Experimental Study on Properties of Syngas, Tar, and Biochar Derived from Different Gasification Methods
Abstract
:1. Introduction
2. Experimental Section
2.1. Raw Material
2.2. Experimental System and Procedure
2.3. Products Analysis
3. Results and Discussion
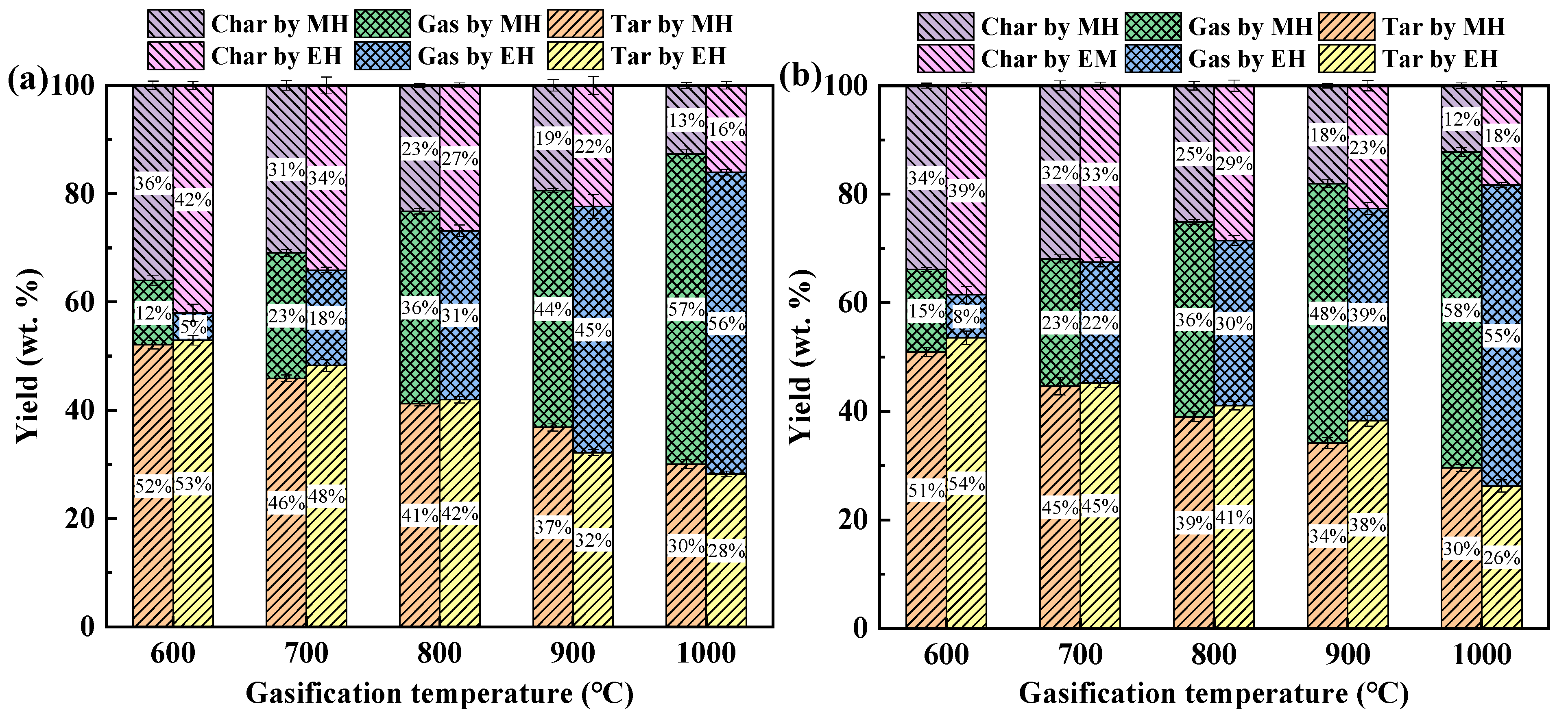
3.1. Analysis of Gasification Product Components
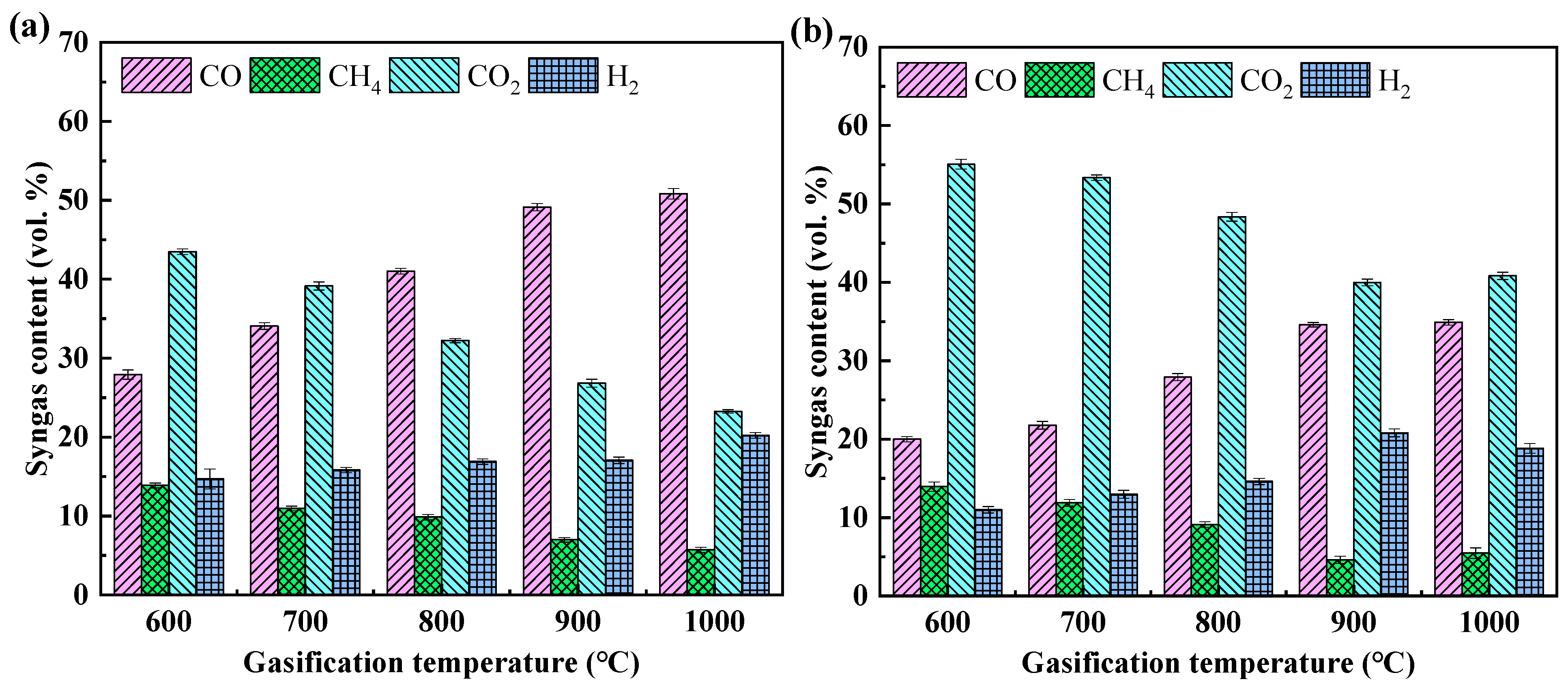
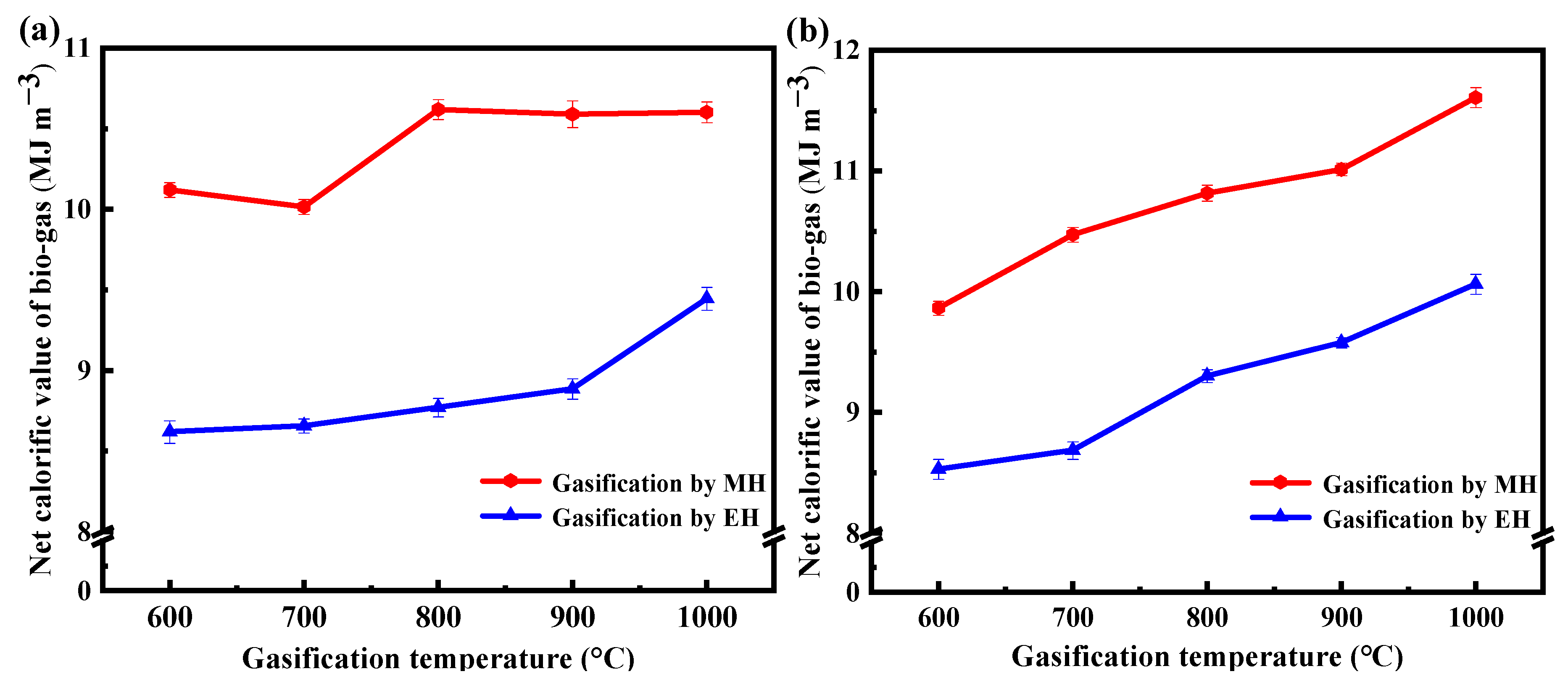
3.2. Analysis of Gaseous Products
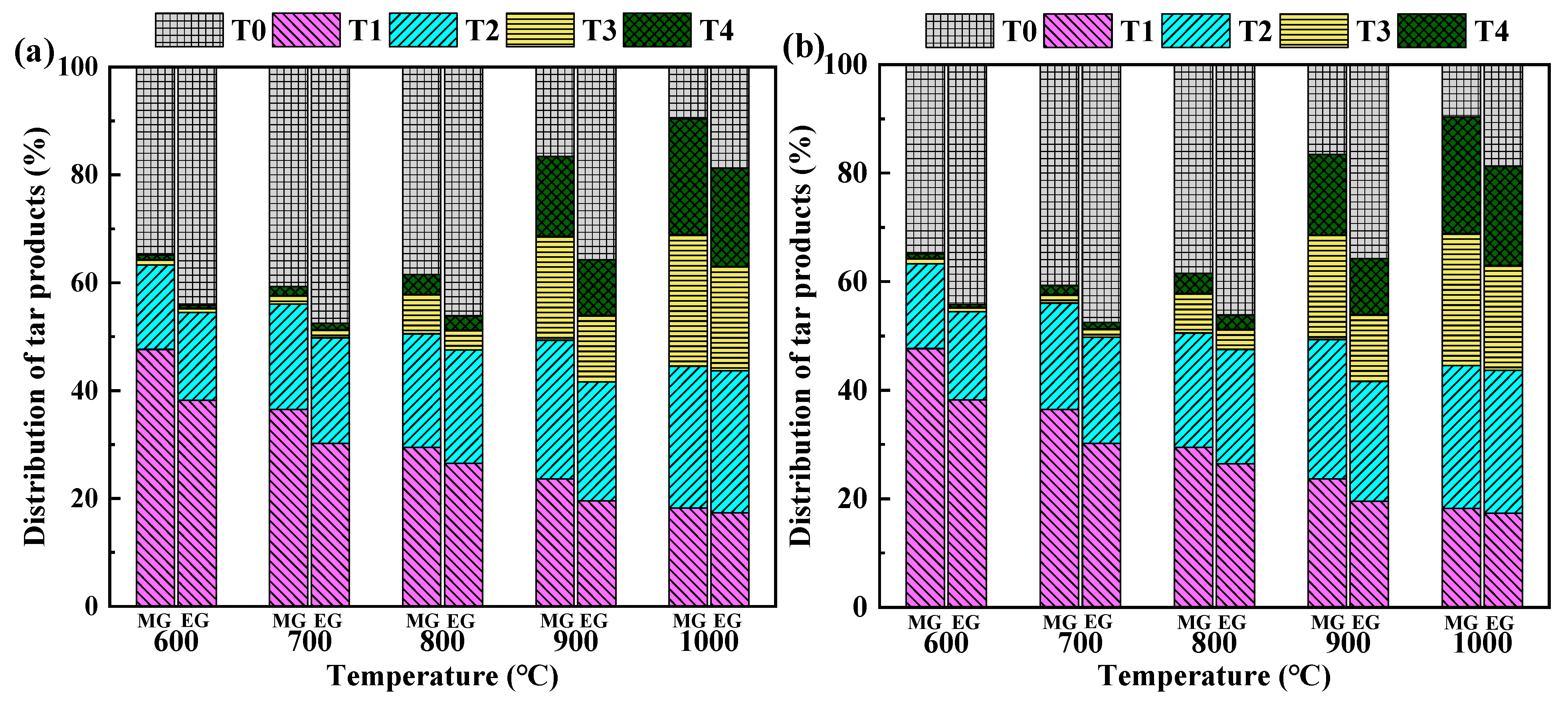
3.3. Analysis of Liquid-Phase Products
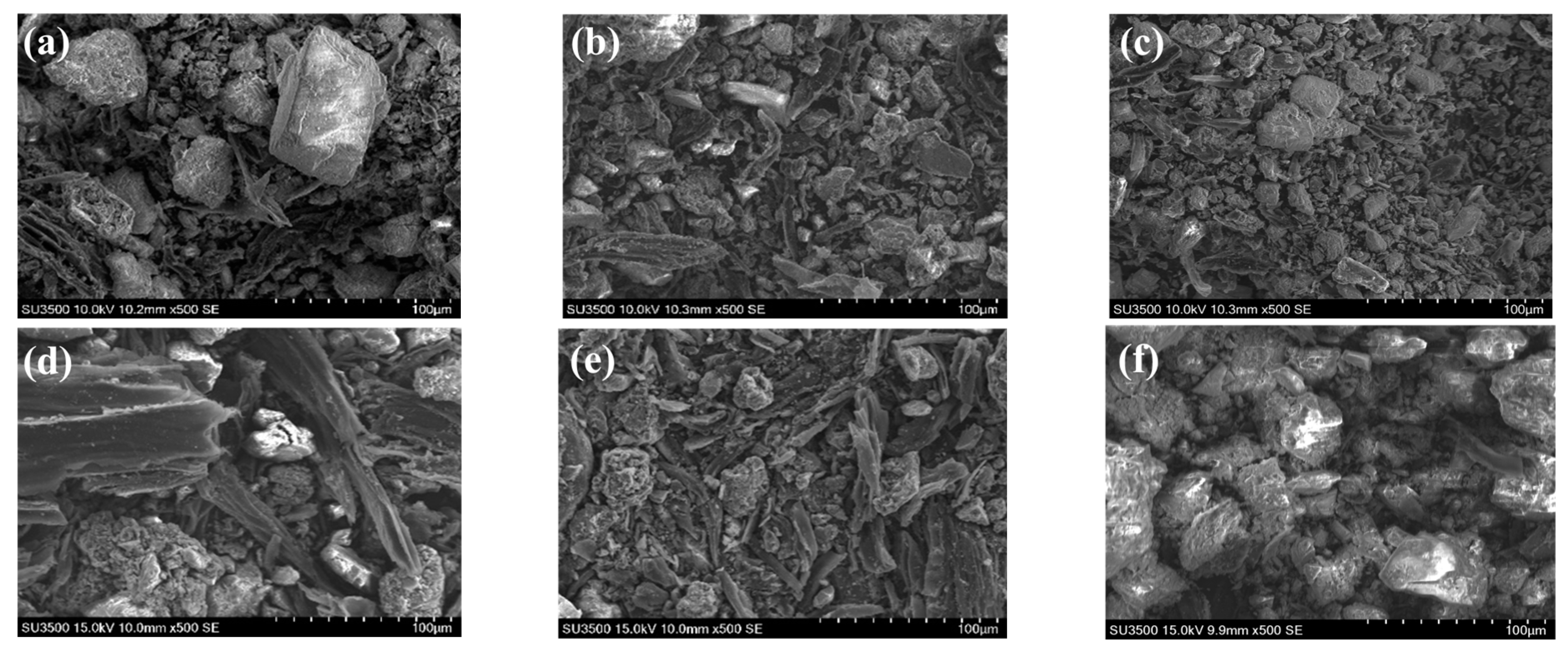
3.4. Characterization of Biochar
3.4.1. SEM-EDS Analysis
3.4.2. BET Analysis
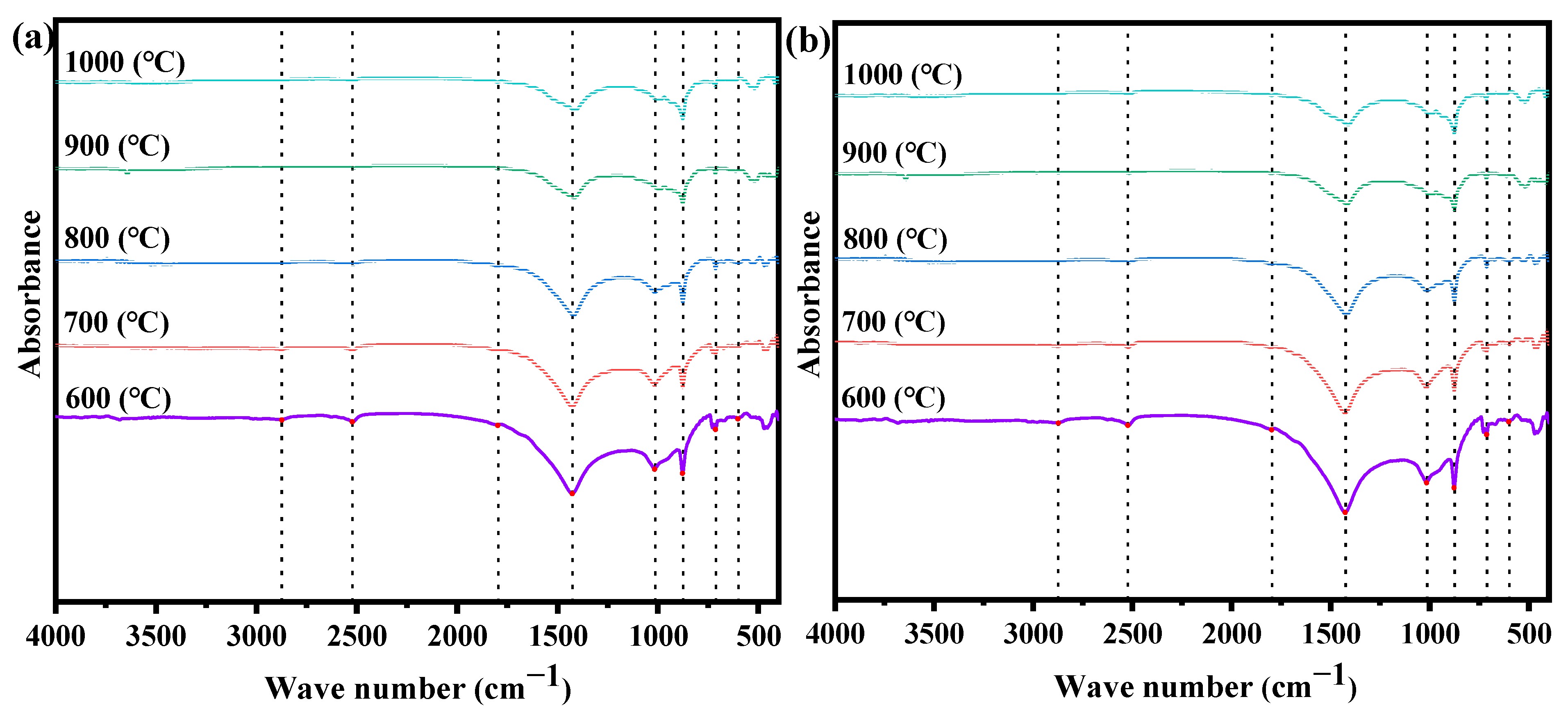
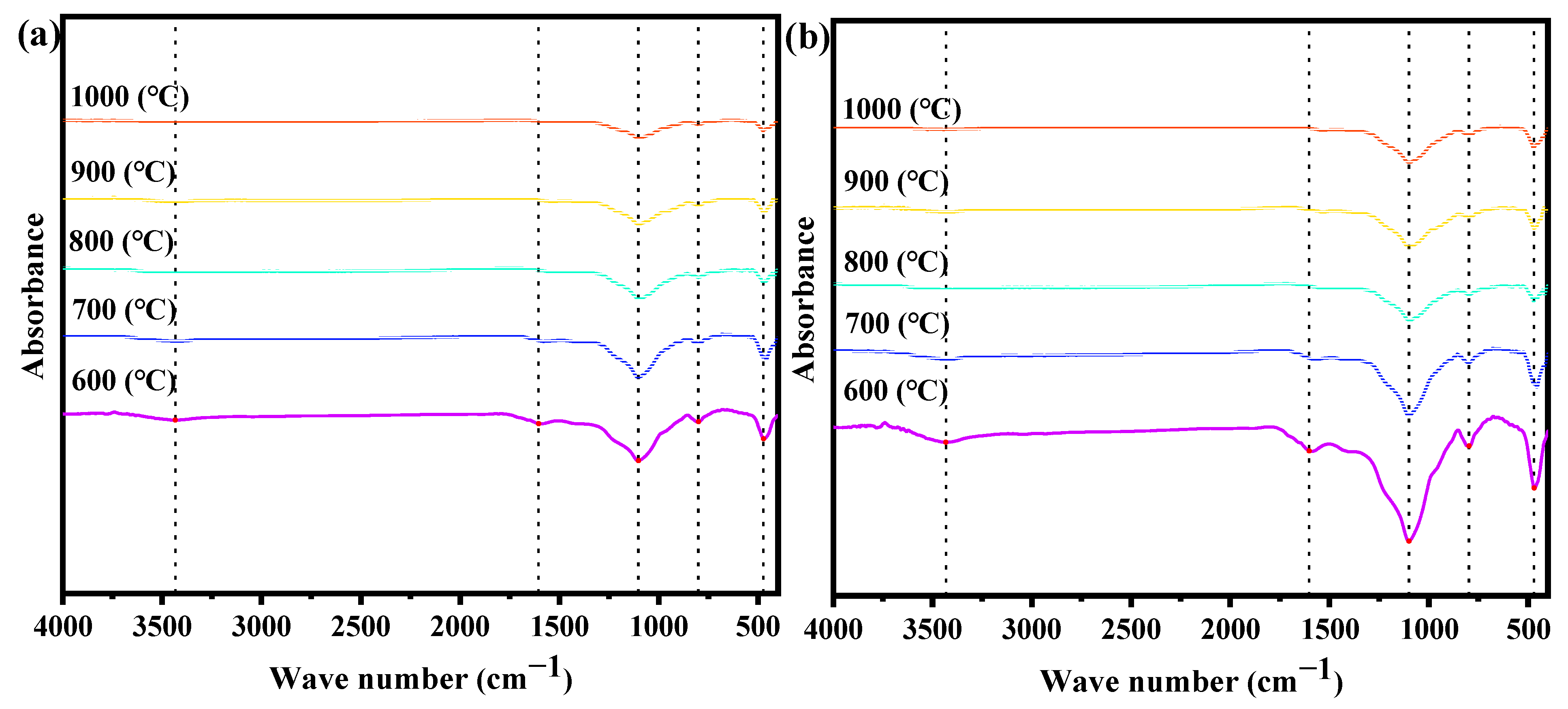
3.4.3. FTIR Analysis
4. Conclusions
- As the gasification temperature increases, the yield of biochar and tar decreases significantly, while the syngas yield experiences a substantial increase. MG produces more syngas and less biochar than EG. Compared to EG, MG of biomass is a better method to be applied to produce the combustible gases to replace the fossil fuels;
- The syngas mainly comprises H2, CH4, CO, and CO2. When the gasification temperature increases from 600 to 1000 °C, the contents of CO and H2 increase, while the contents of CH4 and CO2 decrease. EG produces more CO and less CO2 at the same temperature as MG. Higher gasification temperatures facilitate an increase in the calorific value of the syngas. At 1000 °C, the highest net calorific values of syngas generated from corn stalk and oak MG are 11.61 and 10.60 MJ m−3;
- The tar mainly comprises the benzene ring compounds, the polycyclic aromatic hydrocarbons, and certain aliphatic hydrocarbons. When the gasification temperature increases, more primary tars are converted into PAHs. The yield of T0 tar initially increases and then decreases, while T4 tar continuously increases. MG produces tars with more benzene rings than EG. Employing only CO2 as the gasification agent produces more tar in this study. For subsequent biomass gasification studies, a certain percentage of O2 could be added to the gasification agent to crack some tar;
- MG produces biochar with a richer microporous structure than EG. The biochar from MG has a higher specific surface area, pore volume, and average pore diameter than EG. The specific surface area of biochar from corn stalk MG reaches a maximum value of 525.87 m2 g−1 at 900 °C. The intensity of the characteristic peaks of functional groups on the surface of biochar decreases with increasing gasification temperature. Biochar in a microporous structure obtained via EG would be a promising absorbent during industry.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Nomenclature/Abbreviations
| Nomenclature | |
| carbon conversion/% | |
| volume of the produced gas per unit mass of biomass/L g−1 | |
| atomic weight of carbon/g mol−1 | |
| molar volume at standard conditions/L mol−1 | |
| Abbreviations | |
| electric gasification | |
| microwave gasification | |
| gas chromatography | |
| gas chromatography–mass spectrometry | |
| Fourier transform infrared spectrometer | |
| X-ray photoelectron spectroscopy | |
| scanning electron microscopy | |
| energy dispersive spectrometer | |
| polycyclic aromatic hydrocarbons | |
| alkali and alkaline earth metals |
References
- Global Energy Review 2021; IEA: Paris, France, 2021. Available online: http://www.iea.org/reports/global-energy-review-2021 (accessed on 17 August 2022).
- Alonso, D.M.; Wettstein, S.G.; Dumesic, J.A. Bimetallic catalysts for upgrading of biomass to fuels and chemicals. Chem. Soc. Rev. 2012, 41, 8075–8098. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Depren, S.; Kartal, M.T.; Çoban Çelikdemir, N.; Depren, Ö. Energy consumption and environmental degradation nexus: A systematic review and meta-analysis of fossil fuel and renewable energy consumption. Ecol. Inform. 2022, 70, 101747. [Google Scholar] [CrossRef]
- McKendry, P. Energy production from biomass (part 2): Conversion technologies. Bioresour. Technol. 2002, 83, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Richardson, Y.; Blin, J.; Julbe, A. A short overview on purification and conditioning of syngas produced by biomass gasification: Catalytic strategies, process intensification and new concepts. Prog. Energy Combust. Sci. 2012, 38, 765–781. [Google Scholar] [CrossRef]
- Halder, P. Perceptions of energy production from forest biomass among school students in Finland: Directions for the future bioenergy policies. Renew. Energy 2014, 68, 372–377. [Google Scholar] [CrossRef]
- Uris, M.; Linares, J.I.; Arenas, E. Techno-economic feasibility assessment of a biomass cogeneration plant based on an Organic Rankine Cycle. Renew. Energy 2014, 66, 707–713. [Google Scholar] [CrossRef]
- Xie, Q.; Borges, F.C.; Cheng, Y.; Wan, Y.; Li, Y.; Lin, X.; Liu, Y.; Hussain, F.; Chen, P.; Ruan, R. Fast microwave-assisted catalytic gasification of biomass for syngas production and tar removal. Bioresour. Technol. 2014, 156, 291–296. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, J.; Sun, B.; Yan, Z. Influence of catalyst types on the microwave-induced pyrolysis of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 106, 86–91. [Google Scholar] [CrossRef]
- Zhang, B.; Zhong, Z.; Chen, P.; Ruan, R. Microwave-assisted catalytic fast pyrolysis of biomass for bio-oil production using chemical vapor deposition modified HZSM-5 catalyst. Bioresour. Technol. 2015, 197, 79–84. [Google Scholar] [CrossRef]
- Zhu, L.; Lei, H.; Wang, L.; Yadavalli, G.; Zhang, X.; Wei, Y.; Liu, Y.; Yan, D.; Chen, S.; Ahring, B. Biochar of corn stover: Microwave-assisted pyrolysis condition induced changes in surface functional groups and characteristics. J. Anal. Appl. Pyrolysis 2015, 115, 149–156. [Google Scholar] [CrossRef]
- Purakayastha, T.J.; Bera, T.; Bhaduri, D.; Sarkar, B.; Mandal, S.; Wade, P.; Kumari, S.; Biswas, S.; Menon, M.; Pathak, H.; et al. A review on biochar modulated soil condition improvements and nutrient dynamics concerning crop yields: Pathways to climate change mitigation and global food security. Chemosphere 2019, 227, 345–365. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, K.; Jiang, J.; Zhu, Z.; Deng, L.; Che, D. Catalytic conversion of toluene by biochar modified with KMnO4. Fuel 2023, 332, 126237. [Google Scholar] [CrossRef]
- Chan, Y.H.; Cheah, K.W.; How, B.S.; Loy, A.C.M.; Shahbaz, M.; Singh, H.K.G.; Yusuf, N.R.; Shuhaili, A.F.A.; Yusup, S.; Ghani, W.A.W.A.K.; et al. An overview of biomass thermochemical conversion technologies in Malaysia. Sci. Total Environ. 2019, 680, 105–123. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; He, Q.; Bai, J.; Yu, J.; Kong, L.; Bai, Z.; Li, H.; He, C.; Cao, X.; Ge, Z.; et al. Char reactivity and kinetics based on the dynamic char structure during gasification by CO2. Fuel Process. Technol. 2021, 211, 106583. [Google Scholar] [CrossRef]
- Pinto, F.; André, R.N.; Carolino, C.; Miranda, M. Hot treatment and upgrading of syngas obtained by co-gasification of coal and wastes. Fuel Process. Technol. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Molino, A.; Chianese, S.; Musmarra, D. Biomass gasification technology: The state of the art overview. J. Energy Chem. 2016, 25, 10–25. [Google Scholar] [CrossRef]
- Gilbe, C.; Öhman, M.; Lindström, E.; Boström, D.; Backman, R.; Samuelsson, R.; Burvall, J. Slagging Characteristics during Residential Combustion of Biomass Pellets. Energy Fuels 2008, 22, 3536–3543. [Google Scholar] [CrossRef]
- Su, F.; Sun, S.; Zhao, Y.; Li, L. Studies on mechanism of biomass air gasification. J. Harbin Inst. Technol. 2006, 11, 1898–1902. [Google Scholar] [CrossRef]
- Heidenreich, S.; Foscolo, P.U. New concepts in biomass gasification. Prog. Energy Combust. Sci. 2015, 46, 72–95. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.J.; Fennell, P.S. An overview of advances in biomass gasification. Energy Environ. Sci. 2016, 9, 2939–2977. [Google Scholar] [CrossRef]
- Karatas, H.; Akgun, F. Experimental results of gasification of walnut shell and pistachio shell in a bubbling fluidized bed gasifier under air and steam atmospheres. Fuel 2018, 214, 285–292. [Google Scholar] [CrossRef]
- Jin, X.; Ye, J.; Deng, L.; Che, D. Condensation Behaviors of Potassium during Biomass Combustion. Energy Fuels 2017, 31, 2951–2958. [Google Scholar] [CrossRef]
- Xu, D.; Yang, L.; Ding, K.; Zhang, Y.; Gao, W.; Huang, Y.; Sun, H.; Hu, X.; Syed-Hassan, S.S.A.; Zhang, S.; et al. Mini-Review on Char Catalysts for Tar Reforming during Biomass Gasification: The Importance of Char Structure. Energy Fuels 2020, 34, 1219–1229. [Google Scholar] [CrossRef]
- Spokas, K.A.; Cantrell, K.B.; Novak, J.M.; Archer, D.W.; Ippolito, J.A.; Collins, H.P.; Boateng, A.A.; Lima, I.M.; Lamb, M.C.; McAloon, A.J.; et al. Biochar: A Synthesis of Its Agronomic Impact beyond Carbon Sequestration. J. Environ. Qual. 2012, 41, 973–989. [Google Scholar] [CrossRef]
- Hov, S.; Paniagua, P.; Sætre, C.; Long, M.; Cornelissen, G.; Ritter, S. Stabilisation of Soft Clay, Quick Clay and Peat by Industrial By-Products and Biochars. Appl. Sci. 2023, 13, 9048. [Google Scholar] [CrossRef]
- Fellet, G.; Conte, P.; Marchiol, L. Biochar Effects on Ce Leaching and Plant Uptake in Lepidium sativum L. Grown on a Ceria Nanoparticle Spiked Soil. Appl. Sci. 2023, 13, 6846. [Google Scholar] [CrossRef]
- Wijitkosum, S.; Jiwnok, P. Elemental Composition of Biochar Obtained from Agricultural Waste for Soil Amendment and Carbon Sequestration. Appl. Sci. 2019, 9, 3980. [Google Scholar] [CrossRef]
- Saletnik, B.; Zaguła, G.; Bajcar, M.; Tarapatskyy, M.; Bobula, G.; Puchalski, C. Biochar as a Multifunctional Component of the Environment—A Review. Appl. Sci. 2019, 9, 1139. [Google Scholar] [CrossRef]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Microwave-enhanced CO2 gasification of oil palm shell char. Bioresour. Technol. 2014, 158, 193–200. [Google Scholar] [CrossRef]
- Mašek, O.; Budarin, V.; Gronnow, M.; Crombie, K.; Brownsort, P.; Fitzpatrick, E.; Hurst, P. Microwave and slow pyrolysis biochar—Comparison of physical and functional properties. J. Anal. Appl. Pyrolysis 2013, 100, 41–48. [Google Scholar] [CrossRef]
- Wu, C.; Budarin, V.L.; Gronnow, M.J.; De Bruyn, M.; Onwudili, J.A.; Clark, J.H.; Williams, P.T. Conventional and microwave-assisted pyrolysis of biomass under different heating rates. J. Anal. Appl. Pyrolysis 2014, 107, 276–283. [Google Scholar] [CrossRef]
- Sobhy, A.; Chaouki, J. Microwave-assisted biorefinery. Chem. Eng. Trans. 2010, 19, 25–29. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Fernández, Y.; Pis, J.J.; Nabais, J.M.V.; Carrott, P.J.M.; Carrott, M.M.L.R. Conventional and microwave induced pyrolysis of coffee hulls for the production of a hydrogen rich fuel gas. J. Anal. Appl. Pyrolysis 2007, 79, 128–135. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Inguanzo, M.; Pís, J.J. Production of bio-fuels by high temperature pyrolysis of sewage sludge using conventional and microwave heating. Bioresour. Technol. 2006, 97, 1185–1193. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-H.; Chen, H.-P.; Ding, X.-J.; Yang, H.-P.; Zhang, S.-H.; Shen, Y.-Q. Properties of gas and char from microwave pyrolysis of pine sawdust. BioResources 2009, 4, 946–959. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, J.; Song, Z.; Liu, H.; Li, L.; Ma, C. Microwave pyrolysis of straw bale and energy balance analysis. J. Anal. Appl. Pyrolysis 2011, 92, 43–49. [Google Scholar] [CrossRef]
- Huang, Y.F.; Kuan, W.H.; Lo, S.L.; Lin, C.F. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.K.; Sharma, A.K.; Pulla, R.H.; Sahoo, P.K. Performance analysis of a medium-scale downdraft gasifier using Lantana camera biomass as feeding material. Energy Sources Part Recovery Util. Environ. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Gutiérrez, J.; Rubio-Clemente, A.; Pérez, J.F. Effect of main solid biomass commodities of patula pine on biochar properties produced under gasification conditions. Ind. Crops Prod. 2021, 160, 113123. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.; Liu, H.; Ba, F.; Yan, S.; Hu, J. Pyrolysis/gasification of pine sawdust biomass briquettes under carbon dioxide atmosphere: Study on carbon dioxide reduction (utilization) and biochar briquettes physicochemical properties. Bioresour. Technol. 2018, 249, 983–991. [Google Scholar] [CrossRef]
- Ren, S.; Lei, H.; Wang, L.; Bu, Q.; Chen, S.; Wu, J.; Julson, J.; Ruan, R. Biofuel production and kinetics analysis for microwave pyrolysis of Douglas fir sawdust pellet. J. Anal. Appl. Pyrolysis 2012, 94, 163–169. [Google Scholar] [CrossRef]
- Du, J.; Liu, P.; Liu, Z.; Sun, D.; Tao, C. Fast pyrolysis of biomass for bio-oil with ionic liquid and microwave irradiation. J. Fuel Chem. Technol. 2010, 38, 554–559. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, M.; Liu, H.; Li, L.; Ma, C.; Song, Z. A microwave reactor for characterization of pyrolyzed biomass. Bioresour. Technol. 2012, 104, 673–678. [Google Scholar] [CrossRef] [PubMed]
- Salema, A.A.; Ani, F.N. Microwave-assisted pyrolysis of oil palm shell biomass using an overhead stirrer. J. Anal. Appl. Pyrolysis 2012, 96, 162–172. [Google Scholar] [CrossRef]
- Salema, A.A.; Ani, F.N. Microwave induced pyrolysis of oil palm biomass. Bioresour. Technol. 2011, 102, 3388–3395. [Google Scholar] [CrossRef]
- Zhao, X.; Song, Z.; Liu, H.; Li, Z.; Li, L.; Ma, C. Microwave pyrolysis of corn stalk bale: A promising method for direct utilization of large-sized biomass and syngas production. J. Anal. Appl. Pyrolysis 2010, 89, 87–94. [Google Scholar] [CrossRef]
- Wan, Y.; Chen, P.; Zhang, B.; Yang, C.; Liu, Y.; Lin, X.; Ruan, R. Microwave-assisted pyrolysis of biomass: Catalysts to improve product selectivity. J. Anal. Appl. Pyrolysis 2009, 86, 161–167. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- GB/T 30725-2014; Determination of Ash Composition in Solid Biofuels. China National Coal Association: Beijing, China, 2014.
- Menéndez, J.A.; Arenillas, A.; Fidalgo, B.; Fernández, Y.; Zubizarreta, L.; Calvo, E.G.; Bermúdez, J.M. Microwave heating processes involving carbon materials. Fuel Process. Technol. 2010, 91, 1–8. [Google Scholar] [CrossRef]
- Li, J.; Dai, J.; Liu, G.; Zhang, H.; Gao, Z.; Fu, J.; He, Y.; Huang, Y. Biochar from microwave pyrolysis of biomass: A review. Biomass Bioenergy 2016, 94, 228–244. [Google Scholar] [CrossRef]
- Ren, J.; Yang, K.; Li, Y.; Bai, Y.; Jiang, J.; Huang, X.; Deng, L.; Che, D. Catalytic gasification of large particle-size biomass with loaded AAEMs under oxygen-steam atmosphere. Fuel 2024, 357, 130019. [Google Scholar] [CrossRef]
- Yagmur, E.; Ozmak, M.; Aktas, Z. A novel method for production of activated carbon from waste tea by chemical activation with microwave energy. Fuel 2008, 87, 3278–3285. [Google Scholar] [CrossRef]
- Hanaoka, T.; Inoue, S.; Uno, S.; Ogi, T.; Minowa, T. Effect of woody biomass components on air-steam gasification. Biomass Bioenergy 2005, 28, 69–76. [Google Scholar] [CrossRef]
- Yu, J.; Guo, Q.; Gong, Y.; Ding, L.; Wang, J.; Yu, G. A review of the effects of alkali and alkaline earth metal species on biomass gasification. Fuel Process. Technol. 2021, 214, 106723. [Google Scholar] [CrossRef]
- Tian, T.; Li, Q.; He, R.; Tan, Z.; Zhang, Y. Effects of biochemical composition on hydrogen production by biomass gasification. Int. J. Hydrogen Energy 2017, 42, 19723–19732. [Google Scholar] [CrossRef]
- López-González, D.; Fernandez-Lopez, M.; Valverde, J.L.; Sanchez-Silva, L. Gasification of lignocellulosic biomass char obtained from pyrolysis: Kinetic and evolved gas analyses. Energy 2014, 71, 456–467. [Google Scholar] [CrossRef]
- Menéndez, J.A.; Domínguez, A.; Fernández, Y.; Pis, J.J. Evidence of Self-Gasification during the Microwave-Induced Pyrolysis of Coffee Hulls. Energy Fuels 2007, 21, 373–378. [Google Scholar] [CrossRef]
- Luque, R.; Menéndez, J.A.; Arenillas, A.; Cot, J. Microwave-assisted pyrolysis of biomass feedstocks: The way forward? Energy Environ. Sci 2012, 5, 5481–5488. [Google Scholar] [CrossRef]
- Elnour, A.Y.; Alghyamah, A.A.; Shaikh, H.M.; Poulose, A.M.; Al-Zahrani, S.M.; Anis, A.; Al-Wabel, M.I. Effect of Pyrolysis Temperature on Biochar Microstructural Evolution, Physicochemical Characteristics, and Its Influence on Biochar/Polypropylene Composites. Appl. Sci. 2019, 9, 1149. [Google Scholar] [CrossRef]



| Samples | Proximate Analysis | Ultimate Analysis | |||||||
|---|---|---|---|---|---|---|---|---|---|
| w (FC) | w (V) | w (A) | w (M) | w (C) | w (H) | w (O) | w (N) | w (S) | |
| Oak | 9.43 | 56.54 | 32.65 | 1.38 | 29.60 | 3.01 | 32.84 | 0.48 | 0.04 |
| Corn stalk | 17.49 | 64.19 | 8.80 | 9.52 | 40.34 | 4.63 | 35.36 | 1.17 | 0.18 |
| Compounds of Inorganics | Analysis Standard | Oak | Corn Stalk | |
|---|---|---|---|---|
| Ash analysis | Fe2O3 | GB/T 30725-2014 [50] | 3.87 | 3.67 |
| Al2O3 | 6.17 | 7.69 | ||
| CaO | 20.58 | 18.43 | ||
| MgO | 0.01 | 4.07 | ||
| SiO2 | 36.14 | 46.14 | ||
| TiO2 | 0.37 | 0.50 | ||
| SO3 | 1.45 | 1.65 | ||
| K2O | 0.05 | 5.09 | ||
| Na2O | 0.05 | 2.35 | ||
| P2O5 | 0.95 | 1.49 | ||
| MnO2 | 0.02 | 0.49 |
| Samples | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|
| Corn stalk | 36.5% | 34.9% | 18.8% |
| Oak | 44.9% | 21.5% | 29.3% |
| Name | Carbon Conversion (ηc, %) | ||||
|---|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | |
| CS MG | 63.62 | 69.85 | 73.26 | 80.37 | 72.76 |
| CS EG | 57.13 | 61.35 | 68.56 | 72.65 | 79.78 |
| O MG | 72.68 | 75.27 | 77.69 | 78.13 | 83.18 |
| O EG | 63.26 | 66.92 | 69.97 | 78.56 | 80.53 |
| Compound Name | Relative Content (%) at Different Gasification Heating Temperatures (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | ||||||
| MG | EG | MG | EG | MG | EG | MG | EG | MG | EG | |
| Ethylbenzene | 2.24 | 3.03 | 2.53 | 2.55 | - | 1.21 | - | 1.33 | 1.72 | - |
| 1-Cyclohexene, 1-ethynyl- | 3.46 | 8.43 | 3.74 | 3.24 | 2.46 | 1.34 | - | 2.64 | 1.5 | 1.20 |
| Styrene | 3.87 | 10.59 | 5.15 | 7.10 | 4.75 | 9.25 | - | 9.34 | 3.22 | 8.16 |
| Benzene | - | 2.09 | - | 1.02 | 1.46 | 3.24 | 2.46 | 1.11 | - | 2.85 |
| 3-Methylphenylacetylene | 5.84 | 1.72 | 8.35 | 2.15 | 7.67 | 0.92 | 2.01 | 0.31 | 5.86 | - |
| Biphenylene | 2.35 | 6.31 | - | 4.25 | 1.28 | 2.89 | 2.08 | - | 2.14 | 1.62 |
| Hematoporphyrin | 2.02 | 1.02 | - | 3.24 | 1.52 | 1.11 | - | 4.07 | - | 1.28 |
| Rhodopin | - | 5.19 | - | 1.38 | 1.67 | 3.76 | 1.92 | 5.44 | - | 3.20 |
| 2-Pentanone | 3.06 | 4.14 | - | 2.56 | 4.97 | - | 7.15 | 1.48 | - | 1.50 |
| Compound Name | Relative Content (%) at Different Gasification Heating Temperatures (°C) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | 900 °C | 1000 °C | ||||||
| MG | EG | MG | EG | MG | EG | MG | EG | MG | EG | |
| Phenylephrine | - | - | 0.85 | 1.25 | - | 1.82 | 3.73 | 3.73 | - | - |
| Biphenyl | 1.73 | - | 1.36 | - | 1.68 | 1.58 | 2.52 | 2.01 | - | 2.58 |
| Biphenylene | - | - | 1.68 | - | 8.11 | 2.11 | 4.34 | 4.34 | 5.23 | 5.23 |
| Hematoporphyrin | 0.52 | 3.03 | - | 3.03 | 0.25 | 2.55 | - | 1.21 | 2.33 | 1.33 |
| Tricarbonyl | 4.62 | 1.25 | 2.58 | 1.25 | 4.52 | - | - | 2.58 | 0.48 | 1.52 |
| 3′H-Cycloprop (1,2)-5-coolest-1-en-3-one | 1.44 | 1.44 | 8.43 | 8.43 | 3.24 | 3.24 | 1.34 | 1.34 | 2.64 | 2.64 |
| Hexadecanamide | 2.02 | 2.02 | 2.25 | 2.25 | 2.15 | 2.15 | 3.7 | 3.7 | 1.79 | 1.79 |
| 2-Pentanone | 5.19 | 5.19 | - | - | 1.92 | 1.92 | 1.86 | 1.86 | 1.98 | 1.98 |
| Toluene | 4.21 | 4.21 | 2.09 | 2.09 | 1.02 | 1.02 | 3.24 | 3.24 | 1.11 | 1.32 |
| Furfural | - | - | 3.43 | 3.43 | 3.66 | 3.66 | 4.95 | 4.95 | 4.42 | 4.42 |
| 1,2,4,5-Tetrazine | 3.17 | 3.17 | 1.72 | 1.72 | 2.10 | 2.15 | 0.9 | 1.20 | 0.32 | 2.38 |
| Methoxystyrene | 1.01 | - | - | 1.85 | 0.54 | 0.54 | 2.40 | - | - | 1.66 |
| EDS | Content (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| w (C) | w (O) | w (Mg) | w (Ca) | w (K) | w (Al) | w (Si) | |
| Spot 1 | 31.67 | 22.05 | 15.04 | 29.35 | - | - | 1.89 |
| Spot 2 | 7.58 | 22.04 | 17.63 | 22.19 | - | - | 24.62 |
| Spot 3 | 3.42 | 30.98 | 16.44 | 36.63 | - | 1.00 | 11.53 |
| Spot 4 | 58.45 | 23.64. | 4.36 | 9.42 | 0.49 | 0.39 | 3.25 |
| Spot 5 | 15.07 | 47.74 | 2.89 | 0.93 | 5.87 | 10.26 | 17.25 |
| Spot 6 | 4.93 | 35.60 | 5.40 | 48.90 | - | 0.91 | 4.27 |
| EDS | Content (wt.%) | ||||||
|---|---|---|---|---|---|---|---|
| w (C) | w (O) | w (Mg) | w (Ca) | w (K) | w (Si) | w (P) | |
| Spot 1 | 28.04 | 23.60 | 4.18 | - | 14.03 | 20.60 | 9.55 |
| Spot 2 | 8.02 | 39.10 | - | - | 1.36 | 51.52 | - |
| Spot 3 | 4.12 | 34.68 | - | - | - | 61.21 | - |
| Spot 4 | 39.79 | 33.54 | 0.39 | 0.31 | 1.68 | 23.73 | 0.06 |
| Spot 5 | 23.39 | 40.74 | - | - | 1.07 | 34.80 | - |
| Spot 6 | 4.65 | 50.27 | - | - | 0.3 | 44.78 | - |
| Name | BET (m2 g−1) | Total Pure Volume (cm3 g−1) | Average Pore Diameter (nm) | |||
|---|---|---|---|---|---|---|
| Samples | Oak Chars | Corn Stalk Chars | Oak Chars | Corn Stalk Chars | Oak Chars | Corn Stalk Chars |
| MG (°C) | ||||||
| 600 | 289.54 ± 9.22 | 452.25 ± 10.25 | 0.32 ± 0.06 | 0.32 ± 0.04 | 3.52 ± 0.19 | 3.92 ± 0.21 |
| 700 | 355.52 ± 7.21 | 481.25 ± 6.82 | 0.45 ± 0.03 | 0.42 ± 0.03 | 3.82 ± 0.28 | 4.03 ± 0.15 |
| 800 | 485.02 ± 7.35 | 512.32 ± 11.21 | 0.48 ± 0.04 | 0.52 ± 0.03 | 4.35 ± 0.30 | 4.18 ± 0.32 |
| 900 | 426.52 ± 5.29 | 525.87 ± 8.22 | 0.46 ± 0.02 | 0.62 ± 0.02 | 4.38 ± 0.42 | 4.23 ± 0.22 |
| 1000 | 385.25 ± 9.47 | 489.58 ± 7.56 | 0.42 ± 0.05 | 0.45 ± 0.04 | 4.25 ± 0.25 | 4.20 ± 0.45 |
| EG (°C) | ||||||
| 600 | 264.54 ± 11.25 | 312.25 ± 8.54 | 0.21 ± 0.03 | 0.18 ± 0.02 | 3.12 ± 0.41 | 3.65 ± 0.35 |
| 700 | 306.52 ± 6.74 | 408.26 ± 7.20 | 0.34 ± 0.04 | 0.26 ± 0.05 | 3.43 ± 0.36 | 2.59 ± 0.33 |
| 800 | 368.96 ± 8.96 | 481.31 ± 12.35 | 0.39 ± 0.04 | 0.38 ± 0.03 | 4.16 ± 0.33 | 3.21 ± 0.41 |
| 900 | 326.62 ± 10.11 | 502.52 ± 9.57 | 0.37 ± 0.06 | 0.51 ± 0.04 | 4.22 ± 0.27 | 3.53 ± 0.25 |
| 1000 | 225.25 ± 6.52 | 423.45 ± 10.52 | 0.25 ± 0.05 | 0.35 ± 0.05 | 4.24 ± 0.25 | 3.22 ± 0.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Jin, X.; Deng, L. Experimental Study on Properties of Syngas, Tar, and Biochar Derived from Different Gasification Methods. Appl. Sci. 2023, 13, 11490. https://doi.org/10.3390/app132011490
Yue Y, Jin X, Deng L. Experimental Study on Properties of Syngas, Tar, and Biochar Derived from Different Gasification Methods. Applied Sciences. 2023; 13(20):11490. https://doi.org/10.3390/app132011490
Chicago/Turabian StyleYue, Yang, Xiaoling Jin, and Lei Deng. 2023. "Experimental Study on Properties of Syngas, Tar, and Biochar Derived from Different Gasification Methods" Applied Sciences 13, no. 20: 11490. https://doi.org/10.3390/app132011490





