Phytochemical Profiles and Anti-Glioma Activity of Bearberry Arctostaphylos uva-ursi (L.) Spreng. Leaf Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Plant Material and Extraction of Phytochemicals
2.3. Individual Compounds (HPLC Analysis)
2.4. Total Polyphenols and Flavonoids
2.5. Cells and Culture Conditions
2.6. Drug Treatment and Microscopic Detection of Apoptosis and Necrosis
2.7. Statistical Analysis
3. Results
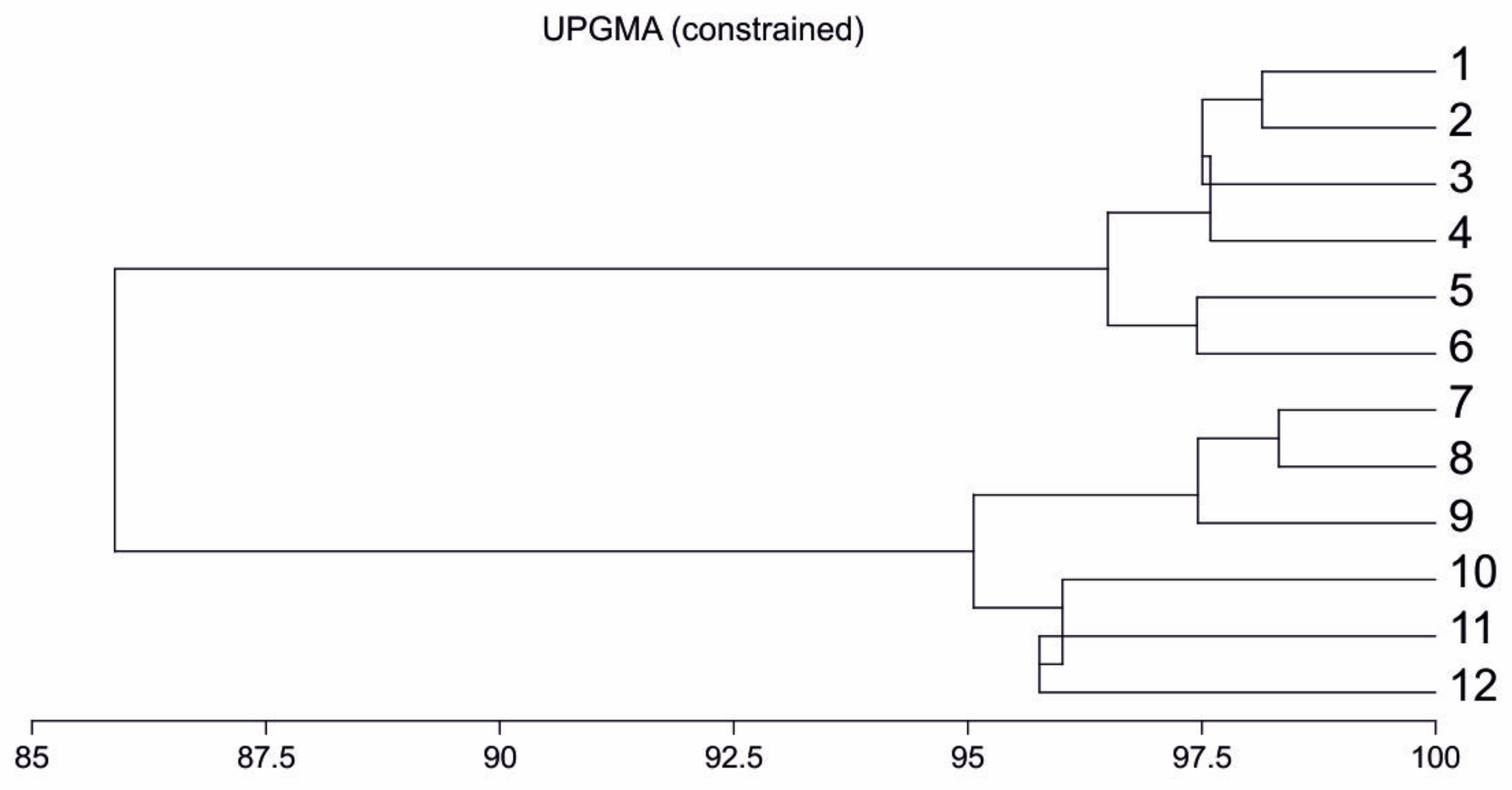
3.1. Characteristics and Differentiation of the Chemical Composition of Bearberry Leaf Extracts
3.2. Antiglioma Activity of Bearberry Extracts
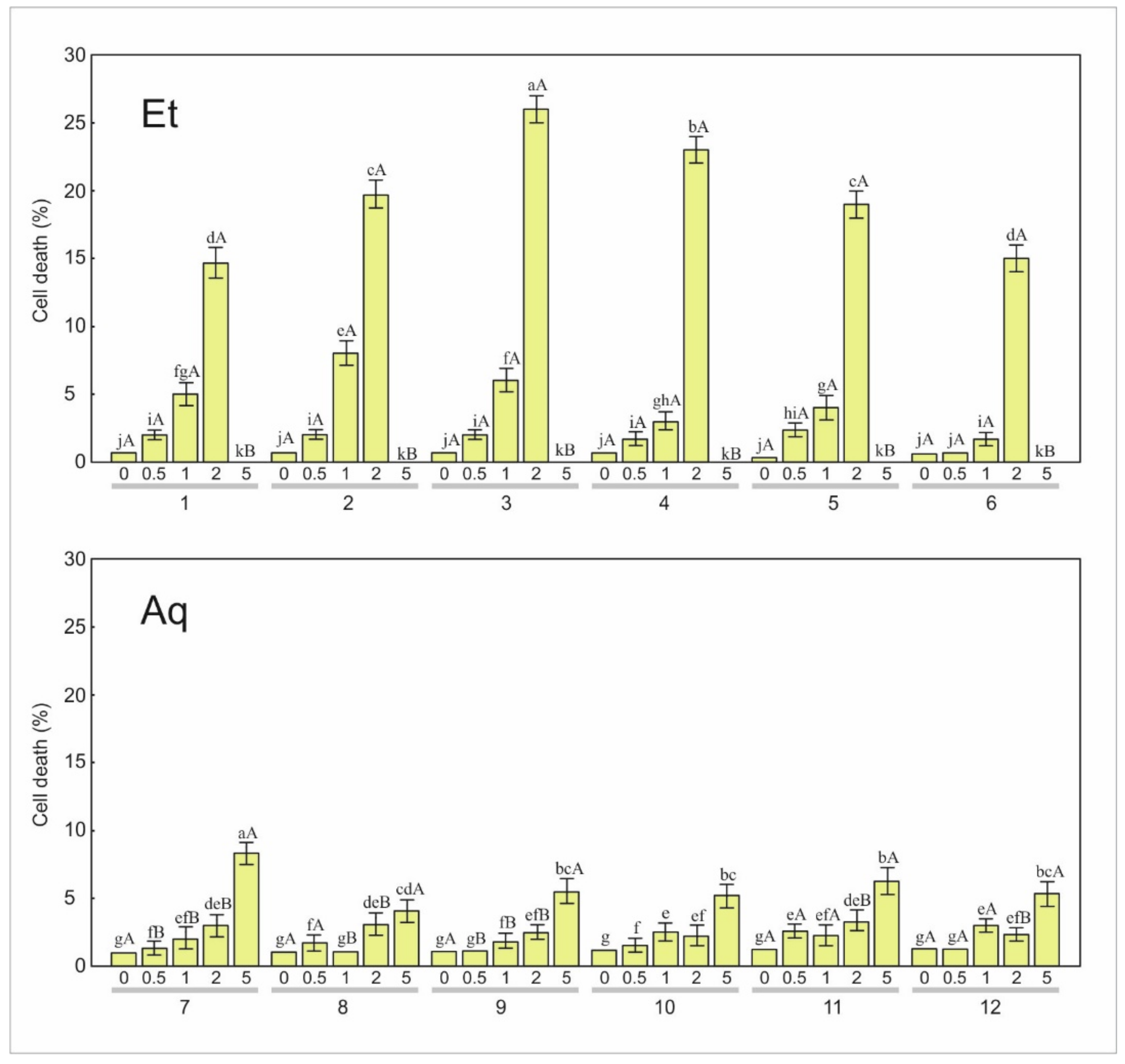
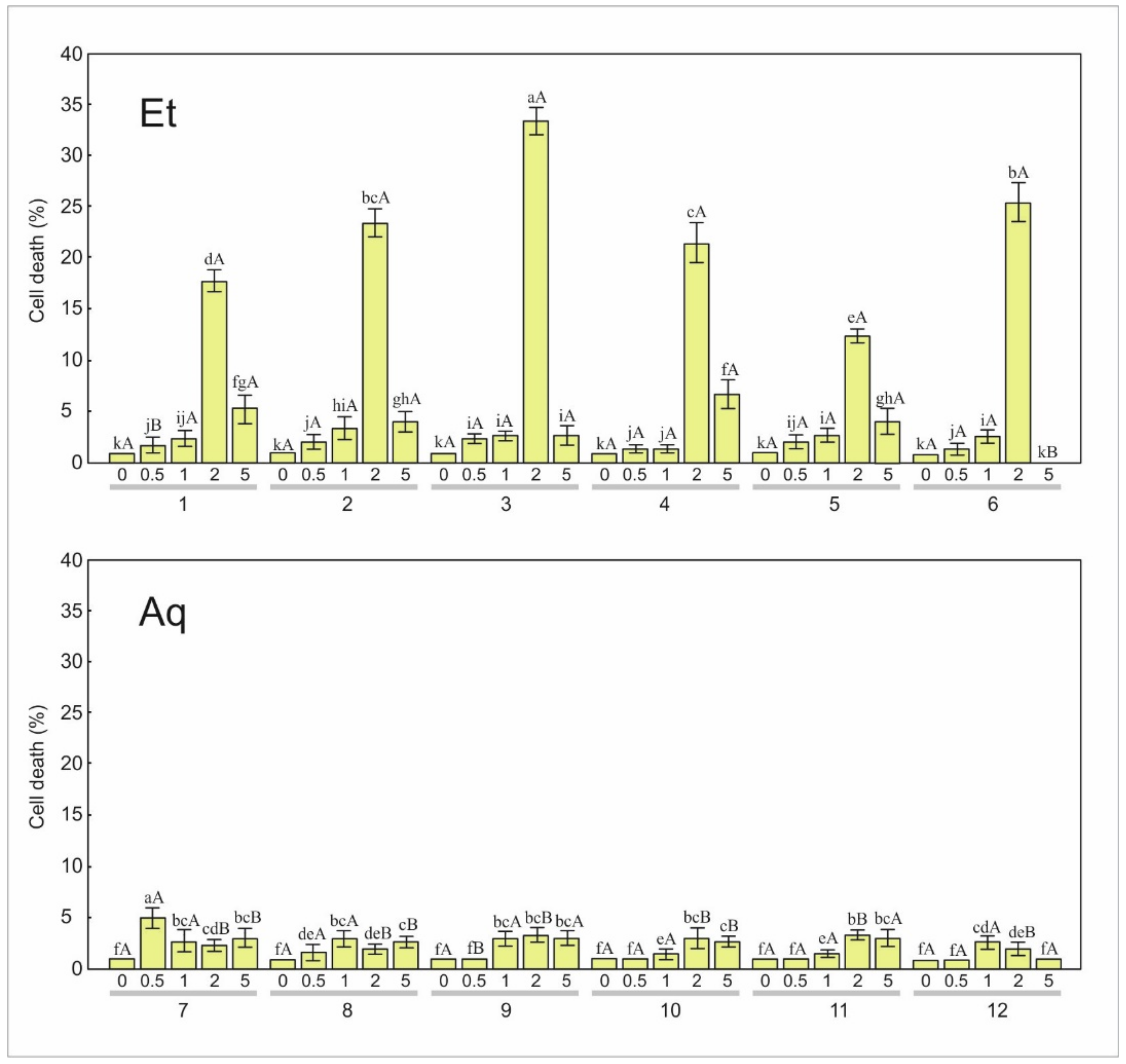
3.2.1. Apoptosis
3.2.2. Necrosis
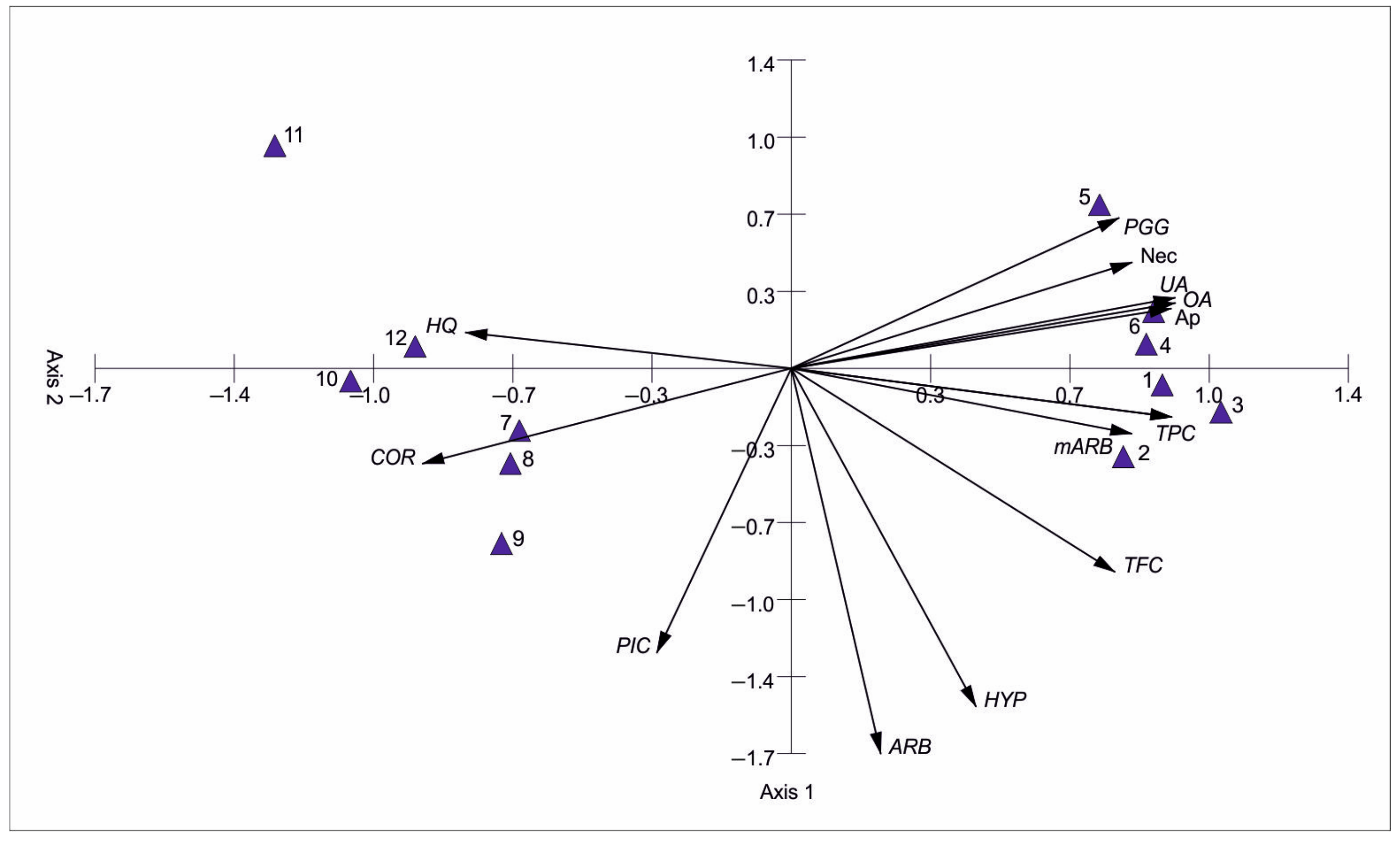
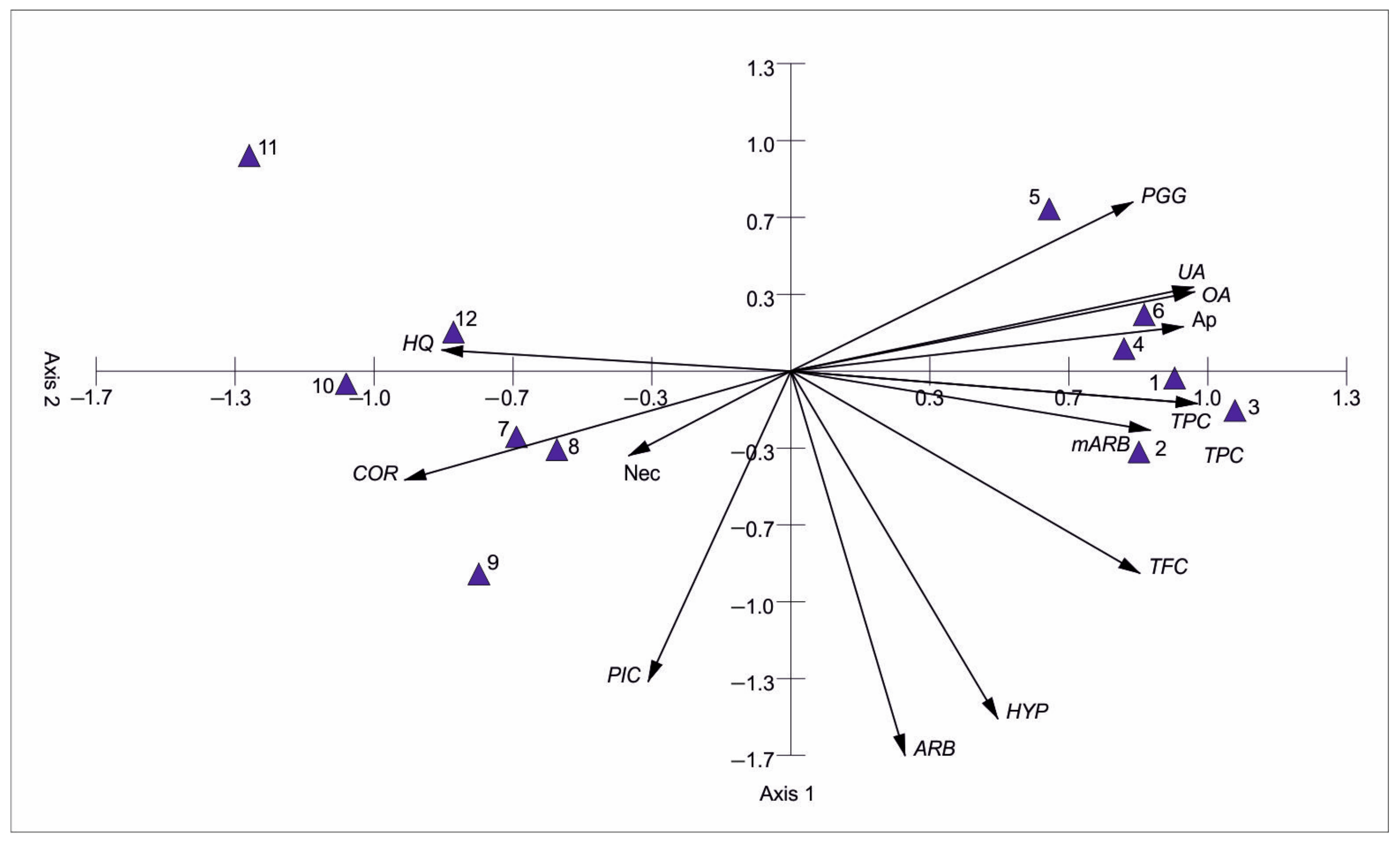
3.3. Bearberry Secondary Metabolites vs. the Level of Apoptosis and Necrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Yadav, B.; Jogawat, A.; Rahman, S.; Narayan, O.P. Secondary metabolites in the drought stress tolerance of crop plants: A review. Gene Rep. 2021, 23, 101040. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981–2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Dutta, S.; Akter, R.; Rahman, M.H.; Karthika, C.; Nagaswarupa, H.P.; Murthy, H.C.A.; Fratila, O.; Brata, R.; Bungau, S. Role of phytonutrients in nutrigenetics and nutrigenomics perspective in curing breast cancer. Biomolecules 2021, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Asma, S.T.; Acaroz, U.; Imre, K.; Morar, A.; Shah, S.R.A.; Hussain, S.Z.; Arslan-Acaroz, D.; Demirbas, H.; Hajrulai-Musliu, Z.; Istanbullugil, F.R.; et al. Natural products/bioactive compounds as a source of anticancer drugs. Cancers 2022, 14, 6203. [Google Scholar] [CrossRef] [PubMed]
- Sivasankarapillai, V.S.; Nair, R.M.K.; Rahdar, A.; Bungau, S.; Zaha, D.C.; Aleya, L.; Tit, D.M. Overview of the anticancer activity of withaferin A, an active constituent of the Indian ginseng Withania somnifera. Environ. Sci. Pollut. Res. 2020, 27, 26025–26035. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.W.; Farooq, M.; Haseeb, M.; Choi, S. Role of plant-derived active constituents in cancer treatment and their mechanisms of action. Cells 2022, 11, 1326. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural products as anticancer agents: Current status and future perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Banerjee, S.; Nau, S.; Hochwald, S.N.; Xie, H.; Zhang, J. Anticancer properties and mechanisms of botanical derivatives. Phytomed. Plus 2023, 3, 100396. [Google Scholar] [CrossRef]
- Chaika, N.; Koshovyi, O.; Raal, A.; Kireyev, I.; Zupanets, A.; Odyntsova, V. Phytochemical profile and pharmacological activity of the dry extract from Arctostaphylos uva-ursi leaves modified with phenylalanine. Sci. Pharm. Sci. 2020, 6, 74–78. [Google Scholar] [CrossRef]
- Recasens, J.; Ninot, P.; Cristóbal, R.; Aymerich, P. Sustainable wild harvesting of Arctostaphylos uva-ursi in the Pyrenees as a conservation practice. J. Herbs Spices Med. Plants 2008, 14, 1–12. [Google Scholar] [CrossRef]
- Allen, D.; Bilz, M.; Leaman, D.J.; Miller, R.M.; Timoshyna, A.; Window, J. European Red List of Medicinal Plants; Publications Office of the European Union: Luxembourg, 2014. [Google Scholar]
- Kaźmierczakowa, R.; Bloch-Orłowska, J.; Celka, Z.; Cwener, A.; Dajdok, Z.; Michalska-Hejduk, D.; Pawlikowski, P.; Szczęśniak, E.; Ziarnek, K. Polska Czerwona Lista Paprotniów i Roślin Kwiatowych. In Polish Red List of Pteridophytes and Flowering Plants; Instytut Ochrony Przyrody Polskiej Akademii Nauk: Kraków, Poland, 2016. [Google Scholar]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer Volumes I–III; Koeltz Scientific Books: Königstein, Germany, 1986. [Google Scholar]
- Asensio, E.; Vitales, D.; Pérez, I.; Peralba, L.; Viruel, J.; Montaner, C.; Vallès, J.; Garnatje, T.; Sales, E. Phenolic compounds content and genetic diversity at population level across the natural distribution range of Bearberry (Arctostaphylos uva-ursi, Ericaceae) in the Iberian Peninsula. Plants 2020, 9, 1250. [Google Scholar] [CrossRef] [PubMed]
- Stefanescu, B.E.; Szabo, K.; Mocan, A.; Crisan, G. Phenolic compounds from five Ericaceae species leaves and their related bioavailability and health benefits. Molecules 2019, 24, 2046. [Google Scholar] [CrossRef]
- Iqbal, A.; Ibrahim, M.; Muhammad, N. Natural approach used for urinary tract infections. Phytopharmacol. Res. J. 2023, 2, 1–17. [Google Scholar]
- Azman, N.A.M.; Gallego, M.G.; Segovia, F.; Abdullah, S.; Shaarani, S.M.; Pablos, M.P.A. Study of the properties of bearberry leaf extract as a natural antioxidant in model foods. Antioxidants 2016, 5, 11. [Google Scholar] [CrossRef]
- Dell’Annunziata, F.; Cometa, S.; Della Marca, R.; Busto, F.; Folliero, V.; Franci, G.; Galdiero, M.; De Giglio, E.; De Filippis, A. In vitro antibacterial and anti-inflammatory activity of Arctostaphylos uva-ursi leaf extract against Cutibacterium acnes. Pharmaceutics 2022, 14, 1952. [Google Scholar] [CrossRef]
- Kravchenko, G.; Krasilnikova, O.; Raal, A.; Mazen, M.; Chaika, N.; Kireyev, I.; Grytsyk, A.; Koshovyi, O. Arctostaphylos uva-ursi L. leaves extract and its modified cysteine preparation for the management of insulin resistance: Chemical analysis and bioactivity. Nat. Prod. Bioprospect. 2022, 12, 30. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Aissani, N.; Albouchi, F.; Sebai, H. Anticancer effect in human glioblastoma and antioxidant activity of Petroselinum crispum L. methanol extract. Nutr. Cancer 2020, 29, 1–9. [Google Scholar] [CrossRef]
- Sugier, P.; Sęczyk, Ł.; Sugier, D.; Krawczyk, R.; Wójcik, M.; Czarnecka, J.; Okoń, S.; Plak, A. Chemical characteristics and antioxidant activity of Arctostaphylos uva-ursi L. Spreng. at the southern border of the geographical range of the species in Europe. Molecules 2021, 26, 7692. [Google Scholar] [CrossRef] [PubMed]
- Sugier, P.; Sęczyk, Ł.; Sugier, D. Variation in population and solvents as factors determining the chemical composition and antioxidant potential of Arctostaphylos uva-ursi (L.) Spreng. leaf extracts. Molecules 2022, 27, 2247. [Google Scholar] [CrossRef] [PubMed]
- Mian, S.Y.; Nambiar, A.; Kaliaperumal, C. Phytotherapy for the treatment of glioblastoma: A review. Front. Surg. 2022, 9, 844993. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phospho- molybdicephosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Lamaison, J.L.; Carnart, A. Teneurs en principaux flavonoïdes des fleurs et feuilles de Crataegeus monogyna Jacq. et de Crataegeus laevigata (Poiret) DC. Pharm. Acta Helv. 1990, 65, 315–320. [Google Scholar]
- Jakubowicz-Gil, J.; Bądziul, D.; Langner, E.; Wertel, I.; Zając, A.; Rzeski, W. Temozolomide and sorafenib as programmed cell death inducers of human glioma cells. Pharmacol. Rep. 2017, 69, 779–787. [Google Scholar] [CrossRef]
- Kovach, W. MVSP—A Multivariate Statistical Package for Windows. Ver. 3.1; Kovach Computing Services: Pentraeth, UK, 1999. [Google Scholar]
- Council of Europe. European Pharmacopoeia, 8.1 Supplement, 8th ed.; Council of Europe: Strasbourg, France, 2014. [Google Scholar]
- Lamien-Meda, A.; Lukas, B.; Schmiderer, C.; Franz, C.; Novak, J. Validation of a quantitative assay of arbutin using gas chromatography in Origanum majorana and Arctostaphylos uva-ursi extracts. Phytochem. Anal. 2009, 20, 416–420. [Google Scholar] [CrossRef]
- Boo, Y.C. Arbutin as a skin depigmenting agent with antimelanogenic and antioxidant properties. Antioxidants 2021, 10, 1129. [Google Scholar] [CrossRef]
- Parejo, I.; Viladomat, F.; Bastida, J.; Codina, C. Variation of the arbutin content in different wild populations of Arctostaphylos uva-ursi in Catalonia, Spain. J. Herbs Spices Med. Plants 2002, 9, 329–333. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.-S.; Ruan, X.; Zhao, Y.-X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Slika, H.; Mansour, H.; Wehbe, N.; Nasser, S.A.; Iratni, R.; Nasrallah, G.; Shaito, A.; Ghaddar, T.; Kobeissy, F.; Eid, A.H. Therapeutic potential of flavonoids in cancer: ROS-mediated mechanisms. Biomed. Pharmacother. 2022, 146, 112442. [Google Scholar] [CrossRef] [PubMed]
- Iman, M.; Taheri, M.; Bahari, Z. The anti-cancer properties of neem (Azadirachta indica) through its antioxidant activity in the liver: Its pharmaceutics and toxic dosage forms. A literature review. J. Complement. Integr. Med. 2022, 19, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ai, Q.; Shi, A.; Wang, N.; Wang, L.; Wei, Y. Oleanolic acid and ursolic acid: Therapeutic potential in neurodegenerative diseases, neuropsychiatric diseases and other brain disorders. Nutr. Neurosci. 2023, 26, 414–428. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ye, X.L.; Liu, R.; Chen, H.L.; Bai, H.; Liang, X.; Zhang, X.D.; Wang, Z.; Li, W.L.; Hai, C.X. Antioxidant activities of oleanolic acid in vitro: Possible role of Nrf2 and MAP kinases. Chem. Biol. Interact. 2010, 184, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, R.; Zhang, W.; Zhang, X.; Liao, N.; Wang, Z.; Li, W.; Qin, X.; Hai, C. Oleanolic acid improves hepatic insulin resistance via antioxidant, hypolipidemic and anti-inflammatory effects. Mol. Cell. Endocrinol. 2013, 376, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Yang, E.J.; Ku, S.K.; Song, K.S.; Bae, J.S. Anti-inflammatory effects of oleanolic acid on LPS-induced inflammation in vitro and in vivo. Inflammation 2013, 36, 94–102. [Google Scholar] [CrossRef]
- Jesus, J.A.; Lago, J.H.G.; Laurenti, M.D.; Yamamoto, E.S.; Passero, L.F.D. Antimicrobial activity of oleanolic and ursolic acids: An update. Evid. Based Complement. Altern. Med. 2015, 2015, 620472. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Zhou, M.; Duan, L.; Wang, W.; Zhang, J.; Wang, D.; Liang, X. Efficient synthesis and anti-fungal activity of oleanolic acid oxime esters. Molecules 2013, 18, 3615–3629. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.Y.; Huang, H.Y.; Wu, Y.L. Anticancer and apoptotic activities of oleanolic acid are mediated through cell cycle arrest and disruption of mitochondrial membrane potential in HepG2 human hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 5012–5018. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, M.; Li, D. Oleanolic acid suppresses the proliferation of lung carcinoma cells by miR-122/Cyclin G1/MEF2D axis. Mol. Cell. Biochem. 2015, 400, 1–7. [Google Scholar] [CrossRef]
- Shanmugam, M.K.; Dai, X.; Kumar, A.P.; Tan, B.K.; Sethi, G.; Bishayee, A. Oleanolic acid and its synthetic derivatives for the prevention and therapy of cancer: Preclinical and clinical evidence. Cancer Lett. 2014, 346, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Janicsák, G.; Veres, K.; Kakasy, A.Z.; Máthé, I. Study of the oleanolic and ursolic acid contents of some species of the Lamiaceae. Biochem. Syst. Ecol. 2006, 34, 392–396. [Google Scholar] [CrossRef]
- Vetal, M.D.; Chavan, R.S.; Rathod, V.K. Microwave assisted extraction of ursolic acid and oleanolic acid from Ocimum sanctum. Biotechnol. Bioprocess Eng. 2014, 19, 720–726. [Google Scholar] [CrossRef]
- Xia, E.Q.; Wang, B.W.; Xu, X.R.; Zhu, L.; Song, Y.; Li, H.B. Microwave-assisted extraction of oleanolic acid and ursolic acid from Ligustrum lucidum Ait. Int. J. Mol. Sci. 2011, 12, 5319–5329. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.R.; Upadhya, V.; Hegde, H.V.; Joshi, R.K.; Kholkute, S.D. Determination of betulinic acid, oleanolic acid and ursolic acid from Achyranthes aspera L. using RP-UFLC-DAD analysis and evaluation of various parameters for their optimum yield. Indian J. Exp. Biol. 2016, 54, 196–202. [Google Scholar] [PubMed]
- Jakubowicz-Gil, J.; Langner, E.; Bądziul, D.; Wertel, I.; Rzeski, W. Quercetin and sorafenib as a novel and effective couple in programmed cell death induction in human gliomas. Neurotox. Res. 2014, 26, 64–77. [Google Scholar] [CrossRef] [PubMed]
- Nghiemphu, P.L.; Ebiana, V.A.; Wen, P.; Gilbert, M.; Abrey, L.E.; Lieberman, F.; DeAngelis, L.M.; Robins, H.I.; Yung, W.K.A.; Chang, S.; et al. Phase I study of sorafenib and tipifarnib for recurrent glioblastoma: NABTC 05–02. J. Neurooncol. 2017, 136, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ni, Q.; Wang, Y.; Fan, H.-W.; Li, Y. Synergistic anticancer effects of formononetin and temozolomide on glioma C6 cells. Biol. Pharm. Bull. 2018, 8, 1194–1202. [Google Scholar] [CrossRef]
- Zając, A.; Sumorek-Wiadro, J.; Maciejczyk, A.; Langner, E.; Wertel, I.; Rzeski, W.; Jakubowicz-Gil, J. LY294002 and sorafenib as inhibitors of intracellular survival pathways in the elimination of human glioma cells by programmed cell death. Cell Tissue Res. 2021, 386, 17–28. [Google Scholar]
- Zając, A.; Maciejczyk, A.; Sumorek-Wiadro, J.; Filipek, K.; Deryło, K.; Langner, E.; Pawelec, J.; Wasiak, M.; Scibiorski, M.; Rzeski, W.; et al. The role of Bcl-2 and Beclin-1 complex in “switching” between apoptosis and autophagy in human glioma cells upon LY294002 and sorafenib treatment. Cells 2023, 12, 2670. [Google Scholar] [CrossRef]
- Schild, L.; Chen, B.H.; Makarov, P.; Kattengell, K.; Heinitz, K.; Keilhoff, G. Selective induction of apoptosis in glioma tumour cells by a Gynostemma pentaphyllum extract. Phytomedicine 2010, 17, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Sitarek, P.; Skała, E.; Toma, M.; Wielanek, M.; Szemraj, J.; Nieborowska-Skorska, M.; Kolasa, M.; Skorski, T.; Wysokińska, H.; Śliwiński, T. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2- p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumor Biol. 2016, 37, 8753–8764. [Google Scholar] [CrossRef] [PubMed]
- Radan, M.; Carev, I.; Tesevic, V.; Politeo, O.; Culic, V.C. Qualitative HPLC-DAD/ESI-TOF-MS analysis, cytotoxic, and apoptotic effects of Croatian endemic Centaurea ragusina L. aqueous extracts. Chem. Biodivers. 2017, 14, e1700099. [Google Scholar] [CrossRef] [PubMed]
- Kubik, J.; Waszak, Ł.; Adamczuk, G.; Humeniuk, E.; Iwan, M.; Adamczuk, K.; Michalczuk, M.; Korga-Plewko, A.; Józefczyk, A. Phytochemical analysis and anti-cancer properties of extracts of Centaurea castriferrei Borbás & Waisb genus of Centaurea L. Molecules 2022, 27, 7537. [Google Scholar] [CrossRef] [PubMed]
- Khwaza, V.; Oyedeji, O.O.; Aderibigbe, B.A. Ursolic acid-based derivatives as potential anti-cancer agents: An update. Int. J. Mol. Sci. 2020, 21, 5920. [Google Scholar] [CrossRef]
- Kornel, A.; Nadile, M.; Tsiani, E. Evidence of the beneficial effects of ursolic acid against lung cancer. Molecules 2022, 27, 7466. [Google Scholar] [CrossRef] [PubMed]
- Panusa, A.; Petrucci, R.; Marrosu, G.; Multari, G.; Gallo, F.R. UHPLC-PDA-ESI-TOF/MS metabolic profiling of Arctostaphylos pungens and Arctostaphylos uva-ursi. A comparative study of phenolic compounds from leaf methanolic extracts. Phytochemistry 2015, 115, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Song, X.C.; Canellas, E.; Dreolin, N.; Nerin, C.; Goshawk, J. Discovery and characterization of phenolic compounds in bearberry (Arctostaphylos uva-ursi) leaves using Liquid Chromatography-Ion Mobility-High-Resolution Mass Spectrometry. J. Agric. Food Chem. 2021, 69, 10856–10868. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
| Et | Aq | |||
|---|---|---|---|---|
| Phytochemicals | Mean | SD | Mean | SD |
| Total flavonoids (mg GAE g−1) | 3.39 a | 0.317 | 2.58 b | 0.424 |
| Total phenolics (mg QE g−1) | 271.68 a | 8.889 | 170.26 b | 21.528 |
| Arbutin (mg g−1) | 88.79 a | 13.757 | 87.08 a | 17.764 |
| Hydroquinone (mg g−1) | 9.22 b | 1.583 | 13.09 a | 1.047 |
| Ursolic acid (mg g−1) | 9.32 a | 0.727 | 0.00 b | 0.000 |
| Hyperoside (mg g−1) | 5.16 a | 1.087 | 4.43 a | 1.175 |
| Methylarbutin (mg g−1) | 5.98 a | 0.990 | 2.21 b | 1.336 |
| PGG (mg g−1) | 3.39 a | 0.317 | 2.58 a | 0.424 |
| Picein (mg g−1) | 1.54 a | 0.109 | 1.66 a | 0.180 |
| Oleanolic acid (mg g−1) | 1.25 a | 0.138 | 0.00 b | 0.000 |
| Corilagin (mg g−1) | 0.85 b | 0.379 | 1.77 a | 0.079 |
| Cell Line | MOGGCCM | LN229 | ||
|---|---|---|---|---|
| Ap | Nec | Ap | Nec | |
| SI | 7.31 | 8.26 | 15.91 | 51.76 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| SO | 540.50 | 1069.24 | 799.29 | 2494.59 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| C | 884.55 | 2580.79 | 822.44 | 3622.77 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| SI × SO | 18.17 | 4.30 | 14.51 | 48.97 |
| p < 0.001 | p < 0.01 | p < 0.001 | p < 0.001 | |
| SI × C | 10.60 | 7.63 | 20.78 | 48.03 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| SO × C | 917.05 | 845.03 | 688.96 | 2602.87 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| SI × SO × C | 11.03 | 3.88 | 18.96 | 46.10 |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Concentration | C | 0.5 | 1 | 2 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 0.3 g | 0.6 | 0.3 g | 0.6 | 3.3 ef | 1.5 | 4.0 e | 1.0 | 91.7 b | 11.8 |
| 2 | 0.0 h | 0.0 | 0.7 g | 0.6 | 3.7 ef | 0.6 | 4.7 e | 1.5 | 100.0 a | 0.0 |
| 3 | 0.0 h | 0.0 | 0.0 h | 0.0 | 1.3 g | 0.6 | 2.7 f | 0.6 | 91.7 b | 6.4 |
| 4 | 0.0 h | 0.0 | 0.3 g | 0.6 | 2.3 f | 1.5 | 5.3 e | 1.5 | 66.0 c | 9.5 |
| 5 | 0.3 g | 0.6 | 0.7 g | 0.6 | 2.0 f | 1.0 | 8.0 d | 1.0 | 92.7 b | 11.8 |
| 6 | 0.0 h | 0.0 | 0.3 g | 0.6 | 2.7 f | 1.5 | 7.0 de | 1.0 | 96.3 b | 6.4 |
| 7 | 0.0 g | 0.0 | 0.3 f | 0.6 | 1.0 f | 0.0 | 1.0 f | 1.0 | 24.0 c | 3.0 |
| 8 | 0.0 g | 0.0 | 0.3 f | 0.6 | 0.7 f | 0.6 | 0.0 g | 0.0 | 26.3 bc | 1.2 |
| 9 | 0.3 g | 0.6 | 1.0 f | 0.0 | 1.0 f | 0.0 | 1.3 f | 0.6 | 27.0 a | 3.6 |
| 10 | 0.0 g | 0.0 | 0.0 g | 0.0 | 0.0 g | 0.0 | 0.3 f | 0.6 | 20.3 d | 1.5 |
| 11 | 0.0 g | 0.0 | 0.3 f | 0.6 | 0.7 f | 0.6 | 1.0 f | 1.0 | 18.3 e | 0.6 |
| 12 | 0.3 f | 0.6 | 0.0 g | 0.0 | 0.7 f | 0.6 | 0.0 g | 0.0 | 30.0 a | 4.4 |
| Concentration | C | 0.5 | 1 | 2 | 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| 1 | 0.0 i | 0.0 | 1.0 h | 0.0 | 1.0 h | 0.0 | 2.3 g | 0.6 | 53.7 e | 2.5 |
| 2 | 0.0 i | 0.0 | 0.3 h | 0.6 | 1.0 g | 1.0 | 2.0 g | 0.0 | 80.7 c | 7.4 |
| 3 | 0.0 i | 0.0 | 1.0 h | 1.0 | 1.3 h | 0.6 | 3.0 g | 1.0 | 95.3 b | 4.0 |
| 4 | 0.0 i | 0.0 | 0.3 h | 0.6 | 0.0 i | 0.0 | 2.7 g | 0.6 | 49.7 f | 4.5 |
| 5 | 0.0 i | 0.0 | 0.7 h | 0.6 | 1.3 g | 1.5 | 2.3 g | 0.6 | 59.3 d | 6.8 |
| 6 | 0.0 i | 0.0 | 1.0 h | 0.0 | 1.3 g | 1.5 | 2.7 g | 0.6 | 100.0a | 0.0 |
| 7 | 0.0 j | 0.0 | 1.7 h | 0.6 | 1.0 i | 0.0 | 2.7 gh | 0.6 | 8.0 b | 1.0 |
| 8 | 0.7 hi | 1.2 | 2.3 g | 0.6 | 1.0 hi | 1.0 | 2.0 gh | 1.0 | 7.7 bc | 1.5 |
| 9 | 0.0 j | 0.0 | 1.0 hi | 1.0 | 1.3 hi | 0.6 | 4.0 e | 1.0 | 9.0 a | 1.0 |
| 10 | 0.0 j | 0.0 | 1.7 hi | 0.6 | 0.0 j | 0.0 | 3.7 e | 1.2 | 6.7 c | 1.5 |
| 11 | 0.0 j | 0.0 | 0.3 i | 0.6 | 1.3 gh | 1.5 | 3.0 fg | 1.0 | 6.0 c | 2.0 |
| 12 | 0.0 j | 0.0 | 0.7 hi | 1.2 | 1.3 gh | 1.5 | 2.3 g | 0.6 | 6.0 c | 1.0 |
| Phytochemicals | Apoptosis | Necrosis | ||
|---|---|---|---|---|
| MOGGCCM | LN229 | MOGGCCM | LN229 | |
| Total flavonoids | 0.787 ** | 0.835 *** | 0.642 * | −0.078 |
| Total phenolics | 0.815 ** | 0.670 * | 0.582 * | −0.473 |
| Arbutin | 0.240 | 0.123 | −0.130 | −0.242 |
| Hydroquinone | −0.829 *** | −0.775 ** | −0.656 * | 0.285 |
| Ursolic acid | 0.810 ** | 0.780 ** | 0.888 *** | −0.587 |
| Hyperoside | 0.441 | 0.604 * | 0.298 | 0.128 |
| Methylarbutin | 0.734 ** | 0.800 ** | 0.758 ** | −0.285 |
| PGG | 0.702 * | 0.663 * | 0.807 ** | −0.337 |
| Picein | −0.448 | −0.505 | −0.319 | 0.174 |
| Oleanolic acid | 0.825 *** | 0.843 *** | 0.788 ** | −0.186 |
| Corilagin | −0.826 *** | −0.684 * | −0.653 * | 0.320 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugier, P.; Jakubowicz-Gil, J.; Zając, A.; Sugier, D.; Wójcik, M.; Czarnecka, J.; Krawczyk, R.; Urban, D.; Sęczyk, Ł. Phytochemical Profiles and Anti-Glioma Activity of Bearberry Arctostaphylos uva-ursi (L.) Spreng. Leaf Extracts. Appl. Sci. 2024, 14, 3418. https://doi.org/10.3390/app14083418
Sugier P, Jakubowicz-Gil J, Zając A, Sugier D, Wójcik M, Czarnecka J, Krawczyk R, Urban D, Sęczyk Ł. Phytochemical Profiles and Anti-Glioma Activity of Bearberry Arctostaphylos uva-ursi (L.) Spreng. Leaf Extracts. Applied Sciences. 2024; 14(8):3418. https://doi.org/10.3390/app14083418
Chicago/Turabian StyleSugier, Piotr, Joanna Jakubowicz-Gil, Adrian Zając, Danuta Sugier, Małgorzata Wójcik, Joanna Czarnecka, Rafał Krawczyk, Danuta Urban, and Łukasz Sęczyk. 2024. "Phytochemical Profiles and Anti-Glioma Activity of Bearberry Arctostaphylos uva-ursi (L.) Spreng. Leaf Extracts" Applied Sciences 14, no. 8: 3418. https://doi.org/10.3390/app14083418





