Deterioration Process of Cementitious Material Properties under Internal Sulphate Attack
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Cement
2.1.2. Gypsum Aggregate
2.1.3. Standard Sand
2.1.4. Tricalcium Aluminate (C3A)
2.2. Mix Proportion
2.3. Formation Conditions of AFt and Gypsum
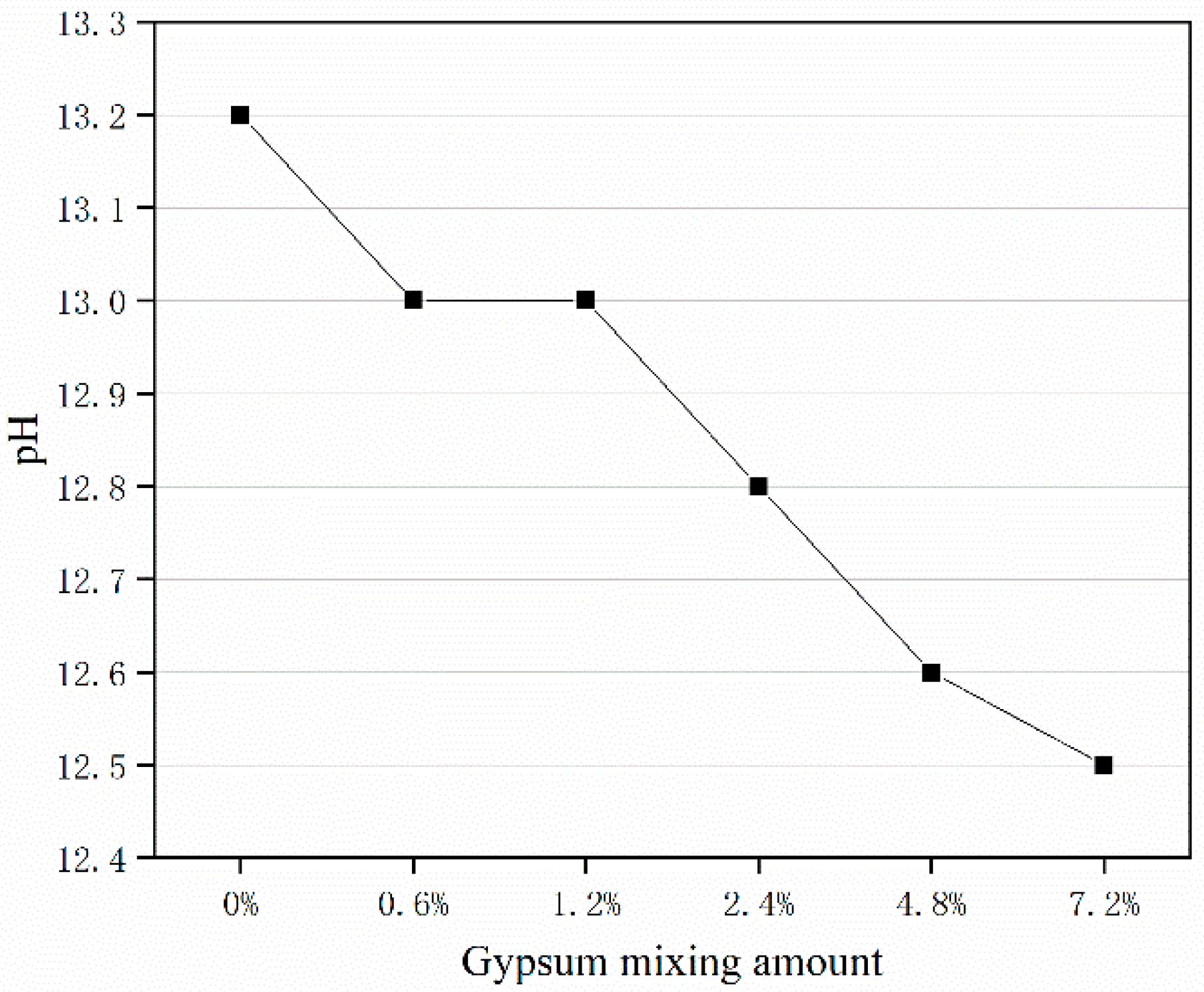
2.4. pH Value of Mortar Test
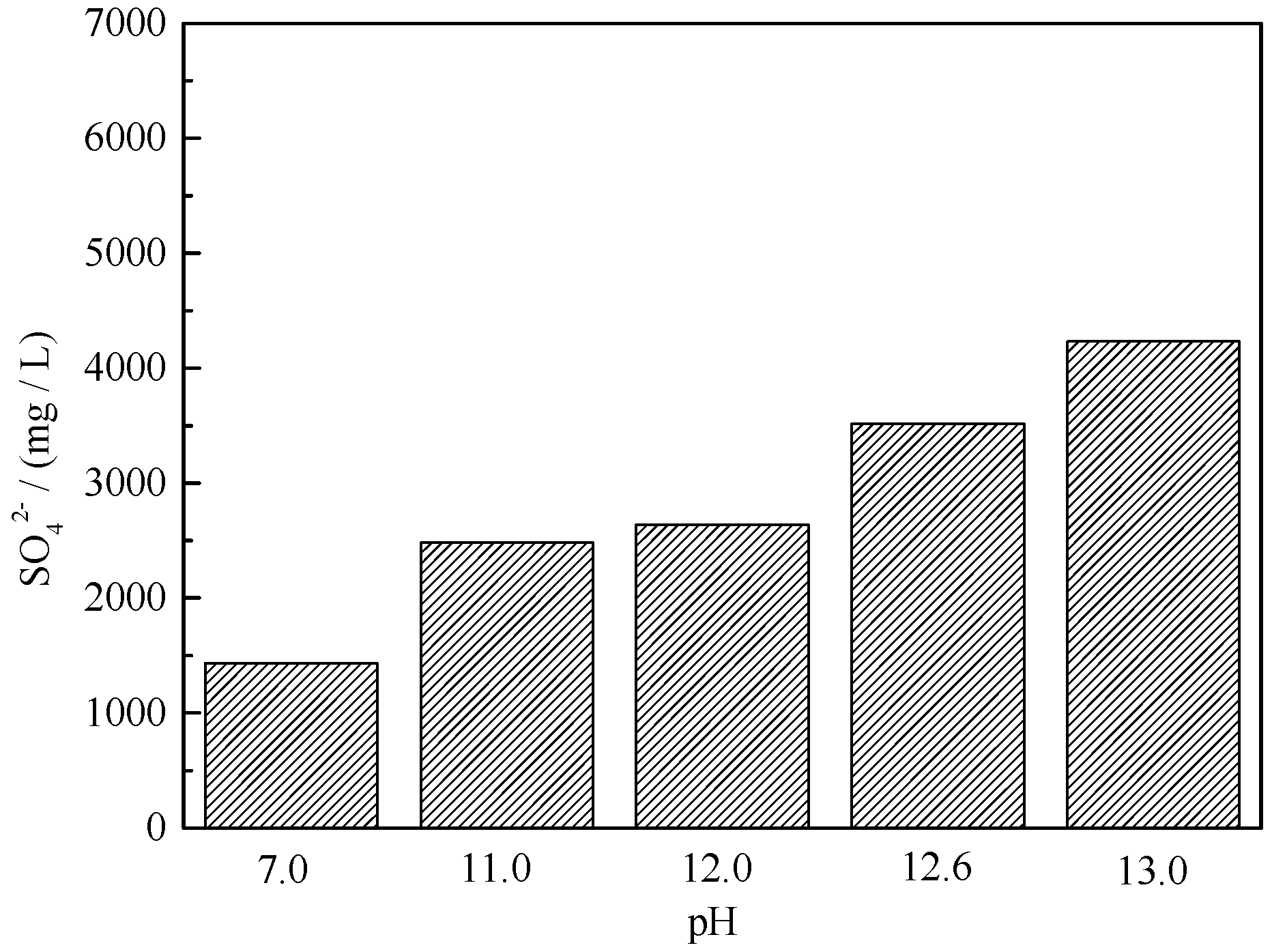
2.5. Dissolution of Gypsum in Solution with Different pH Values
2.6. Test Methods
2.6.1. Expansion Measurement
2.6.2. Strength Test
2.6.3. Mineral Components Analysis
2.6.4. SEM Analysis
2.6.5. MIP Analysis
3. Results and Discussion
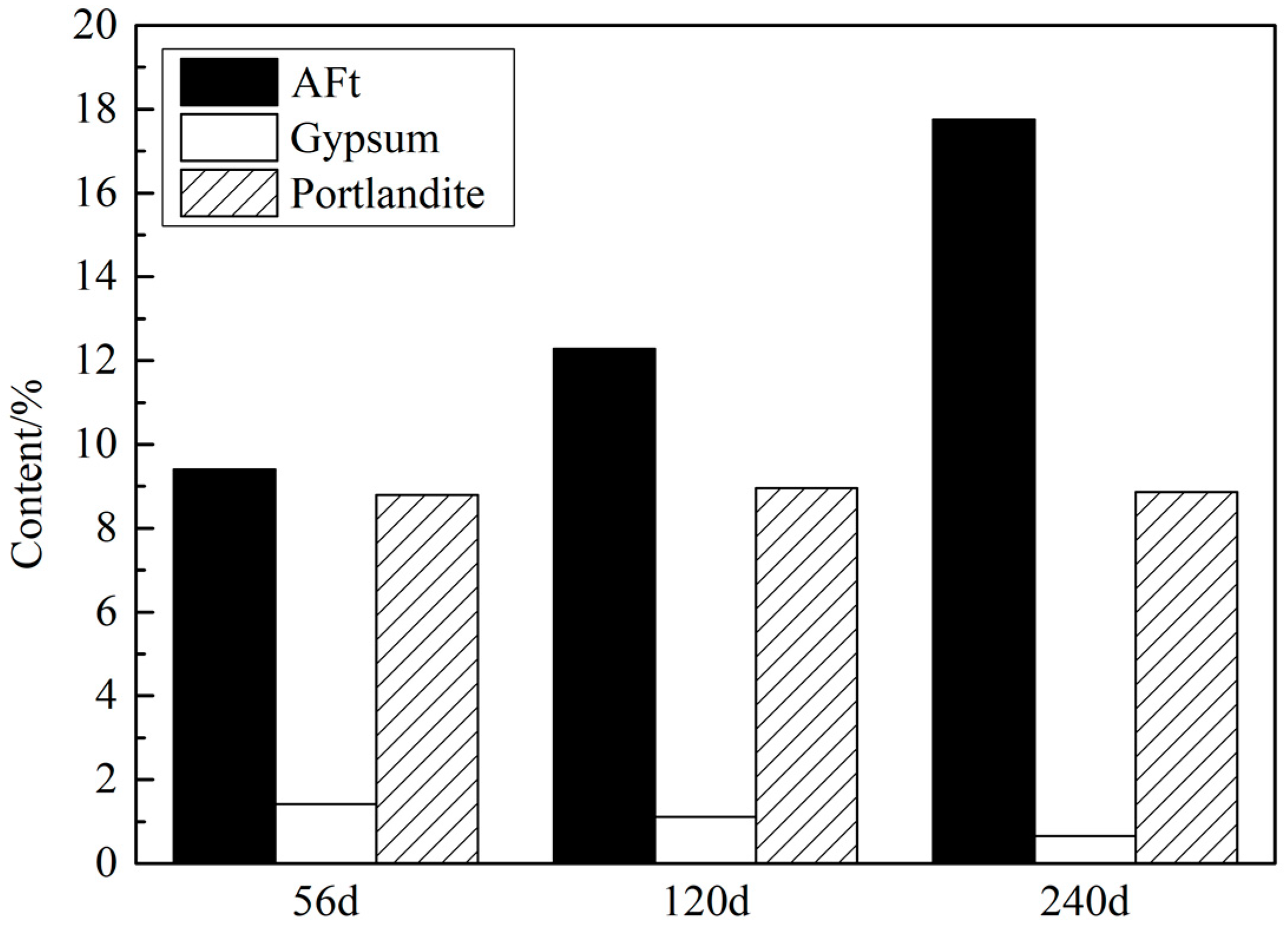
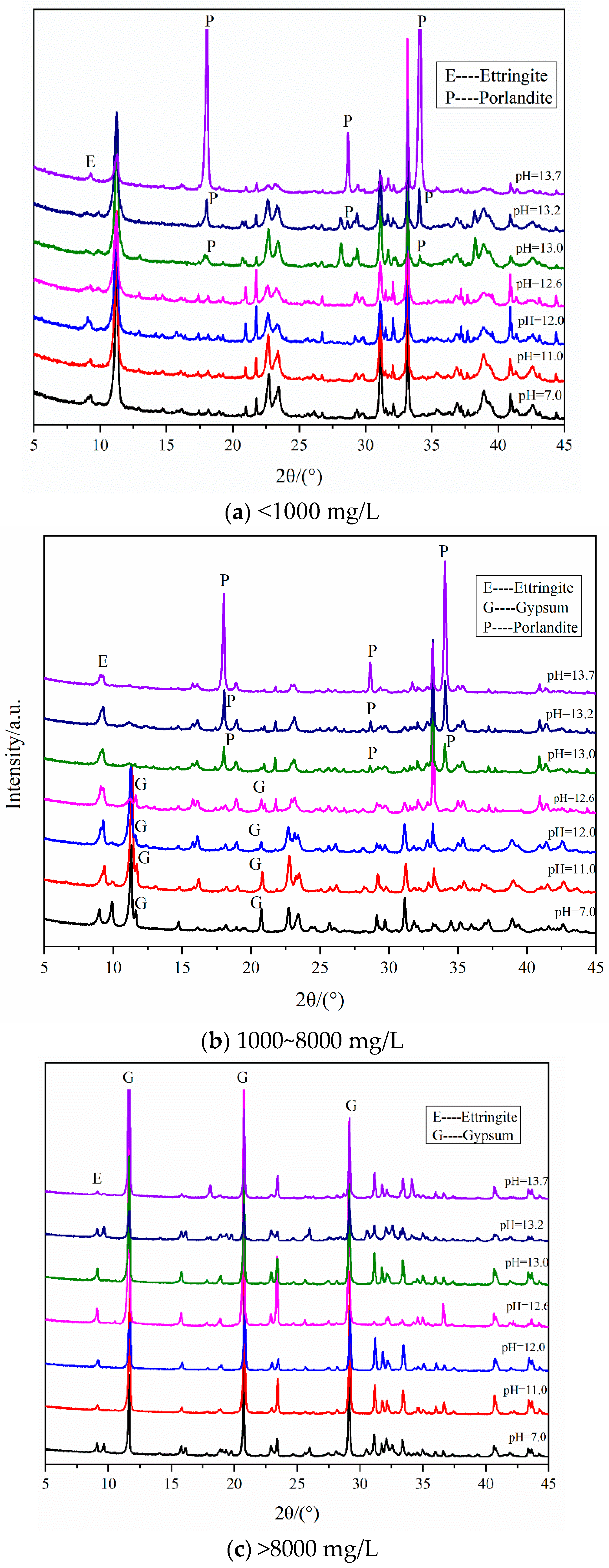
3.1. XRD Analysis
3.1.1. Samples with Different Contents of Gypsum
3.1.2. Samples Curing in Different Temperatures
3.1.3. Samples with Different Size of Gypsum Sand (<0.3 mm)
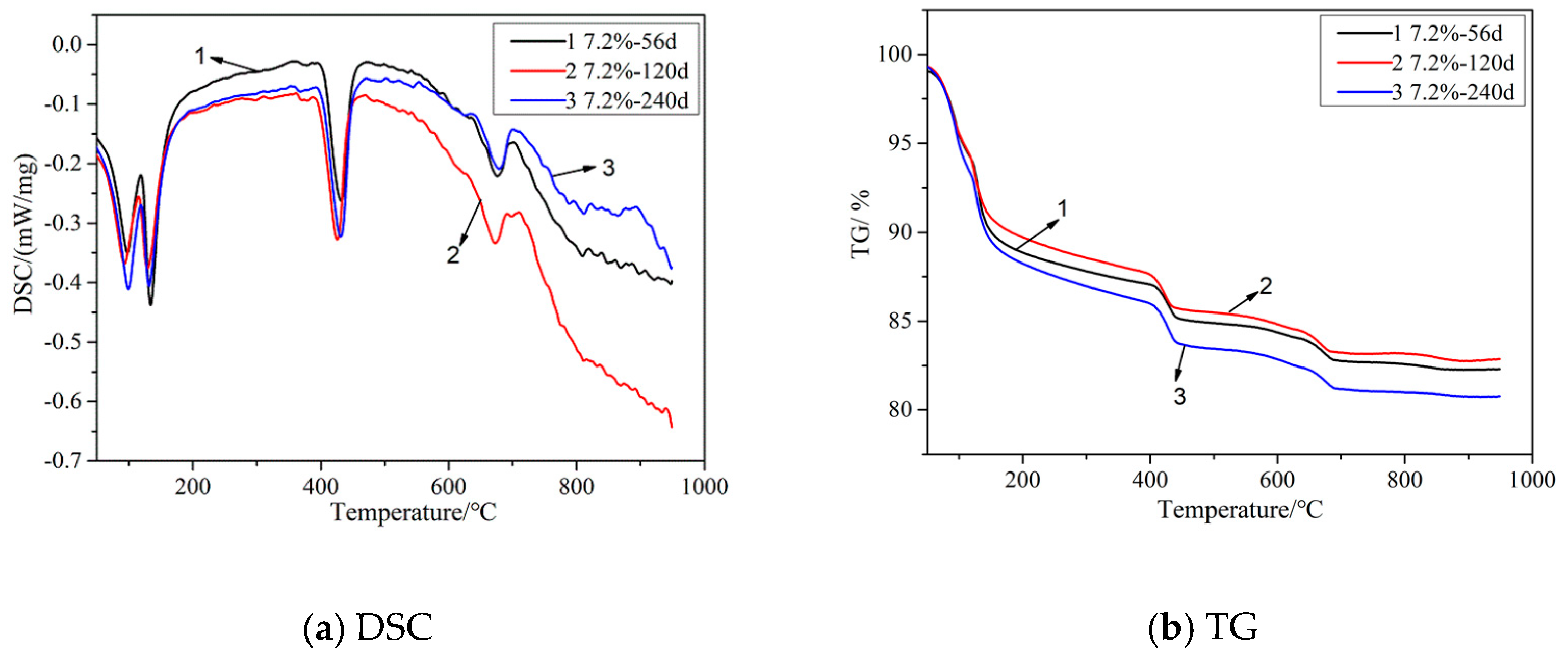
3.2. TG–DSC Analysis
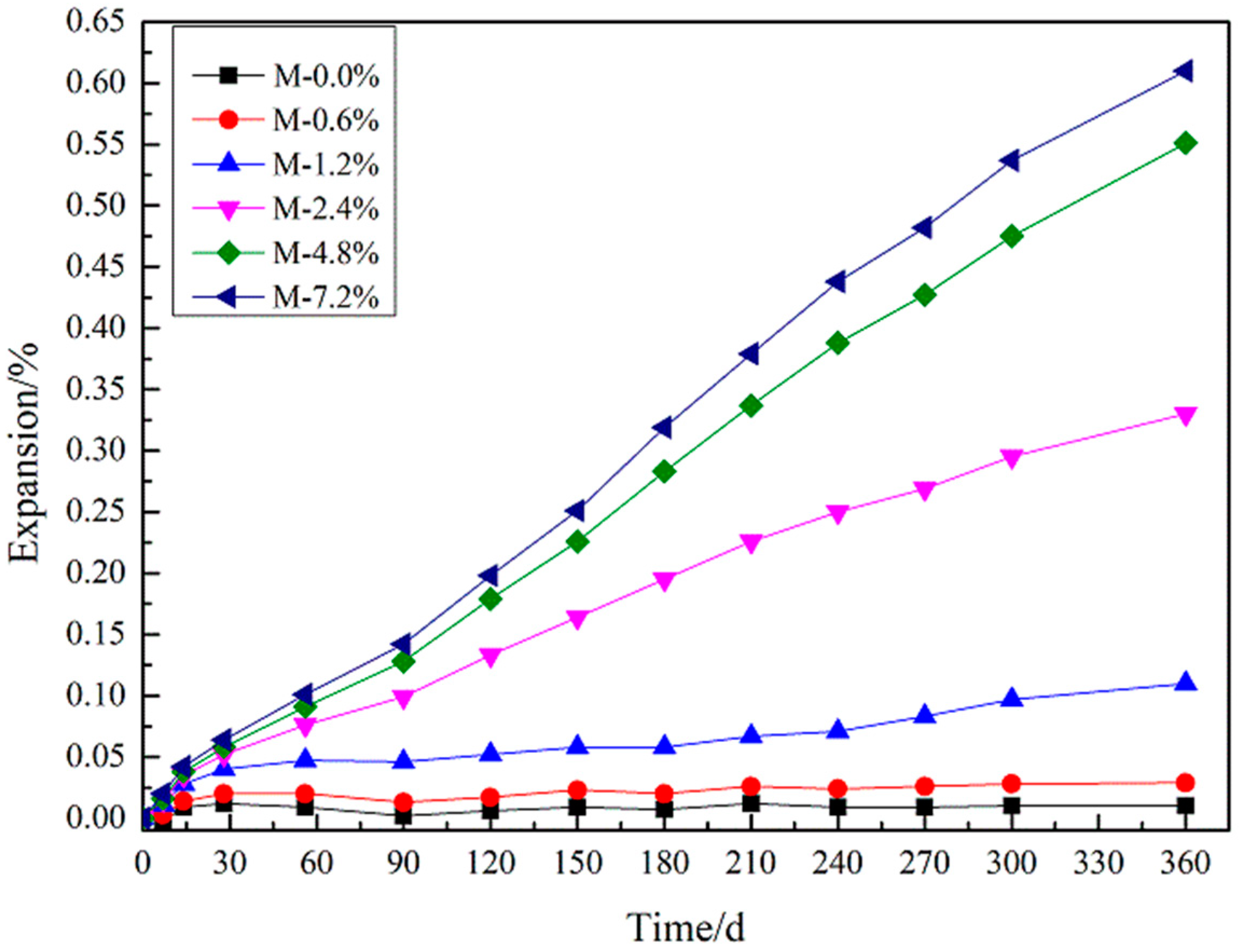
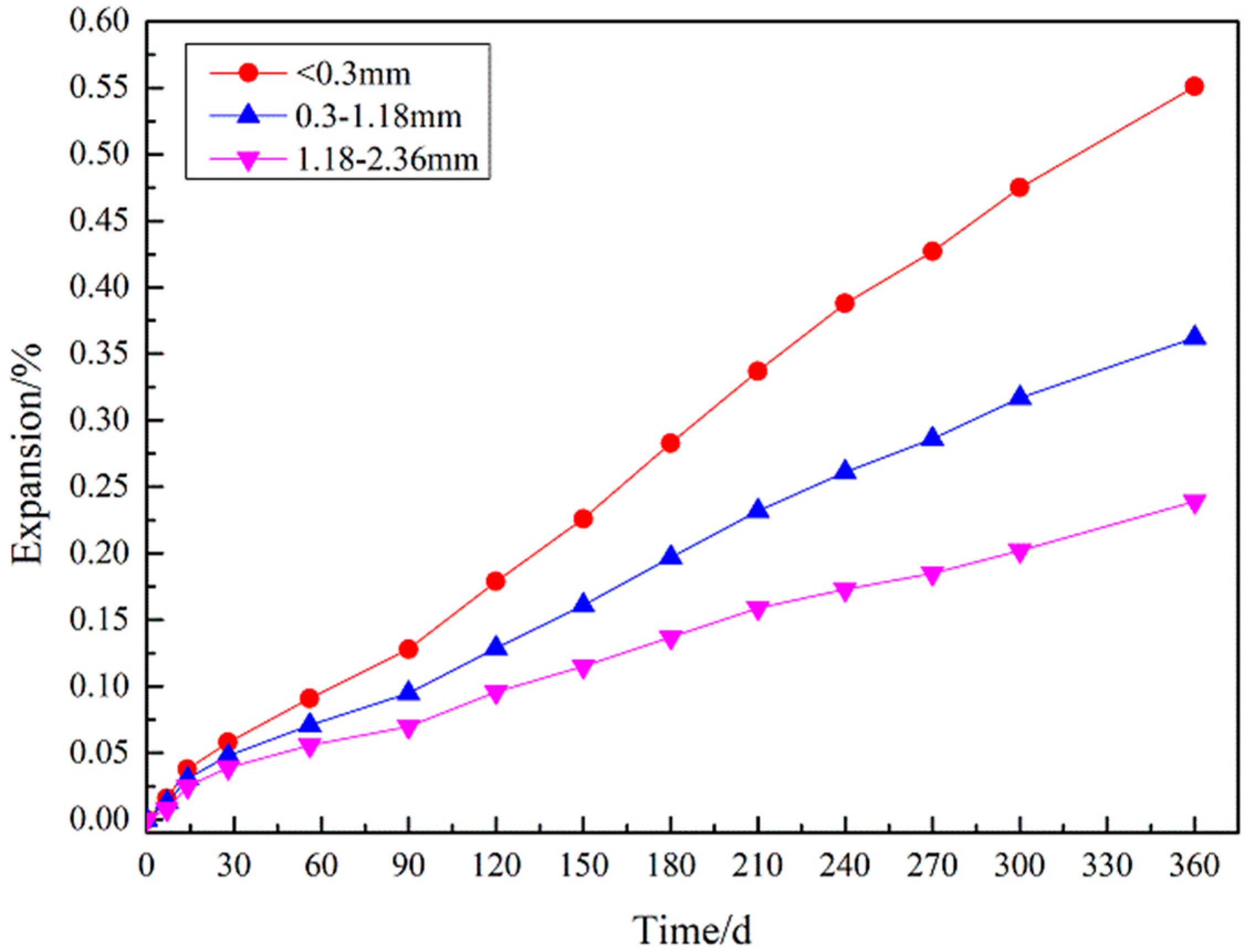
3.3. Expansion Measurements
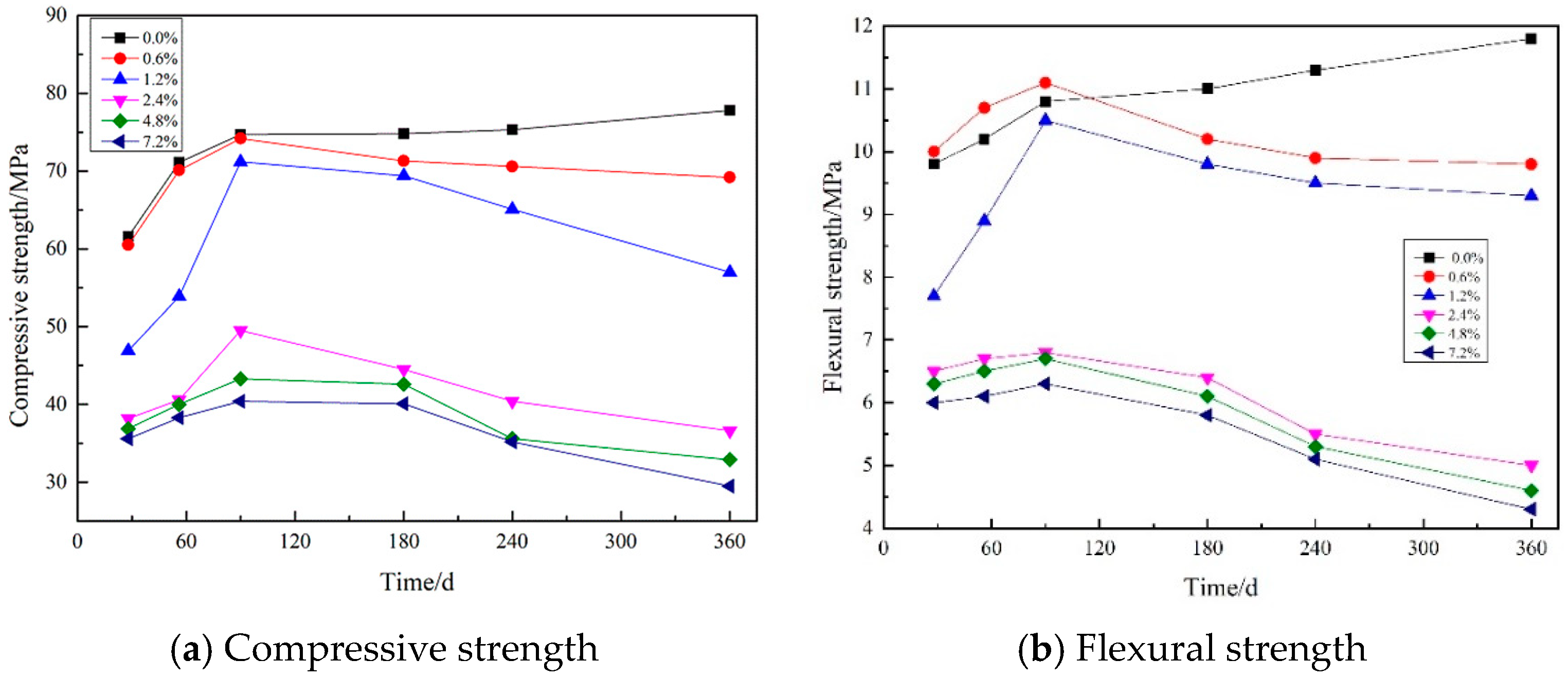
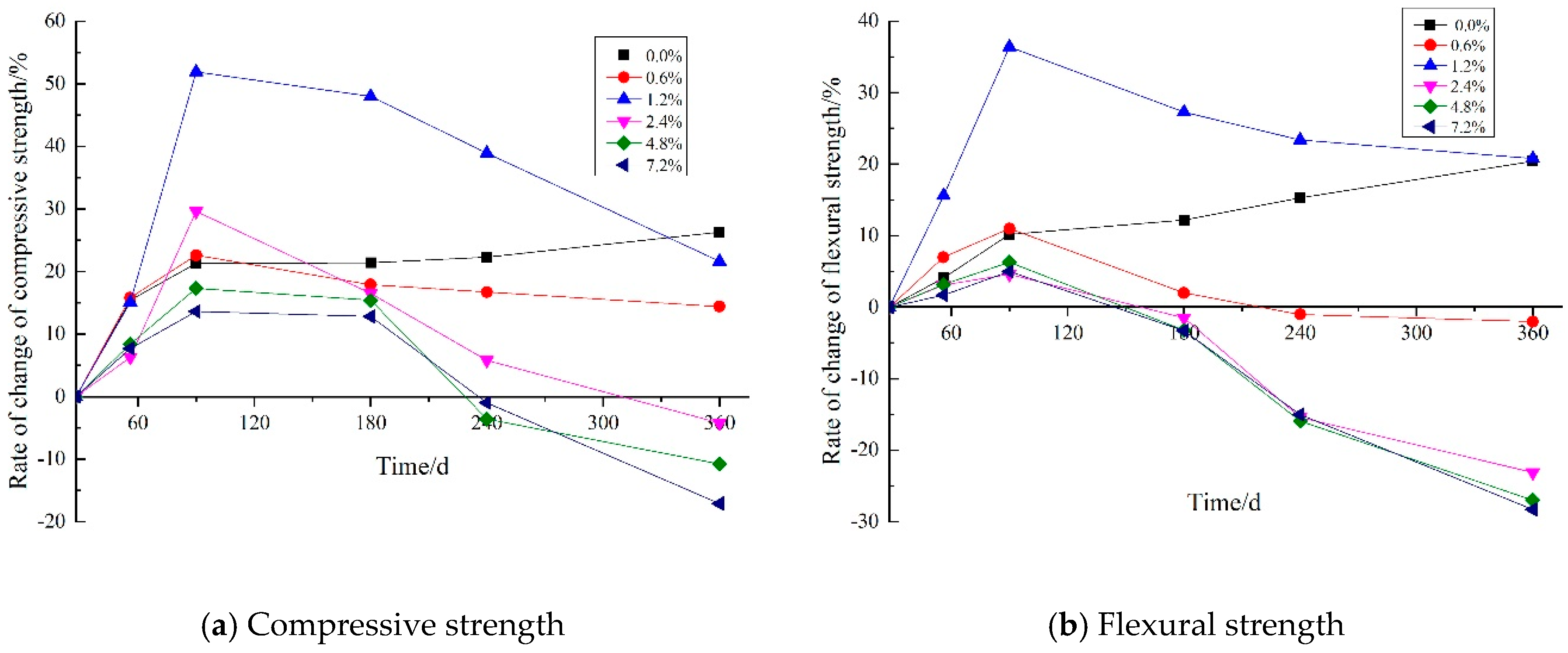
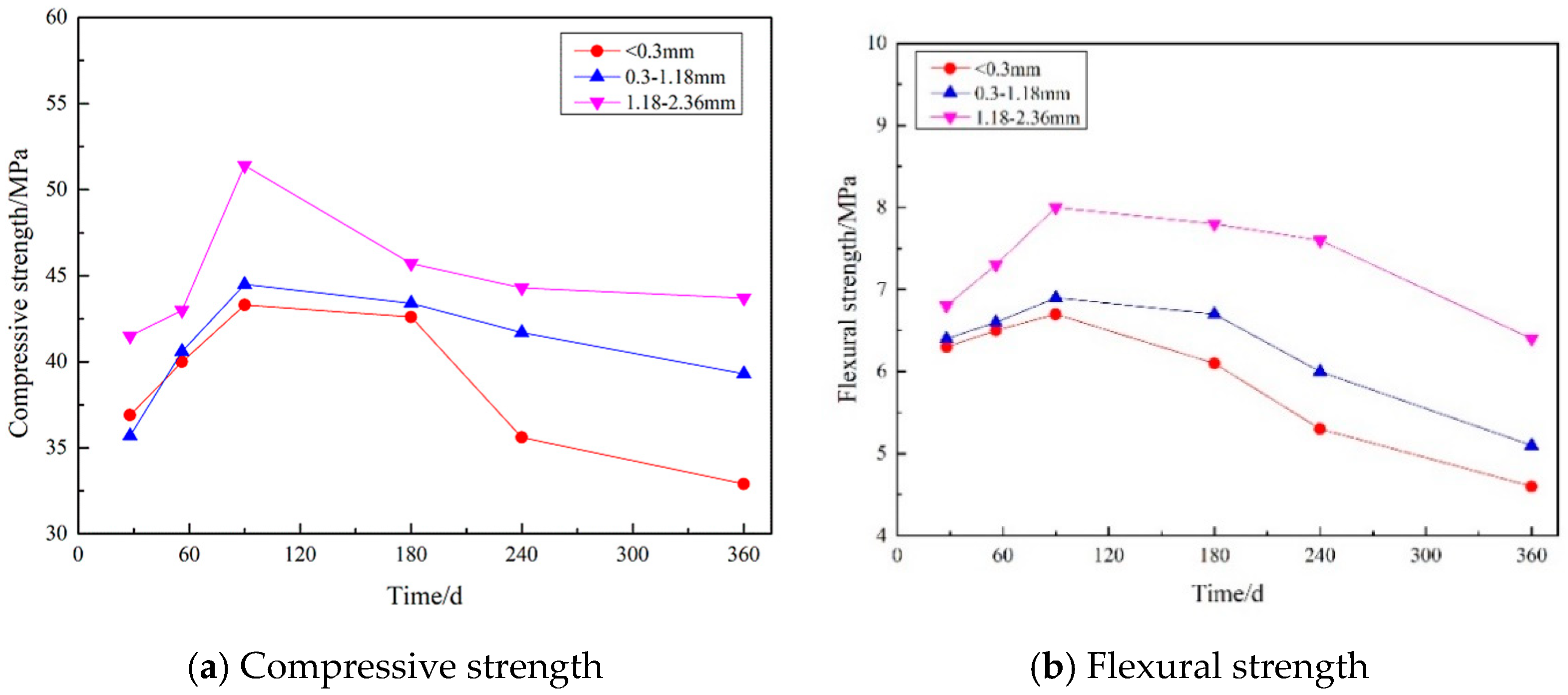
3.4. Strength Measurements
3.4.1. The Effect of Maintenance Temperature on Strength
3.4.2. Effect of Gypsum Sand Particle Size on Strength
3.5. SEM Analysis
3.6. Deterioration Mechanism Analysis
3.6.1. Formation of AFt and Gypsum
3.6.2. Dissolution of Gypsum
3.6.3. pH Value in Mortar
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yishen, D. Experimental Study on the Durability of Concrete under Sulfate Attack; Zhejiang University: Hangzhou, China, 2011. [Google Scholar]
- Xiaobin, C.; Mengxiong, T.; Kunlin, M. Durability experiments on sulfate and chloride salt attack of underground concrete structures. J. Cent. South Univ. 2012, 43, 2803–2812. [Google Scholar]
- Meyer, Y.A.; Menezes, I.; Bonatti, R.S.; Bortolozo, A.D.; Osório, W.R. EIS investigation of the corrosion behavior of steel bars embedded into modified concretes with eggshell contents. Metals 2022, 12, 417. [Google Scholar] [CrossRef]
- Jia-Ming, Z. Study on the Effect of Dry and Wet Cycles and Carbonation on Sulfate Attack of Concrete; Yangzhou University: Yangzhou, China, 2009. [Google Scholar]
- Yin, G.J.; Zuo, X.B.; Tang, Y.J.; Ayinde, O.; Wang, J.L. Numerial simulation on time-dependent mechanical behavior of concrete under coupled axial loading and sulfate attack. Ocean. Eng. 2017, 142, 115–124. [Google Scholar] [CrossRef]
- Yang, Z.; Ye, H.; Yuan, Q.; Li, B.; Li, Y.; Zhou, D. Factors influencing the hydration, dimensional stability, and strength development of the OPC-CSA-anhydrite ternary system. Materials 2021, 14, 7001. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Testing and Analysis of Microstructure Evolution of Cementitious Materials under Sulfate Attack; Southeast University: Nanjing, China, 2012. [Google Scholar]
- Horkoss, S.; Lteif, R.; Rizk, T. Influence of the clinker SO3 on the cement characteristics. Cem. Concr. Res. 2011, 41, 913–919. [Google Scholar] [CrossRef]
- Lei, Z.; Dingyi, Y. Research progress on the process and main products of concrete sulfate attack. Concr. Cem. Prod. 2006, 19–22. [Google Scholar] [CrossRef]
- Oliveira Tassiane, A.; Pinkoski Igor, M.; D’Orey Gaivão Portella Bragança, M.; André, A.; Oliveira, I.C.; Eduardo, P. Use of Raman spectroscopy to characterize the effect of nanomagnetite as an addition to Portland cement paste on mitigating internal sulfate attack. Constr. Build. Mater. 2020, 262, 120803. [Google Scholar] [CrossRef]
- Lin-N, L.; Yong-Jia, H. Sulfate attack mechanism of concrete and its influencing factors. J. Jiaozuo Eng. Coll. 2003, 465–468. [Google Scholar] [CrossRef]
- Elias, P.; Eduardo, P.; Antonio, P.S.; Miranda, F.M.; D’Orey Gaivão Portella Bragança, M.; Oliveira, I.C. Combined effect of alkali-aggregate reaction (AAR) and internal sulfate attack (ISA): Microstructural and porous structure modifications of portland cement mortars. Constr. Build. Mater. 2023, 362, 129676. [Google Scholar]
- Yinliang, W. Research on the Engineering Geological Characteristics of Gypsum Rock and its Hazard Mechanism to Tunnel Concrete Structure; China University of Geosciences: Wuhan, China, 2013. [Google Scholar]
- Tu, Y.L.; Wu, Y.L. Experimental study on the deterioration of gypsum rock on tunnel concrete. Southwest Highw. 2017, 14–20, CNKI:SUN:XNGL.0.2017-03-003. [Google Scholar]
- Horkoss, S.; Escadeillas, G.; Rizk, T.; Lteif, R. The effect of the source of cement SO3 on the expansion of mortars. Case Stud. Constr. Mater. 2016, 4, 62–72. [Google Scholar]
- Chongbang, X.; Huajun, W. Geological characteristics of gypsum-bearing marl and analysis of the impact of tunneling. J. Undergr. Space Eng. 2020, 16, 227–233. [Google Scholar]
- Gesoglu, M.; Güneyisi, E.; Nahhab, A.H.; Yazıcı, H. The effect of aggregates with high gypsum content on the performance of ultra-high strength concretes and Portland cement mortars. Constr. Build. Mater. 2016, 110, 346–354. [Google Scholar] [CrossRef]
- Crammond, N.J. The thaumasite form of sulfate attack in the UK. Cem. Concr. Compos. 2003, 25, 809–818. [Google Scholar] [CrossRef]
- Geng, W.N.; Shang, J.L.; Chen, Q.S. Experimental study on the corrosion of concrete due to sulphate containing aggregates. Constr. Technol. 1992, 19, 472–474. [Google Scholar]
- Fu, K.; Liu, J.; Hu, H. Research and Engineering Countermeasures of “Ash Baobao” in Yi-Ba Expressway. In Proceedings of the 2010 Annual Conference of Computer Application Branch of China Highway Society, Taiyuan, China, 22–24 October 2010; pp. 151–153. [Google Scholar]
- Xing, C.-S.; Deng, M.; Wang, A.-G.; Liu, K. Internal sulfate attack of concrete caused by gypsum-containing aggregates. J. Constr. Mater. 2014, 30–34. [Google Scholar] [CrossRef]
- Skalny, J.; Marchand, J.; Odler, I. Sulfate Attack on Concrete; Spon Press: London, UK, 2002. [Google Scholar]
- Jian, L. Paste-bearing rock systems and their influence on tunneling. J. Southwest Jiaotong Univ. 1978, 63–72, CNKI:SUN:XNJT.0.1978-01-004. [Google Scholar]
- Biyu, Y.; Maojing, W. The Chengkun Railway through the “Geological Museum”. J. Railw. Eng. 2005, 210–215. [Google Scholar] [CrossRef]
- GB/T 749-2008; Test Method for Determining Capability of Resisting Sulfate Corrode of Cement. National Standard of the People’s Republic of China: Beijing, China, 2017.
- GB/T17671-1999; Method of Testing Cements-Determination of Strength. Test Method for Determining Capability of Resisting Sulfate Corrode of Cement. National Standard of the People’s Republic of China: Beijing, China, 2017.
- Clark, B.A.; Brown, P.W. Formation of ettringite from monosubstituted calcium sulfoaluminate hydrate and gypsum. J. Am. Ceram. Soc. 1999, 82, 2900–2905. [Google Scholar] [CrossRef]
- Liu, S.H.; Rao, M.J. Judgment criteria and evaluation of alkali-aggregate reaction. Highway 2010, 139–142, CNKI:SUN:GLGL.0.2010-01-036. [Google Scholar]
- Yuexue, Y. Process and Mechanism of Sulfate Attack within Gypsum-Containing Aggregates; Nanjing University of Technology: Nanjing, China, 2017. [Google Scholar]


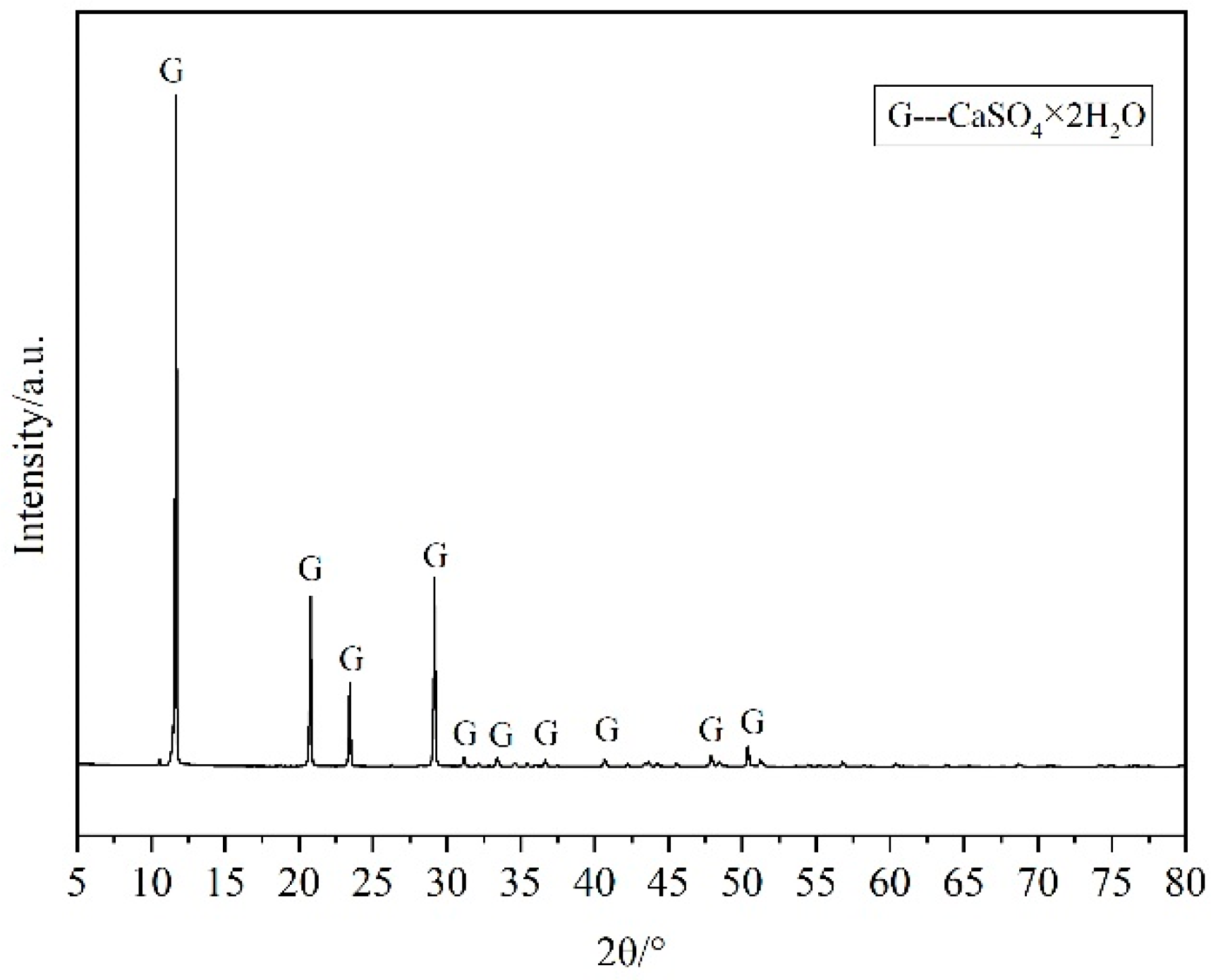
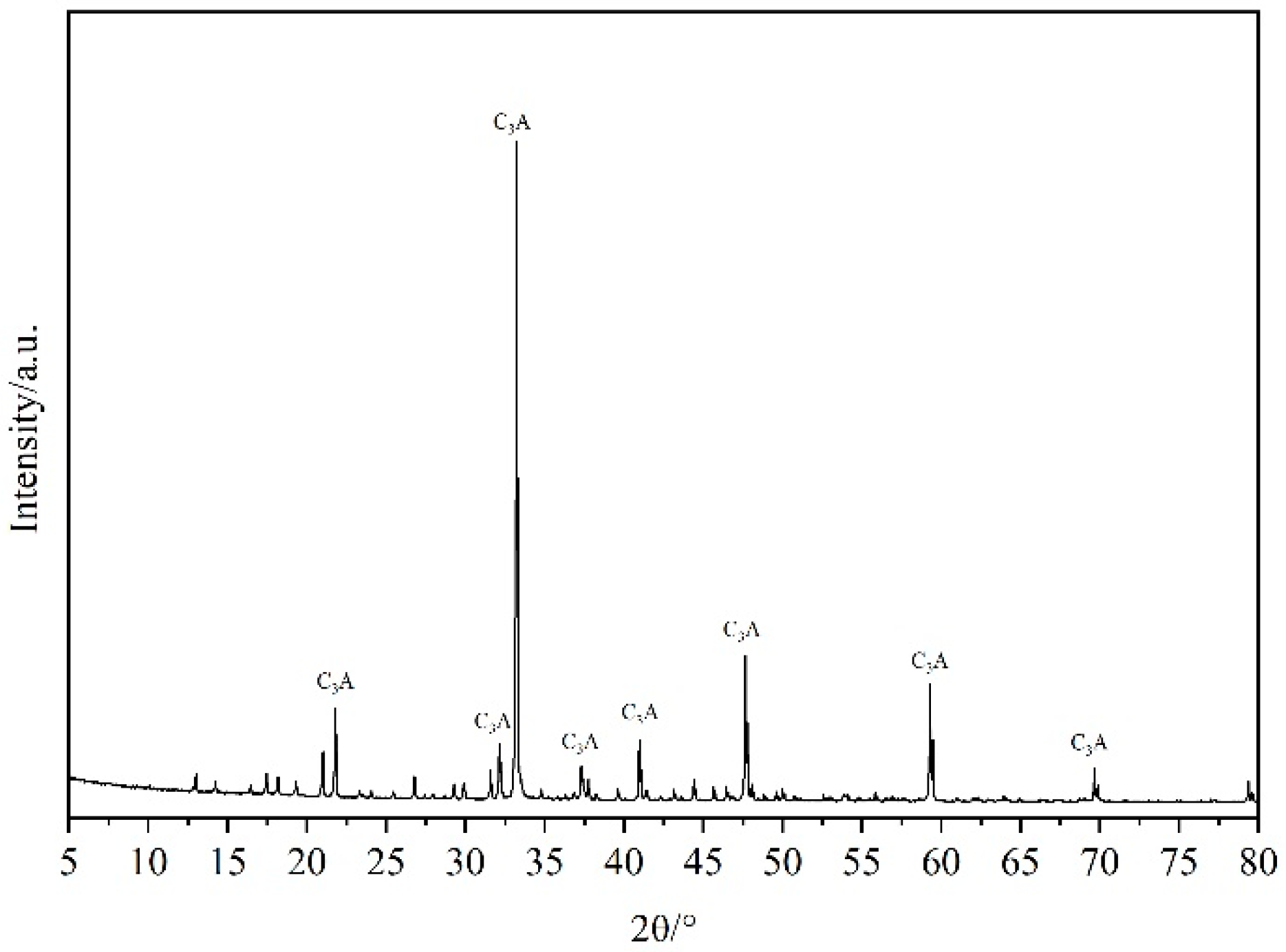

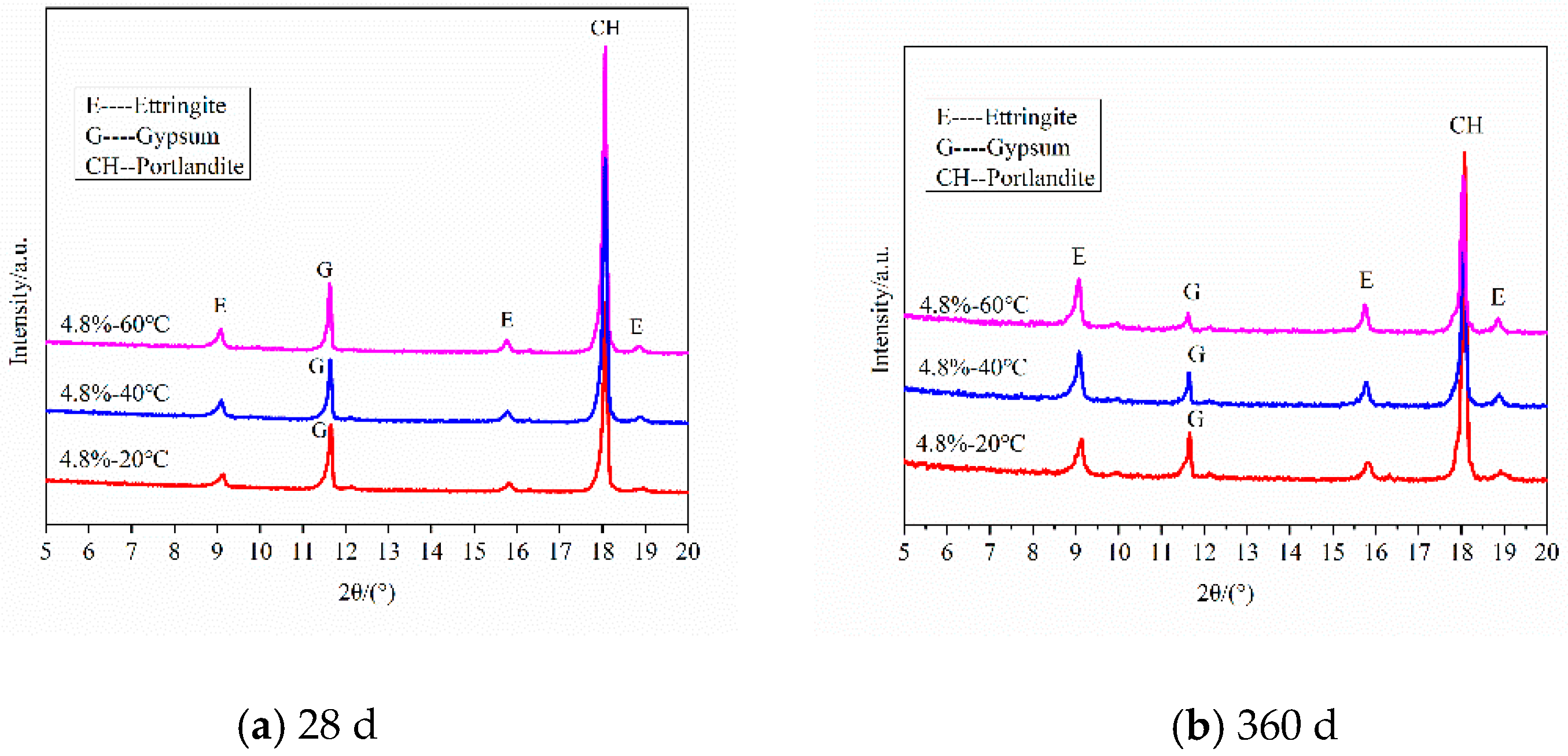
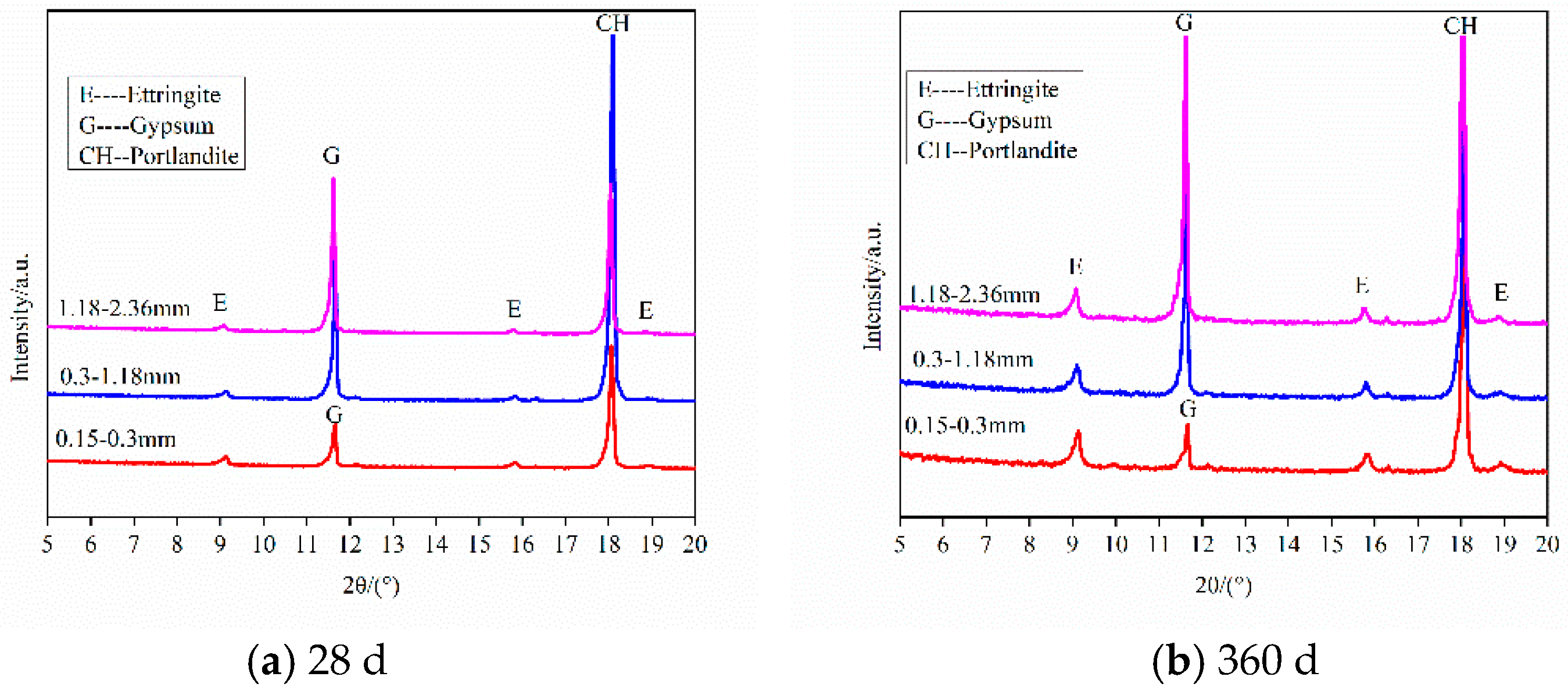



| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | SO3 | Loss |
|---|---|---|---|---|---|---|
| 64.00 | 19.46 | 4.73 | 2.96 | 2.35 | 2.59 | 2.81 |
| CaO | SiO2 | Fe2O3 | Al2O3 | MgO | SO3 |
|---|---|---|---|---|---|
| 34.52 | 0.14 | 0.00 | 0.33 | 0.11 | 45.65 |
| Quality Index | ||
|---|---|---|
| SiO2 Content/% | Burning Loss/% | Mud Content/% |
| >96 | ≤0.40 | ≤0.20 |
| Samples | < 0.08 mm | 0.08~0.16 mm | 0.16~0.5 mm | 0.5~1 mm | 1~1.6 mm | 1.6~2 mm | >2 mm |
|---|---|---|---|---|---|---|---|
| ISO sand | 0 | 6.68 | 22.79 | 24.72 | 23.86 | 9.14 | 12.81 |
| Samples | SO3/% | W/C | Gypsum Particle Size/mm | Water/g | Cement/g | Sand/g | Gypsum/g |
|---|---|---|---|---|---|---|---|
| M0 | 0 | 0.5 | — | 250 | 500 | 1250 | - |
| M06 | 0.6 | 0.15–0.30 | 250 | 500 | 1233.6 | 16.4 | |
| M12 | 1.2 | 0.15–0.30 | 250 | 500 | 1217.1 | 32.9 | |
| M24 | 2.4 | 0.15–0.30 | 250 | 500 | 1184.3 | 65.7 | |
| M48 | 4.8 | 0.15–0.30 | 250 | 500 | 1118.6 | 131.4 | |
| M72 | 7.2 | 0.15–0.30 | 250 | 500 | 1052.8 | 197.2 | |
| ML1.18 | 4.8 | 0.30–1.18 | 250 | 500 | 1118.6 | 131.4 | |
| ML2.36 | 4.8 | 1.18–2.36 | 250 | 500 | 1118.6 | 131.4 | |
| M48-40 °C | 4.8 | 0.15–0.30 | 250 | 500 | 1118.6 | 131.4 | |
| M48-60 °C | 4.8 | 0.15–0.30 | 250 | 500 | 1118.6 | 131.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, C.; Huang, B. Deterioration Process of Cementitious Material Properties under Internal Sulphate Attack. Appl. Sci. 2023, 13, 3982. https://doi.org/10.3390/app13063982
Zhong C, Huang B. Deterioration Process of Cementitious Material Properties under Internal Sulphate Attack. Applied Sciences. 2023; 13(6):3982. https://doi.org/10.3390/app13063982
Chicago/Turabian StyleZhong, Chao, and Bei Huang. 2023. "Deterioration Process of Cementitious Material Properties under Internal Sulphate Attack" Applied Sciences 13, no. 6: 3982. https://doi.org/10.3390/app13063982




