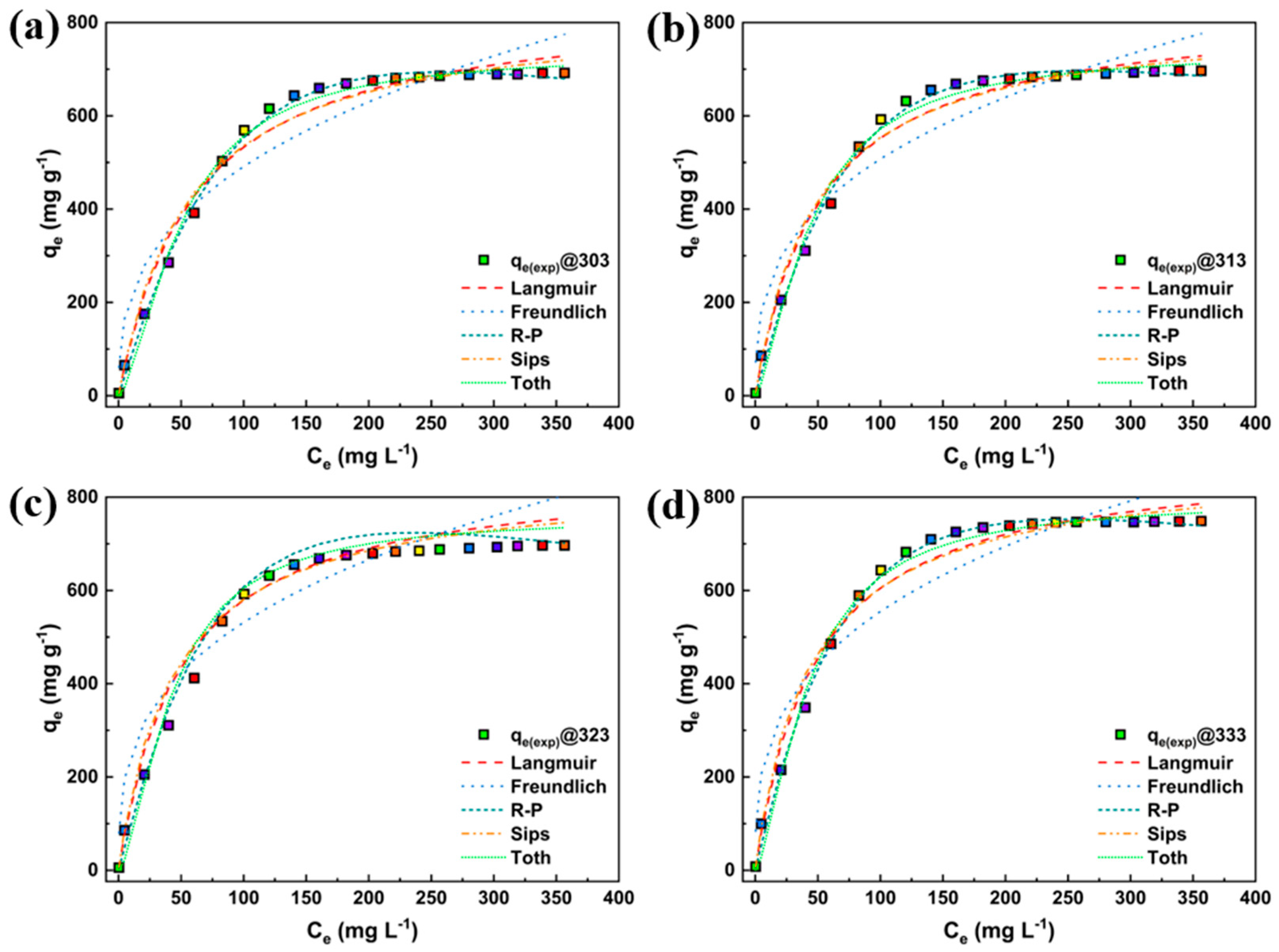


| Particulars | Unit |
|---|---|
| Surface area | 589.06 m2 g−1 |
| Average pore size | 4.49 nm |
| Pore volume | 5.62 × 10−1 cm3 g−1 |
| Crystal size | 4.49 Å |
| Crystallite lattice sizes | 15 nm |
| C | 88.13% |
| O | 11.5% |
| N | 0.37% |
| Model | Nonlinear | Linear | Linear Plot | Parameters |
|---|---|---|---|---|
| Langmuir | Type-1 | vs. | KL, qm | |
| Type-2 | vs. | |||
| Type-3 | qe vs. | |||
| Type-4 | vs. | |||
| Type-5 | ||||
| Type-6 | Ce vs. | |||
| Freundlich | Lnqe vs. LnCe | KF, n | ||
| R-P | A, B, g | |||
| Sips | qm, bs, n | |||
| Toth | qm, KT, n |
| Models | Parameters | Temperature (K) | |||
|---|---|---|---|---|---|
| 303 | 313 | 323 | 333 | ||
| Langmuir Type-1 | qmax | 660.491 | 766.344 | 721.527 | 727.711 |
| KL | 0.027 | 0.025 | 0.033 | 0.037 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Langmuir Type-2 | qmax | 661.636 | 766.768 | 722.122 | 728.578 |
| KL | 0.027 | 0.025 | 0.033 | 0.037 | |
| R2 | 0.999 | 0.999 | 0.999 | 0.999 | |
| Langmuir Type-3 | qmax | 810.821 | 797.165 | 807.475 | 828.349 |
| KL | 0.0194 | 0.023 | 0.026 | 0.029 | |
| R2 | 0.889 | 0.915 | 0.888 | 0.884 | |
| Langmuir Type-4 | qmax | 844.915 | 820.192 | 837.234 | 858.515 |
| KL | 0.017 | 0.021 | 0.023 | 0.025 | |
| R2 | 0.889 | 0.915 | 0.888 | 0.884 | |
| Langmuir Type-5 | qmax | 820.772 | 801.373 | 816.299 | 851.26 |
| KL | 0.019 | 0.023 | 0.026 | 0.026 | |
| R2 | 0.990 | 0.993 | 0.991 | 0.993 | |
| Langmuir Type-6 | qmax | 812.614 | 796.02 | 855.012 | 845.473 |
| KL | 0.019 | 0.024 | 0.021 | 0.027 | |
| R2 | 0.990 | 0.993 | 0.991 | 0.993 | |
| Freundlich | 1/n | 0.666 | 0.642 | 0.623 | 0.612 |
| KF | 19.509 | 22.705 | 26.035 | 28.779 | |
| R2 | 0.956 | 0.942 | 0.943 | 0.944 | |
| Sips | qm | 691.9 | 696.456 | 714.864 | 748.873 |
| n | 0.996 | 0.991 | 0.99 | 1.001 | |
| bs | 0.019 | 0.023 | 0.026 | 0.027 | |
| R2 | 0.985 | 0.986 | 0.977 | 0.982 | |
| R-P | g | 0.737 | 1.073 | 0.837 | 0.814 |
| B | 0.096 | 0.017 | 0.071 | 0.09 | |
| A | 18.67 | 19.09 | 23.9 | 27.39 | |
| R2 | 0.987 | 0.98 | 0.99 | 0.991 | |
| Toth | qm | 692.907 | 700.333 | 716.741 | 749.844 |
| n | 1.004 | 1.009 | 1.01 | 0.999 | |
| KT | 0.019 | 0.023 | 0.026 | 0.027 | |
| R2 | 0.985 | 0.986 | 0.977 | 0.982 | |
| Models | Parameters | Temperature (K) | |||
|---|---|---|---|---|---|
| 303 | 313 | 323 | 333 | ||
| Langmuir | qmax | 849.367 | 831.971 | 855.012 | 889.211 |
| KL | 0.017 | 0.020 | 0.021 | 0.021 | |
| R2 | 0.981 | 0.982 | 0.975 | 0.982 | |
| RL | 0.995 | 0.994 | 0.994 | 0.994 | |
| Freundlich | 1/n | 0.358 | 0.334 | 0.325 | 0.324 |
| KF | 94.514 | 108.839 | 119.043 | 124.809 | |
| R2 | 0.916 | 0.915 | 0.900 | 0.910 | |
| Sips | qm | 832.966 | 821.569 | 844.092 | 795.438 |
| n | 0.996 | 0.991 | 0.990 | 0.655 | |
| bs | 0.019 | 0.023 | 0.025 | 0.003 | |
| R2 | 0.98 | 0.980 | 0.973 | 0.991 | |
| R-P | g | 1.44 | 1.359 | 1.418 | 1.347 |
| B | 7.33 × 10−4 | 1.44 × 10−3 | 1.02 × 10−3 | 1.66 × 10−3 | |
| A | 8.66 | 10.066 | 10.343 | 11.508 | |
| R2 | 0.998 | 0.995 | 0.995 | 0.997 | |
| Toth | qm | 697.951 | 704.157 | 721.800 | 760.118 |
| n | 3.019 | 2.660 | 3.210 | 2.583 | |
| KT | 0.010 | 0.012 | 0.012 | 0.013 | |
| R2 | 0.998 | 0.995 | 0.995 | 0.996 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deb, H.; Hasan, K.; Islam, M.Z.; Kai, L.; Yang, S.; Zhang, Y.; Yao, J. The Statistical Error Optimization of Dye Sorption Equilibria for the Precise Prediction of Adsorption Isotherms on Activated Graphene. Appl. Sci. 2023, 13, 8106. https://doi.org/10.3390/app13148106
Deb H, Hasan K, Islam MZ, Kai L, Yang S, Zhang Y, Yao J. The Statistical Error Optimization of Dye Sorption Equilibria for the Precise Prediction of Adsorption Isotherms on Activated Graphene. Applied Sciences. 2023; 13(14):8106. https://doi.org/10.3390/app13148106
Chicago/Turabian StyleDeb, Hridam, K. Hasan, Md Zahidul Islam, Lv Kai, Shujuan Yang, Yong Zhang, and Juming Yao. 2023. "The Statistical Error Optimization of Dye Sorption Equilibria for the Precise Prediction of Adsorption Isotherms on Activated Graphene" Applied Sciences 13, no. 14: 8106. https://doi.org/10.3390/app13148106





