Kinetics of Ions in Post-Lithium Batteries
Abstract
:1. Introduction
2. Materials and Methods
Molecular Dynamics and Density Functional Theory
3. Post-Lithium Ionic Conductors
3.1. Sodium-Ion Batteries
3.2. Magnesium-Ion Batteries
4. Oxygen Ion Batteries
4.1. Oxygen-Ion Diffusion
4.2. Oxygen Battery
5. Tuning the Ionic Diffusion
6. Summary, Perspective, and Future Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Steele, B.C.H. Appraisal of Ce1-yGdyO2-y/2 electrolytes for IT-SOFC operation at 500 °C. Solid State Ion. 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Sata, N.; Eberman, K.; Eberl, K.; Maier, J. Mesoscopic fast ion conduction in nanometre scale planar heterostructures. Nature 2000, 408, 946–949. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.C. Advances in solid oxide fuel cell technology. Solid State Ion. 2000, 135, 305–313. [Google Scholar] [CrossRef]
- Sickafus, K.E.; Minervini, L.; Grimes, R.W.; Valdez, J.A.; Ishimaru, M.; Li, F.; McClellan, K.J.; Hartmann, T. Radiation tolerance of complex oxides. Science 2000, 289, 748–751. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Garcia-Barriocanal, J.; Rivera-Calzada, A.; Varela, M.; Sefrioui, Z.; Iborra, E.; Leon, C.; Pennycook, S.J.; Santamaria, J. Colossal ionic conductivity at interfaces of epitaxial ZrO2:Y2O3/SrTiO3 heterostructures. Science 2008, 321, 676–680. [Google Scholar] [CrossRef]
- Kilner, J.A. Ionic conductors: Feel the strain. Nat. Mater. 2008, 7, 838. [Google Scholar] [CrossRef] [PubMed]
- Vovk, R.V.; Obolenskii, M.A.; Zavgorodniy, A.A.; Goulatis, I.L.; Beleskii, V.I.; Chroneos, A. Structural relaxation, metal to insulator transition and pseudo-gap in oxygen deficient HoBa2Cu3O7-δ single crystals. Phys. C 2009, 469, 203–206. [Google Scholar] [CrossRef]
- Vovk, R.V.; Vovk, N.R.; Shekhovtsov, O.V.; Goulatis, I.L.; Chroneos, A. c-axis hopping conductivity in heavily Pr-doped YBCO single crystals. Semicond. Sci. Technol. 2013, 26, 085017. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A.; Skinner, S.J.; Kilner, J.A.; Grimes, R.W. Effect of strain on the oxygen diffusion in yttria and gadolinia co-doped ceria. Solid State Ion. 2013, 230, 37–42. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Chroneos, A. Impact of uniaxial strain and doping on oxygen diffusion in CeO2. Sci. Rep. 2014, 4, 6068. [Google Scholar] [CrossRef]
- Lumley, S.C.; Grimes, R.W.; Murphy, S.T.; Burr, P.A.; Chroneos, A.; Chard-Tuckey, P.R.; Wenman, M.R. The thermodynamics of hydride precipitation: The importance of entropy, enthalpy and disorder. Acta Mater. 2014, 79, 351–362. [Google Scholar] [CrossRef]
- Tomkiewicz, A.C.; Tamimi, A.; Huq, A.; McIntosh, S. Oxygen transport pathways in Ruddlesden–Popper structured oxides revealed via in situ neutron diffraction. J. Mater. Chem. A 2015, 3, 21864–21874. [Google Scholar] [CrossRef]
- Ning, D.; Baki, A.; Scherb, T.; Song, J.; Fantin, A.; Liu, X.Z.; Schumacher, G.; Banhart, J.; Bouwmeester, H.J.M. Influence of A-site deficiency on structural evolution of Pr2−xNiO4+δ with temperature. Solid State Ion. 2019, 342, 115056. [Google Scholar] [CrossRef]
- Solovjov, A.L.; Petrenko, E.V.; Omelchenko, L.V.; Vovk, R.V.; Goulatis, I.L.; Chroneos, A. Effect of annealing on a pseudogap state in untwinned YBa2Cu3O7-δ single crystals. Sci. Rep. 2019, 9, 9274. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Iyngaran, P.; Vovk, R.; Chroneos, A. Defects, dopants and Mg diffusion in MgTiO3. Sci. Rep. 2019, 9, 4394. [Google Scholar] [CrossRef]
- Chiabrera, F.; Garbayo, I.; Lopez-Conesa, L.; Martin, G.; Ruiz-Caridad, A.; Walls, M.; Ruiz-Gonzalez, L.; Kordatos, A.; Nunez, M.; Morata, A.; et al. Engineering transport in manganites by tuning local nonstoichiometry in grain boundaries. Adv. Mater. 2019, 31, 1805360. [Google Scholar] [CrossRef]
- Acosta, M.; Baiutti, F.; Tarancon, A.; MacManus-Driscoll, J.L. Nanostructured materials and interfaces for advanced ionic electronic conducting oxides. Adv. Mater. Interfaces 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Zou, Z.; Ma, N.; Wang, A.; Ran, Y.; Song, T.; Jiao, Y.; Liu, J.; Zhou, H.; Shi, W.; He, B.; et al. Relationships between Na+ distribution, concerted migration, and diffusion properties in rhombohedral NASICON. Adv. Energy Mater. 2020, 10, 2001486. [Google Scholar] [CrossRef]
- Shi, J.; Han, C.; Niu, H.; Zhu, Y.; Yun, S. Theoretical investigation of proton diffusion in Dion-Jacobson layered perovskite RbBiNb2O7. Nanomaterials 2021, 11, 1953. [Google Scholar] [CrossRef]
- Wang, F.; Xing, Y.; Hu, E.; Wang, J.; Shi, J.; Yun, S.; Zhu, B. PN heterostructure interface-facilitated proton conduction in 3C-SiC/Na0.6CoO2 electrolyte for fuel cell application. ACS Appl. Energy Mater. 2021, 4, 7519–7525. [Google Scholar] [CrossRef]
- Grieshammer, S.; Belova, I.V.; Murch, G.E. Thermodiffusion and ion transport in doped ceria by molecular dynamics simulations. Acta Mater. 2021, 210, 116802. [Google Scholar] [CrossRef]
- Varley, J.B.; Shen, B.; Higashiwaki, M. Wide bandgap semiconductor materials and devices. J. Appl. Phys. 2022, 131, 230401. [Google Scholar] [CrossRef]
- Hassan, J.Z.; Raza, A.; Qumar, U.; Li, G. Recent advances in engineering strategies of Bi-based photocatalysts for environmental remediation. Sust. Mater. Technol. 2022, 33, e00478. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Seymour, I.D.; Skinner, S.J. Neutron diffraction and DFT studies of oxygen defect and transport in higher-order Ruddlesden–Popper phase materials. RSC Adv. 2023, 13, 13786–13797. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, J.; NuLi, Y.; Wang, J. Sol–gel synthesis of Mg1.03Mn0.97SiO4 and its electrochemical intercalation behavior. J. Power Sources 2008, 184, 604–609. [Google Scholar] [CrossRef]
- Schichtel, N.; Korte, C.; Hesse, D.; Janek, J. Elastic strain at interfaces and its influence on ionic conductivity in nanoscaled solid electrolyte thin films- theoretical considerations and experimental studies. Phys. Chem. Chem. Phys. 2009, 11, 3043–3048. [Google Scholar] [CrossRef]
- Vovk, R.V.; Nazyrov, Z.F.; Obolenskii, M.A.; Goulatis, I.L.; Chroneos, A.; Simoes, V.M.P. Phase separation in oxygen deficient HoBa2Cu3O7-δ single crystals: Effect of pressure and twin boundaries. Phil. Mag. 2011, 91, 2291–2302. [Google Scholar] [CrossRef]
- Serras, P.; Palomares, V.; Goñi, A.; Gil de Muro, I.; Kubiak, P.; Lezama, L.; Rojo, T. High voltage cathode materials for Na-ion batteries of general formula Na3V2O2x(PO4)2F3-2x. J. Mater. Chem. 2012, 22, 22301–22308. [Google Scholar] [CrossRef]
- Tripathi, R.; Wood, S.M.; Islam, M.S.; Nazar, L.F. Na-ion mobility in layered Na2FePO4F and olivine Na[Fe,Mn]PO4. Energy Environ. Sci. 2013, 6, 2257–2264. [Google Scholar] [CrossRef]
- Clark, J.M.; Barpanda, P.; Yamada, A.; Islam, M.S. Sodium-ion battery cathodes Na2FeP2O7 and Na2MnP2O7: Diffusion behavior for high rate performance. J. Mater. Chem. A 2014, 2, 11807–11812. [Google Scholar] [CrossRef]
- Orikasa, Y.; Masese, T.; Koyama, Y.; Mori, T.; Hattori, M.; Yamamoto, K.; Okado, T.; Huang, Z.D.; Minato, T.; Tassel, C.; et al. High energy density rechargeable magnesium battery using earth-abundant and non-toxic elements. Sci. Rep. 2014, 4, 5622. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; DiLeo, R.A.; Marschilok, A.C.; Takeuchi, K.J.; Takeuchi, E.S. Sol gel based synthesis and electrochemistry of magnesium vanadium oxide: A promising cathode material for secondary magnesium ion batteries. ECS Electrochem. Lett. 2014, 3, A87. [Google Scholar] [CrossRef]
- Huang, Z.D.; Masese, T.; Orikasa, Y.; Mori, T.; Minato, T.; Tassel, C.; Kobayashi, Y.; Kageyama, H.; Uchimoto, Y. MgFePO4F as a feasible cathode material for magnesium batteries. J. Mater. Chem. A 2014, 2, 11578. [Google Scholar] [CrossRef]
- Jay, E.E.; Rushton, M.J.D.; Chroneos, A.; Grimes, R.W.; Kilner, J.A. Genetics of superionic conductivity in lithium lanthanum titanates. Phys. Chem. Chem. Phys. 2015, 17, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.; Zhou, X.; Even, J.; Hagfeldt, A. Theoretical treatment of CH3NH3PbI3 perovskite solar cells. Angew. Chem. 2017, 56, 15806–15817. [Google Scholar] [CrossRef]
- Zhu, J.; Vasilopoulou, M.; Davazoglou, D.; Kennou, S.; Chroneos, A.; Schwingenschlögl, U. Intrinsic defects and H doping in WO3. Sci. Rep. 2017, 7, 40882. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, G.; Kim, H.; Park, Y.U.; Kang, K. Na3V(PO4)2: A new layered-type cathode material with high water stability and power capability for Na-ion batteries. Chem. Mater. 2018, 30, 3683–3689. [Google Scholar] [CrossRef]
- Kuganathan, N.; Kordatos, A.; Fitzpatrick, M.E.; Vovk, R.V.; Chroneos, A. Defect process and lithium diffusion in Li2TiO3. Solid State Ion. 2018, 327, 93–98. [Google Scholar] [CrossRef]
- Kuganathan, N.; Kordatos, A.; Chroneos, A. Li2SnO3 as a cathode material for lithium-ion batteries: Defects, lithium ion diffusion and dopants. Sci. Rep. 2018, 8, 12621. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defects, dopants and sodium mobility in Na2MnSiO4. Sci. Rep. 2018, 8, 14669. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Chroneos, A. Defects and dopant properties of Li3V2(PO4)3. Sci. Rep. 2019, 9, 333. [Google Scholar]
- Liu, T.; Zhang, Y.; Jiang, Z.; Zeng, X.; Ji, J.; Li, Z.; Gao, X.; Sun, M.; Lin, Z.; Ling, M.; et al. Exploring competitive features of stationary sodium ion batteries for electrochemical energy storage. Energy Environ. Sci. 2019, 12, 1512–1533. [Google Scholar] [CrossRef]
- Tsuruaka, T.; Tsujita, T.; Su, J.; Nishitani, Y.; Hamamura, T.; Inatomi, Y.; Nakura, K.; Terabe, K. Fabrication of a magnesium-ion conducting magnesium phosphate electrolyte film using atomic layer deposition. Jpn. J. Appl. Phys. 2020, 59, SIIG08. [Google Scholar] [CrossRef]
- Kuganathan, N.; Davazoglou, K.; Chroneos, A. Computer modelling investigation of MgV2O4 for Mg-ion batteries. J. Appl. Phys. 2020, 127, 035106. [Google Scholar] [CrossRef]
- Kuganathan, N.; Rushton, M.J.D.; Grimes, R.W.; Kilner, J.A.; Gkanas, E.I.; Chroneos, A. Self-diffusion in garnet-type Li7La3Zr2O12 solid electrolytes. Sci. Rep. 2021, 11, 451. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defects, diffusion, dopants and encapsulation of Na in NaZr2(PO4)3. Materialia 2021, 16, 101039. [Google Scholar] [CrossRef]
- Hasa, I.; Mariyappan, S.; Saurel, D.; Adelhelm, P.; Koposov, A.Y.; Masquelier, C.; Croguennec, L.; Casas-Cabanas, M. Challenges of today for Na-based batteries of the future: From materials to cell metrics. J. Power Sources 2021, 482, 228872. [Google Scholar] [CrossRef]
- Rex, K.A.; Iyngaran, P.; Kuganathan, N.; Chroneos, A. Defect properties and lithium incorporation in Li2ZrO3. Energies 2021, 14, 3963. [Google Scholar] [CrossRef]
- Schmid, A.; Krammer, M.; Fleig, J. Rechargeable oxide ion batteries based on mixed conducting oxide electrodes. Adv. Energy Mater. 2023, 13, 2203789. [Google Scholar] [CrossRef]
- Smith, W.; Forester, T.R. DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. J. Mol. Graph. 1996, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.D. GULP: A computer program for the symmetry-adapted simulation of solids. J. Chem. Soc. Faraday Trans. 1997, 93, 629. [Google Scholar] [CrossRef]
- Catlow, C.R.A. (Ed.) Computer Modelling in Inorganic Crystallography, 1st ed.; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Hasnip, P.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous electron gas. Phys. Rev. 1964, 136, B864. [Google Scholar] [CrossRef]
- Kohn, W. Nobel Lecture: Electronic structure of matter—Wave functions and density functionals. Rev. Mod. Phys. 1998, 71, 1253. [Google Scholar] [CrossRef]
- Koch, W.; Holthausen, M.C. A Chemist’s Guide to Density Functional Theory; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901. [Google Scholar] [CrossRef]
- Born, M.; Mayer, J.E. Zur Gittertheorie der Ionenkristalle. Z. Phys. 1932, 75, 1. [Google Scholar] [CrossRef]
- Buckingham, R.A. The classical equation of state of gaseous helium, neon and argon. Proc. R. Soc. Lond. Ser. A 1938, 168, 264. [Google Scholar]
- Grimes, R.W.; Busker, G.; McCoy, M.A.; Chroneos, A.; Kilner, J.A.; Chen, S.P. The effect of ion size on solution mechanism and defect cluster geometry. Ber. Bunsenges. Phys. Chem. 1997, 101, 1204. [Google Scholar] [CrossRef]
- Busker, G.; Chroneos, A.; Grimes, R.W.; Chen, I.W. Solution Mechanisms for Dopant Oxides in Yttria. J. Am. Ceram. Soc. 1999, 82, 1553. [Google Scholar] [CrossRef]
- Varotsos, P. Calculation of the migration volume of vacancies in ionic solids from macroscopic parameters. Phys. Stat. Sol. 1978, 47, K133–K136. [Google Scholar] [CrossRef]
- Varotsos, P. Comparison of models that interconnect point defect parameters in solids with bulk properties. J. Appl. Phys. 2007, 101, 123503. [Google Scholar] [CrossRef]
- Varotsos, P. Point defect parameters in β-PbF2 revisited. Solid State Ion. 2008, 179, 438–441. [Google Scholar] [CrossRef]
- Zhang, B.; Wu, X.; Xu, J.; Zhou, R. Application of the cBΩ model for the calculation of oxygen self-diffusion coefficients in minerals. J. Appl. Phys. 2010, 108, 053505. [Google Scholar] [CrossRef]
- Vallianatos, F.; Saltas, V. Application of the cBΩ model to the calculation of diffusion parameters of He in olivine. Phys. Chem. Miner. 2014, 41, 181–188. [Google Scholar] [CrossRef]
- Cooper, M.W.D.; Grimes, R.W.; Fitzpatrick, M.E.; Chroneos, A. Modeling oxygen self-diffusion in UO2 under pressure. Solid State Ion. 2015, 282, 26–30. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, S. Application of the cBΩ model to the calculation of diffusion parameters of Si in silicates. Geochem. Geophys. Geosyst. 2015, 16, 705–718. [Google Scholar] [CrossRef]
- Chroneos, A.; Vovk, R.V. Modeling self-diffusion in UO2 and ThO2 by connecting point defect parameters with bulk properties. Solid State Ion. 2015, 274, 1–3. [Google Scholar] [CrossRef]
- Parfitt, D.C.; Cooper, M.W.D.; Rushton, M.J.D.; Christopoulos, S.-R.G.; Fitzpatrick, M.E.; Chroneos, A. Thermodynamic calculations of oxygen self-diffusion in mixed-oxide nuclear fuels. RSC Adv. 2016, 6, 74018–74028. [Google Scholar] [CrossRef]
- Saltas, V.; Chroneos, A.; Vallianatos, F.A. A thermodynamic approach of self- and hetero-diffusion in GaAs: Connecting point defect parameters with bulk properties. RSC Adv. 2016, 6, 53324–53330. [Google Scholar] [CrossRef]
- Chroneos, A. Connecting point defect parameters with bulk properties to describe diffusion in solids. Appl. Phys. Rev. 2016, 3, 041304. [Google Scholar] [CrossRef]
- Sarlis, N.V.; Skordas, E.S. Estimating the compressibility of osmium from recent measurements of Ir-Os alloys under high pressure. J. Phys. Chem. A 2016, 120, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Skordas, E.S.; Sarlis, N.V.; Varotsos, P.A. Applying the cBΩ thermodynamical model to LiF using its equation of state obtained from high pressure diamond anvil cell measurements. Solid State Ion. 2020, 354, 115404. [Google Scholar] [CrossRef]
- Sanchez, J.M.; Ducastelle, F.; Gratias, D. Generalized cluster description of multicomponent systems. Phys. A 1984, 128, 334–350. [Google Scholar] [CrossRef]
- Wei, S.H.; Ferreira, L.G.; Bernard, J.E.; Zunger, A. Electronic properties of random alloys: Special quasirandom structures. Phys. Rev. B 1990, 42, 9622–9649. [Google Scholar] [CrossRef]
- Zunger, A.; Wei, S.H.; Ferreira, L.G.; Bernard, J.E. Special quasirandom structures. Phys. Rev. Lett. 1990, 65, 353–356. [Google Scholar] [CrossRef]
- Laks, D.B.; Ferreira, L.G.; Froyen, S.; Zunger, A. Efficient cluster expansion for substitutional systems. Phys. Rev. B 1992, 46, 12587–12605. [Google Scholar] [CrossRef]
- Wolverton, C.; Zunger, A. Ising-like description of structurally released ordered and disordered alloys. Phys. Rev. Lett. 1995, 75, 3162–3165. [Google Scholar] [CrossRef]
- Jiang, C.; Sordelet, D.J.; Gleeson, B. First-principles study of phase stability in pseudobinary (Ni1-xPtx)3Al. Phys. Rev. B 2005, 72, 184203. [Google Scholar] [CrossRef]
- Chroneos, A.; Jiang, C.; Grimes, R.W.; Schwingenschlögl, U.; Bracht, H. Defect interactions in Sn1-xGex alloys. Appl. Phys. Lett. 2009, 94, 252104. [Google Scholar] [CrossRef]
- Chroneos, A.; Jiang, C.; Grimes, R.W.; Schwingenschlögl, U.; Bracht, H. E centers in Si1-x-yGexSny alloys. Appl. Phys. Lett. 2009, 95, 112101. [Google Scholar] [CrossRef]
- Murphy, S.T.; Chroneos, A.; Jiang, C.; Schwingenschlögl, U.; Grimes, R.W. Deviations from Vegard’s law in ternary III-V alloys. Phys. Rev. B 2010, 82, 073201. [Google Scholar] [CrossRef]
- Murphy, S.T.; Chroneos, A.; Grimes, R.W.; Jiang, C.; Schwingenschlögl, U. Phase stability and the arsenic vacancy defect in InxGa1-xAs. Phys. Rev. B 2011, 84, 184108. [Google Scholar] [CrossRef]
- Wang, H.; Chroneos, A.; Jiang, C.; Schwingenschlögl, U. Modelling zirconium hydrides using the special quasirandom structure approach. Phys. Chem. Chem. Phys. 2013, 15, 7599–7603. [Google Scholar] [CrossRef]
- Chroneos, A.; Rushton, M.J.D.; Jiang, C.; Tsoukalas, L.H. Nuclear wasteform materials: Atomistic simulation case studies. J. Nucl. Mater. 2013, 441, 29–39. [Google Scholar] [CrossRef]
- Jiang, C.; Chroneos, A. Ab initio modelling of MAX phase solid solutions using the special quasirandom structure approach. Phys. Chem. Chem. Phys. 2018, 20, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Matsumoto, K.; Nohira, T.; Hagiwara, R. Na2MnSiO4 as a positive electrode material for sodium secondary batteries using an ionic liquid electrolyte. Electrochem. Commun. 2014, 45, 63–66. [Google Scholar] [CrossRef]
- Kosova, N.V.; Podugolnikov, V.R.; Devyatkina, E.T.; Slobodyuk, A.B. Structure and electrochemistry of NaFePO4 and Na2FePO4F cathode materials prepared via mechanochemical route. Mater. Res. Bull. 2014, 60, 849–857. [Google Scholar] [CrossRef]
- Han, M.H.; Gonzalo, E.; Singh, G.; Rojo, T. A comprehensive review of sodium layered oxides: Powerful cathodes for Na-ion batteries. Energy Environ. Sci. 2015, 8, 81–102. [Google Scholar] [CrossRef]
- Fang, Y.; Liu, Q.; Xiao, L.; Ai, X.; Yang, H.; Cao, Y. High-Performance Olivine NaFePO4 Microsphere Cathode Synthesized by Aqueous Electrochemical Displacement Method for Sodium Ion Batteries. ACS Appl. Mater. Interfaces 2015, 7, 17977–17984. [Google Scholar] [CrossRef]
- Hang, X.; Rui, X.; Chen, D.; Tan, H.; Yang, D.; Huang, S.; Yu, Y. Na3V2(PO4)3: An advanced cathode for sodium-ion batteries. Nanoscale 2019, 11, 2556–2576. [Google Scholar]
- Kuganathan, N.; Chroneos, A. Na3V(PO4)2 cathode material for Na ion batteries: Defect, dopants and Na diffusion. Solid State Ion. 2019, 336, 75–79. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defect chemistry and Na-ion diffusion in Na3Fe2(PO4)3. Materials 2019, 12, 1348. [Google Scholar] [CrossRef]
- Kaushalya, R.; Iyngaran, P.; Kuganathan, N.; Chroneos, A. Defect, diffusion and dopant properties of NaNiO2: Atomistic simulation study. Energies 2019, 12, 3094. [Google Scholar] [CrossRef]
- Kuganathan, N.; Kelaidis, N.; Chroneos, A. Defect chemistry, sodium diffusion and doping behaviour in NaFeO2. Materials 2019, 12, 3243. [Google Scholar] [CrossRef]
- Pak, Y.C.; Rim, C.H.; Hwang, S.G.; Ri, K.C.; Yu, C.J. Defect formation and ambivalent effects on electrochemical performance in layered sodium titanate Na2Ti3O7. Phys. Chem. Chem. Phys. 2023, 25, 3420–3431. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, I.N.; Apostolov, A.T.; Wesselinowa, J.M. Band gap energy of ion-doped multiferroic NaFeO2 nanoparticles. Phys. Status Solidi RRL 2023, 2300159. [Google Scholar] [CrossRef]
- Wu, H.L.; Chen, Y.Q.; Wen, T.Z.; Chen, L.; Pu, X.J.; Chen, Z.X. Advances in vanadium-redoxed polyanions for high-voltage sodium-ion batteries. Batteries 2023, 9, 56. [Google Scholar] [CrossRef]
- Paidi, A.K.; Sharma, A.; Paidi, V.K.; Illa, M.P.; Lee, K.S.; Lee, S.S.; Ahn, D.; Mukhopadhyay, A. Na2ZrFe(PO4)3- A Rhombohedral NASICON-Structured Material: Synthesis, Structure and Na-Intercalation Behavior. Inorg. Chem. 2023, 62, 4124–4135. [Google Scholar] [CrossRef]
- Ji, Y.Z.; Honma, T.; Komatsu, T. Formation of sodium ion conductive NaZr2(PO4)3 composite via liquid phase sintering method with sodium disilicate glass. Solid State Ion. 2023, 395, 116213. [Google Scholar] [CrossRef]
- Satrughna, J.A.K.; Kanwade, A.; Srivastava, A.; Tiwara, M.K.; Yadav, S.C.; Akula, S.T.; Shirage, P.M. Experimental and ab initio based DFT calculation of NaFe0.5Co0.5O2 as an excellent cathode material for futuristic sodium ion batteries. J. Energy Storage 2023, 65, 107371. [Google Scholar] [CrossRef]
- Gomez-Garduno, N.; Araiza, D.G.; Celaya, C.A.; Muniz, J.; Pfeiffer, H. Unveiling the different physicochemical properties of M-doped beta-NaFeO2 (where M = Ni or Cu) materials evaluated as CO2 sorbents: A combined experimental and theoretical analysis. J. Mater. Chem. A 2023, 11, 10938–10954. [Google Scholar] [CrossRef]
- Zhang, X.T.; Tian, H.L.; Zhang, Y.H.; Cai, Y.J.; Yao, X.; Su, Z. Diatomic-doped carbon layer decorated Na3V2(PO4)2F3 as a durable ultrahigh-stability cathode for sodium ion batteries. New J. Chem. 2023, 47, 9611–9617. [Google Scholar] [CrossRef]
- Aurbach, D.; Gofer, Y.; Lu, Z.; Schechter, A.; Chusid, O.; Gizbar, H.; Cohen, Y.; Ashkenazi, V.; Moshkovich, M.; Turgeman, R.; et al. A short review on the comparison between Li battery systems and rechargeable magnesium battery technology. J. Power Sources 2001, 97–98, 28–32. [Google Scholar] [CrossRef]
- Muldoon, J.; Bucur, C.B.; Gregory, T. Quest for Nonaqueous Multivalent Secondary Batteries: Magnesium and Beyond. Chem. Rev. 2014, 114, 11683–11720. [Google Scholar] [CrossRef] [PubMed]
- Ponrouch, A.; Frontera, C.; Bardé, F.; Palacín, M.R. Towards a calcium-based rechargeable battery. Nat. Mater. 2015, 15, 169–172. [Google Scholar] [CrossRef]
- Gummow, R.J.; Vamvounis, G.; Kannan, M.B.; He, Y. Calcium-Ion Batteries: Current State-of-the-Art and Future Perspectives. Adv. Mater. 2018, 30, 1801702. [Google Scholar] [CrossRef]
- Kuganathan, N.; Gkanas, E.I.; Chroneos, A. Mg6MnO8 as a magnesium-ion battery material: Defects, dopants and Mg-ion transport. Energies 2019, 12, 3213. [Google Scholar] [CrossRef]
- Kuganathan, N.; Ganeshalingam, S.; Chroneos, A. Defect, transport, and dopant properties of andradite garnet Ca3Fe2Si3O12. AIP Adv. 2020, 10, 075004. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defect and dopants in CaFeSi2O6: Classical and DFT simulations. Energies 2020, 13, 1285. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Atomic-scale studies of garnet-type Mg3Fe2Si3O12: Defect chemistry, diffusion and dopant properties. J. Power Sources Adv. 2020, 3, 100016. [Google Scholar] [CrossRef]
- Kuganathan, N.; Chroneos, A. Defect and dopant properties in CaMnO3. AIP Adv. 2021, 11, 055106. [Google Scholar] [CrossRef]
- Torres, A.; Casals, J.L.; Arroyo de Dompablo, M.E. Enlisting potential cathode materials for rechargeable Ca batteries. Chem. Mater. 2021, 33, 2488–2497. [Google Scholar] [CrossRef]
- Tekliye, D.B.; Kumar, A.; Weihang, X.; Mercy, T.D.; Canepa, P.; Gautam, G.S. Exploration of NASICON frameworks as calcium-ion battery electrodes. Chem. Mater. 2022, 34, 10133–10143. [Google Scholar] [CrossRef]
- Liu, D.W.; Chen, X.H.; Zhang, Q.J.; Li, J.C.; Yan, F.F.; Dai, H.Y.; Wang, X.Z.; Chen, J.; Zhai, X.Z. Effects of Mg-doping on distorted structure and enhanced electrochemical performance of V1-xMgxO2 nanorods. Mater. Today Energy 2022, 33, 104948. [Google Scholar]
- Zhang, X.; Li, D.; Ruan, Q.; Liu, L.; Wang, B.; Xiong, F.; Huang, C.; Chu, P. Vanadium-based cathode materials for rechargeable magnesium batteries. Mater. Today Energy 2023, 32, 101232. [Google Scholar] [CrossRef]
- Lagunas, F.; Alexander, G.; Punaro, A.L.; Moscosa, C.; Hu, L.H.; Cabana, J.; Klie, R.F. Structural transformations at the atomic scale in spinel vanadium oxides upon Mg2+ extraction. ACS Appl. Energy Mater. 2023, 6, 5681–5689. [Google Scholar] [CrossRef]
- Mauvy, F.; Bassat, J.M.; Boehm, E.; Dordor, P.; Grenier, J.C.; Loup, J.P. Chemical oxygen diffusion coefficient measurement by conductivity relaxation—Correlation between tracer diffusion coefficient and chemical diffusion coefficient. J. Eur. Ceram. Soc. 2004, 24, 1265–1269. [Google Scholar] [CrossRef]
- Boehm, E.; Bassat, J.M.; Dordor, P.; Mauvy, F.; Grenier, J.C.; Stevens, P. Oxygen diffusion and transport properties in non-stoichiometric Ln2−xNiO4+δ oxides. Solid State Ion. 2005, 176, 2717–2725. [Google Scholar] [CrossRef]
- Tarancon, A.; Marrero-Lopez, D.; Pena-Martinez, J.; Ruiz-Morales, J.C.; Nunez, P. Effect of phase transition on high-temperature electrical properties of GdBaCo2O5+x layered perovskite. Solid State Ion. 2008, 179, 611–618. [Google Scholar] [CrossRef]
- Rupasov, D.; Chroneos, A.; Parfitt, D.; Kilner, J.A.; Grimes, R.W.; Istomin, S.Y.; Antipov, E.V. Oxygen diffusion in Sr0.75Y0.25CoO2.625: A molecular dynamics study. Phys. Rev. 2009, 79, 172102. [Google Scholar] [CrossRef]
- Krammer, M.; Schmid, A.; Kubicek, M.; Feig, J. Utilizing oxygen gas storage in rechargeable oxygen ion batteries. J. Power Sources 2023, 577, 233167. [Google Scholar] [CrossRef]
- Korte, C.; Schichtel, N.; Hesse, D.; Janek, J. Influence of interface structure on mass transport in phase boundaries between different ionic materials: Experimental studies and formal considerations. Monatsh. Chem. 2009, 140, 1069–1080. [Google Scholar] [CrossRef]
- Cavallaro, A.; Burriel, M.; Roqueta, J.; Apostolidis, A.; Bernardi, A.; Tarancόn, A.; Srinivasan, R.; Cook, S.N.; Fraser, H.L.; Kilner, J.A.; et al. Electronic nature of the enhanced conductivity in YSZ-STO multilayers deposited by PLD. Solid State Ion. 2010, 181, 592–601. [Google Scholar] [CrossRef]
- Pennycook, T.J.; Beck, M.J.; Varga, K.; Varela, M.; Pennycook, S.J.; Pantelides, S.T. Origin of colossal ionic conductivity in oxide multilayers: Interface induced sublattice disorder. Phys. Rev. Lett. 2010, 104, 115901. [Google Scholar] [CrossRef]
- De Souza, R.A.; Ramadan, A.; Hörner, S. Modifying the barriers for oxygen-vacancy migration in fluorite-structured CeO2 electrolytes through strain; A computer simulation study. Energy Environ. Sci. 2012, 5, 5445–5453. [Google Scholar] [CrossRef]
- Navickas, E.; Huber, T.M.; Chen, Y.; Hetaba, W.; Holzlechner, G.; Rupp, G.; Stöger-Pollach, M.; Friedbacher, G.; Hutter, H.; Yildiz, B.; et al. Fast oxygen exchange and diffusion kinetics of grain boundaries in Sr-doped LaMnO3 thin films. Phys. Chem. Chem. Phys. 2015, 17, 7659–7669. [Google Scholar] [CrossRef]
- Saranya, A.M.; Pla, D.; Morata, A.; Cavallaro, A.; Canales-Vazquez, J.; Kilner, J.A.; Burriel, M.; Tarancon, A. Engineering mixed ionic electronic conduction in La0.8Sr0.2MnO3+δ nanostructures through fast grain boundary oxygen diffusivity. Adv. Energy Mater. 2015, 5, 1500377. [Google Scholar] [CrossRef]
- Sun, L.; Marrocchelli, D.; Yildiz, B. Edge dislocation slows down oxide ion diffusion in doped CeO2 by segregation of charged defects. Nat. Commun. 2015, 6, 6294. [Google Scholar] [CrossRef]
- Ma, W.; Kim, J.J.; Tsvetkov, N.; Daio, T.; Kuru, Y.; Cai, Z.; Chen, Y.; Sasaki, K.; Tuller, H.L.; Yildiz, B. Vertically aligned nanocomposite La0.8Sr0.2CoO3/(La0.5Sr0.5)2CoO4 cathodes—Electronic structure, surface chemistry and oxygen reduction kinetics. J. Mater. Chem. A 2015, 3, 207–219. [Google Scholar] [CrossRef]
- Marrocchelli, D.; Sun, L.; Yildiz, B. Dislocation in SrTiO3: Easy to reduce but not so fast for oxygen transport. J. Am. Chem. Soc. 2015, 137, 4735–4748. [Google Scholar] [CrossRef] [PubMed]
- Saranya, A.M.; Morata, A.; Pla, D.; Burriel, M.; Chiabrera, F.; Garbayo, I.; Hornes, A.; Kilner, J.A.; Tarancon, A. Unveiling the outstanding oxygen mass transport properties of Mn-rich perovskites in grain boundary-dominated La0.8Sr0.2(Mn1-xCox)0.85O3±δ nanostructures. Chem. Mater. 2018, 30, 5621–5629. [Google Scholar] [PubMed]
- Lee, D.; Gao, X.; Sun, L.; Jee, Y.; Poplawsky, J.; Farmer, T.O.; Fan, L.; Guo, E.-J.; Lu, Q.; Heller, W.T.; et al. Colossal oxygen vacancy formation at a fluorite-bixbyite interface. Nat. Commun. 2021, 11, 1371. [Google Scholar] [CrossRef]
- Baiutti, F.; Chiabrera, F.; Acosta, M.; Diercks, D.; Parfitt, D.; Santiso, J.; Wang, X.; Cavallaro, A.; Morata, A.; Wang, H.; et al. A high-entropy manganite in an ordered nanocomposite for long-term application in solid oxide cells. Nat. Commun. 2021, 12, 2660. [Google Scholar] [CrossRef] [PubMed]
- Kuganathan, N.; Baiutti, F.; Tarancon, A.; Fleig, J.; Chroneos, A. Defects energetics in the SrTiO3-LaCrO3 system. Solid State Ion. 2021, 361, 115570. [Google Scholar] [CrossRef]
- Saravanan, K.; Mason, C.W.; Rudola, A.; Wong, K.H.; Balaya, P. The First Report on Excellent Cycling Stability and Superior Rate Capability of Na3V2(PO4)3 for Sodium Ion Batteries. Adv. Energy Mater. 2013, 3, 444–450. [Google Scholar] [CrossRef]
- Serras, P.; Palomares, V.; Goñi, A.; Kubiak, P.; Rojo, T. Electrochemical performance of mixed valence Na3V2O2x(PO4)2F3-2x/C as cathode for sodium-ion batteries. J. Power Sources 2013, 241, 56–60. [Google Scholar] [CrossRef]
- Sharma, N.; Serras, P.; Palomares, V.; Brand, H.E.A.; Alonso, J.; Kubiak, P.; Fdez-Gubieda, M.L.; Rojo, T. Sodium distribution and reaction mechanisms of a Na3V2O2(PO4)2F Electrode during use in a sodium-ion battery. Chem. Mater. 2014, 26, 3391–3402. [Google Scholar] [CrossRef]
- Bui, K.M.; Dinh, V.A.; Okada, S.; Ohno, T. Hybrid functional study of the NASICON-type Na3V2(PO4)3: Crystal and electronic structures, and polaron-Na vacancy complex diffusion. Phys. Chem. Chem. Phys. 2015, 17, 30433–30439. [Google Scholar] [CrossRef]
- Li, H.; Peng, L.; Zhu, Y.; Chen, D.; Zhang, X.; Yu, G. An advanced high-energy sodium ion full battery based on nanostructured Na2Ti3O7/VOPO4 layered materials. Energy Environ. Sci. 2016, 9, 3399–3405. [Google Scholar] [CrossRef]
- Shen, W.; Li, H.; Guo, Z.; Wang, C.; Li, Z.; Xu, Q.; Liu, H.; Wang, Y.; Xia, Y. Double-Nanocarbon Synergistically Modified Na3V2(PO4)3: An Advanced Cathode for High-Rate and Long-Life Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15341–15351. [Google Scholar] [CrossRef] [PubMed]
- Vellaisamy, M.; Reddy, M.V.; Chowdari, B.V.R.; Kalaiselvi, N. Exploration of AVP2O7/C (A = Li, Li0.5Na0.5, and Na) for High-Rate Sodium-Ion Battery Applications. J. Phys. Chem. C 2018, 122, 24609–24618. [Google Scholar] [CrossRef]
- Jin, T.; Li, H.; Zhu, K.; Wang, P.F.; Liu, P.; Jiao, L. Polyanion-type cathode materials for sodium-ion batteries. Chem. Soc. Rev. 2020, 49, 2342–2377. [Google Scholar] [CrossRef] [PubMed]
- Zakharkin, M.V.; Drozhzhin, O.A.; Ryazantsev, S.V.; Chernyshov, D.; Kirsanova, M.A.; Mikheev, I.V.; Pazhetnov, E.M.; Antipov, E.V.; Stevenson, K.J. Electrochemical properties and evolution of the phase transformation behavior in the NASICON-type Na3+xMnxV2-x(PO4)3 (0≤x≤1) cathodes for Na-ion batteries. J. Power Sources 2020, 470, 228231. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Chotard, J.-N.; Fauth, F.; Masquelier, C. Na7V3(P2O7)4 as a high voltage electrode material for Na-ion batteries: Crystal structure and mechanism of Na+ extraction/insertion by operando X-ray diffraction. J. Mater. Chem. A 2020, 8, 21110–21121. [Google Scholar] [CrossRef]
- Zhao, D.; Wang, C.; Ding, Y.; Ding, M.; Cao, Y.; Chen, Z. Will Vanadium-Based Electrode Materials Become the Future Choice for Metal-Ion Batteries? ChemSusChem 2022, 15, e202200479. [Google Scholar] [CrossRef]


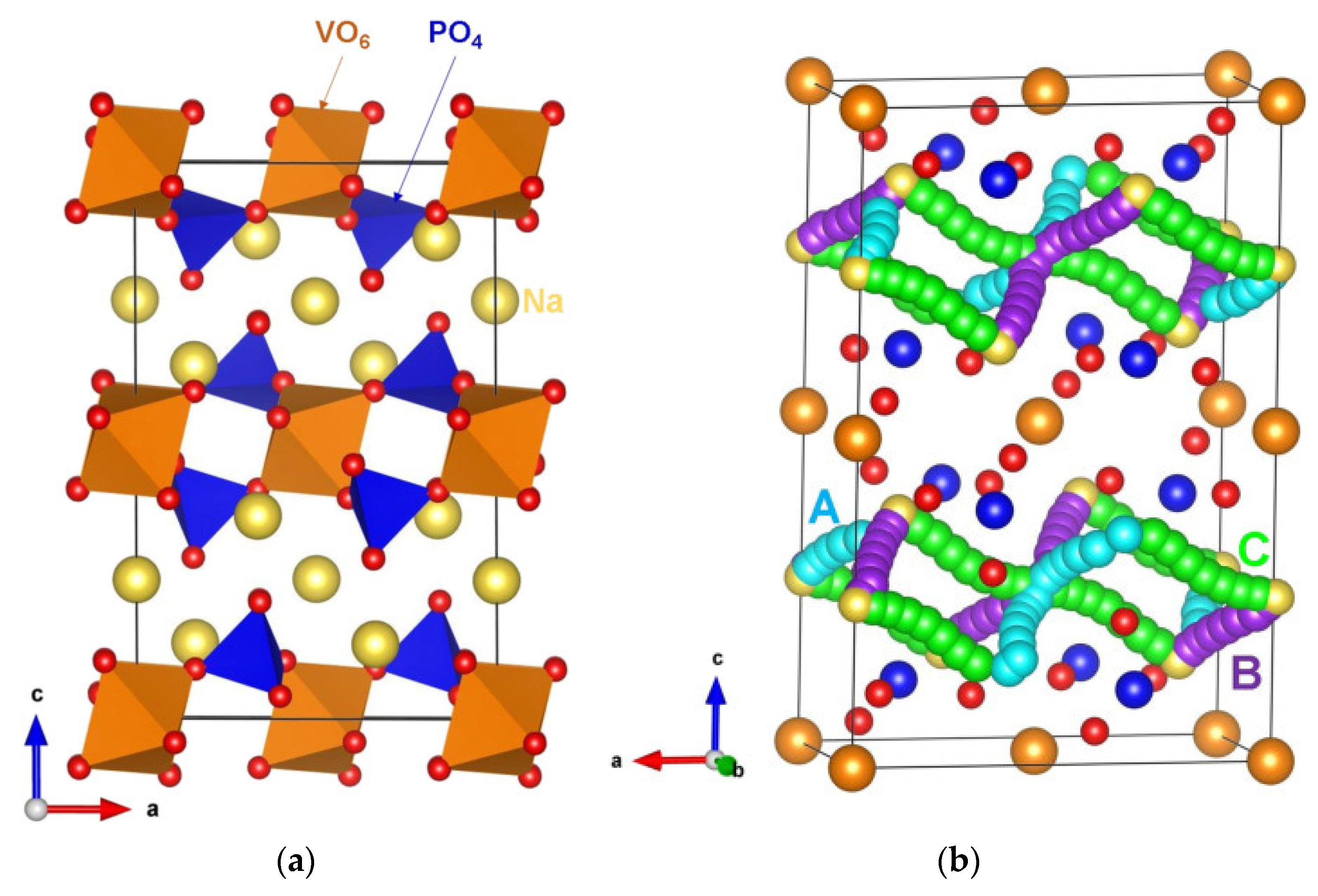
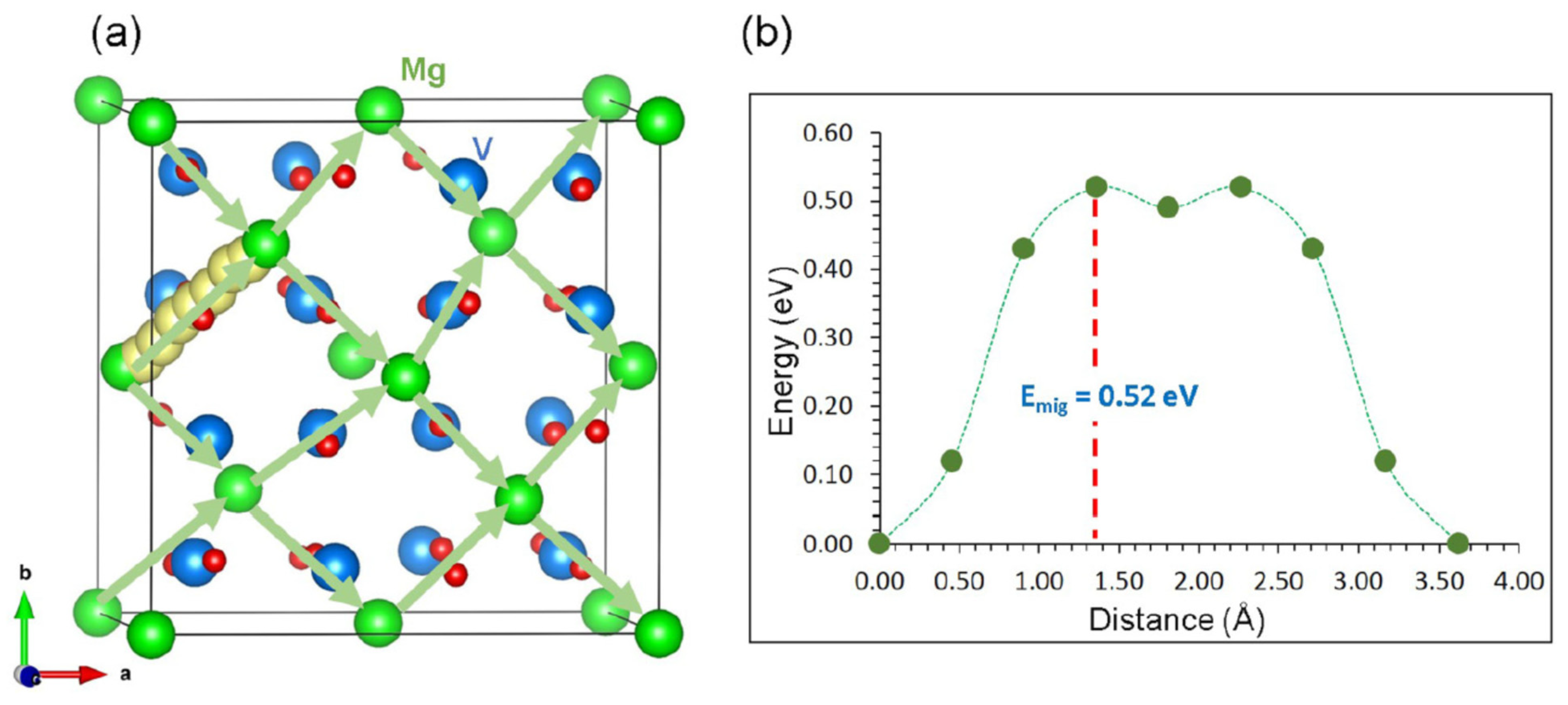

| Material | Activation Energy/eV | Comments |
|---|---|---|
| NaZr2(PO4)3 | 0.26 | Ref. [47], vacancy mechanism |
| Na3Fe2(PO4)3 | 0.45 | Ref. [95], vacancy mechanism |
| Na3V(PO4)2 | 0.59 | Ref. [94], vacancy mechanism |
| NaNiO2 | 0.67 | Ref. [96], ab-plane |
| NaFeO2 | 0.65–0.67 | Ref. [97], vacancy mechanism |
| Na2MnSiO4 | 0.81 | Ref. [41], vacancy mechanism |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sgourou, E.N.; Daskalopulu, A.; Tsoukalas, L.H.; Goulatis, I.L.; Vovk, R.V.; Chroneos, A. Kinetics of Ions in Post-Lithium Batteries. Appl. Sci. 2023, 13, 9619. https://doi.org/10.3390/app13179619
Sgourou EN, Daskalopulu A, Tsoukalas LH, Goulatis IL, Vovk RV, Chroneos A. Kinetics of Ions in Post-Lithium Batteries. Applied Sciences. 2023; 13(17):9619. https://doi.org/10.3390/app13179619
Chicago/Turabian StyleSgourou, Efstratia N., Aspassia Daskalopulu, Lefteri H. Tsoukalas, Ioannis L. Goulatis, Ruslan V. Vovk, and Alexander Chroneos. 2023. "Kinetics of Ions in Post-Lithium Batteries" Applied Sciences 13, no. 17: 9619. https://doi.org/10.3390/app13179619








