Establishment of Effective Callus Induction in the Economically Important Brown Seaweed Ecklonia cava
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Pre-Processing of E. cava Thalli
2.3. Experimental Conditions
2.4. Plasma Treatment
2.4.1. Indirect Plasma Treatment
2.4.2. Direct Plasma Treatment
2.5. Statistical Analysis
3. Results and Discussion
3.1. Effect of Media Type
3.2. Effect of Agar Concentration
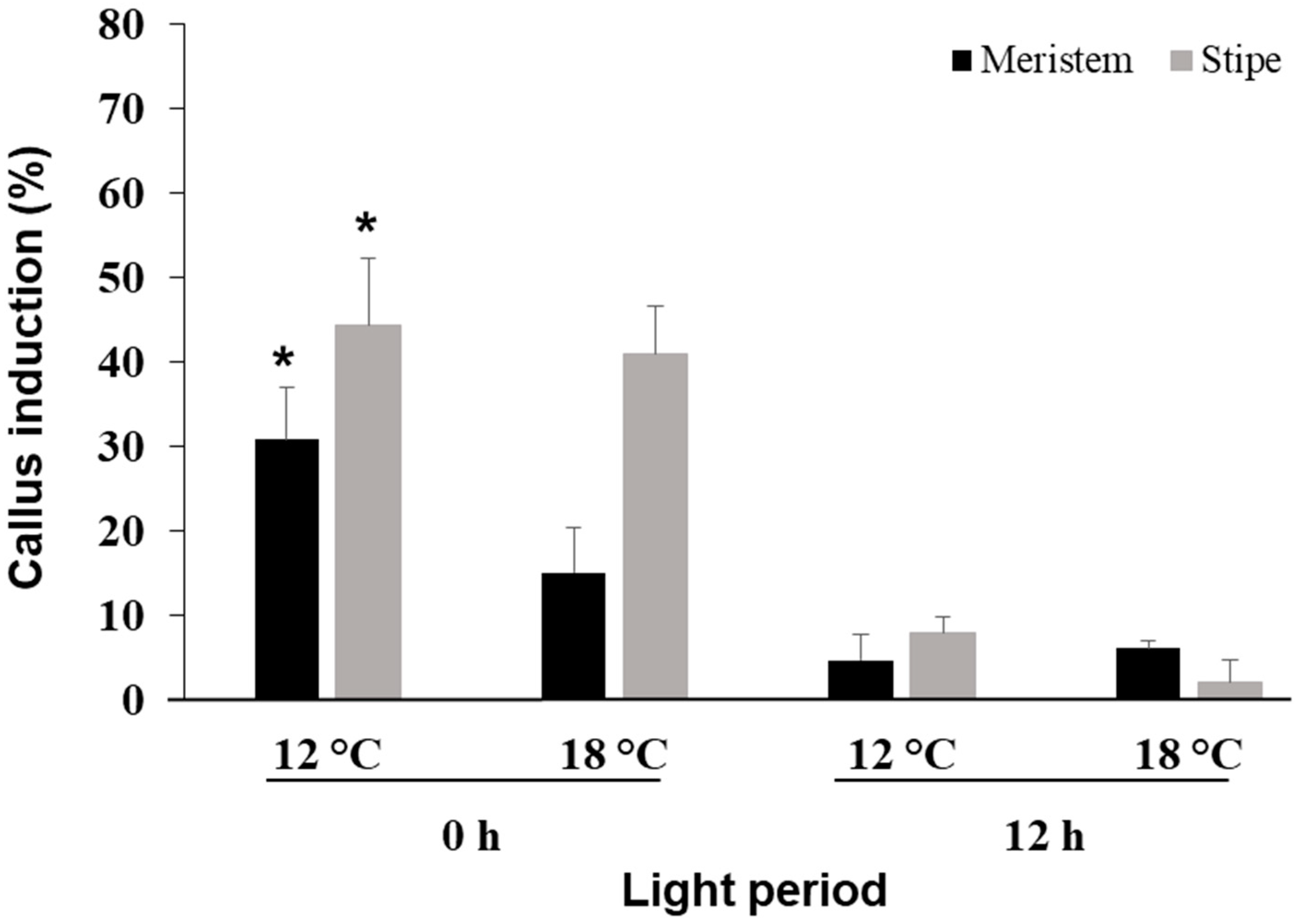
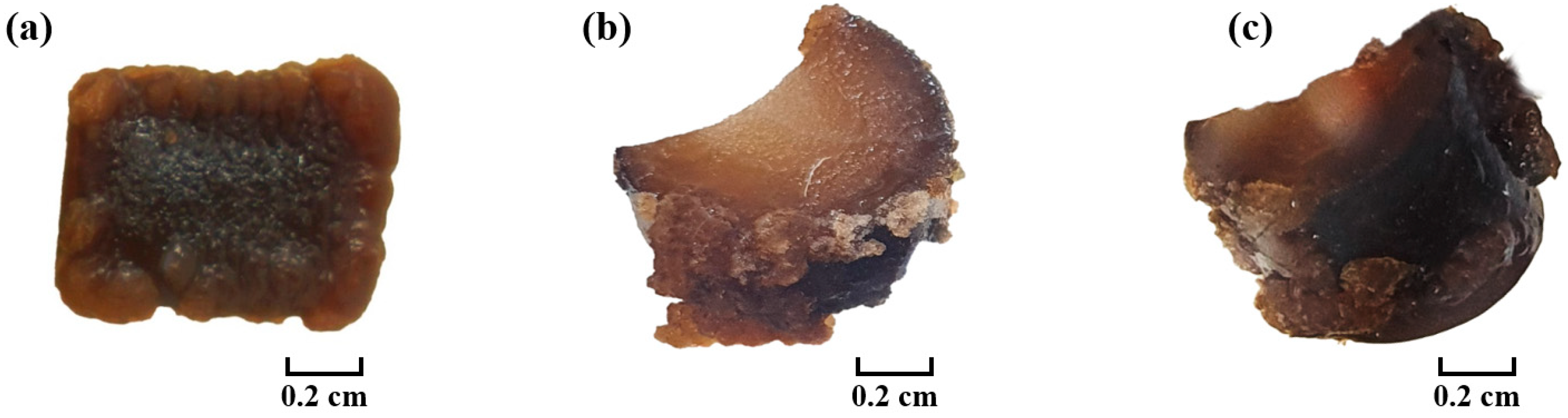
3.3. Effect of Photoperiod and Temperature
3.4. Effect of Growth Regulator
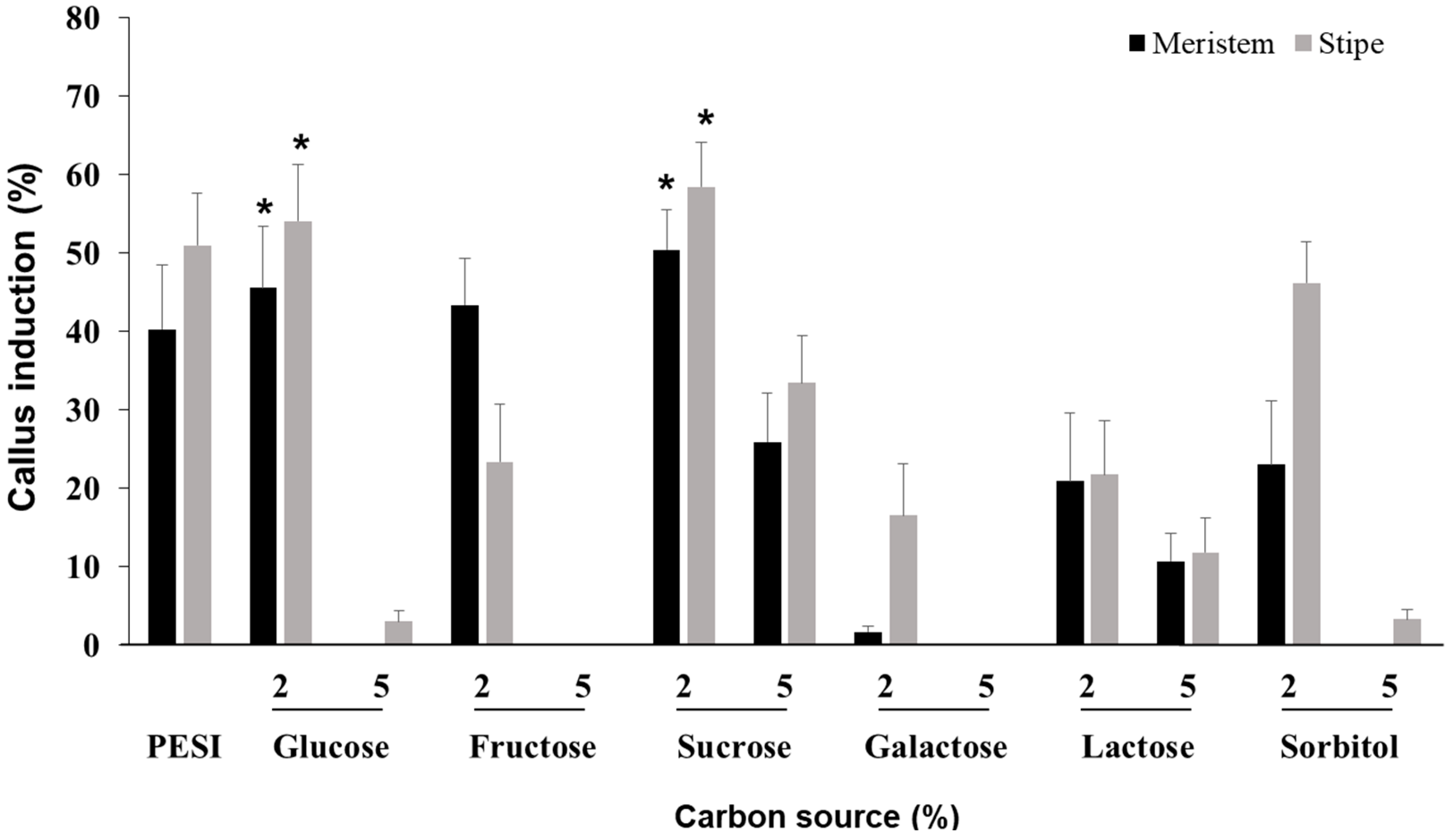
3.5. Effect of Carbon Source
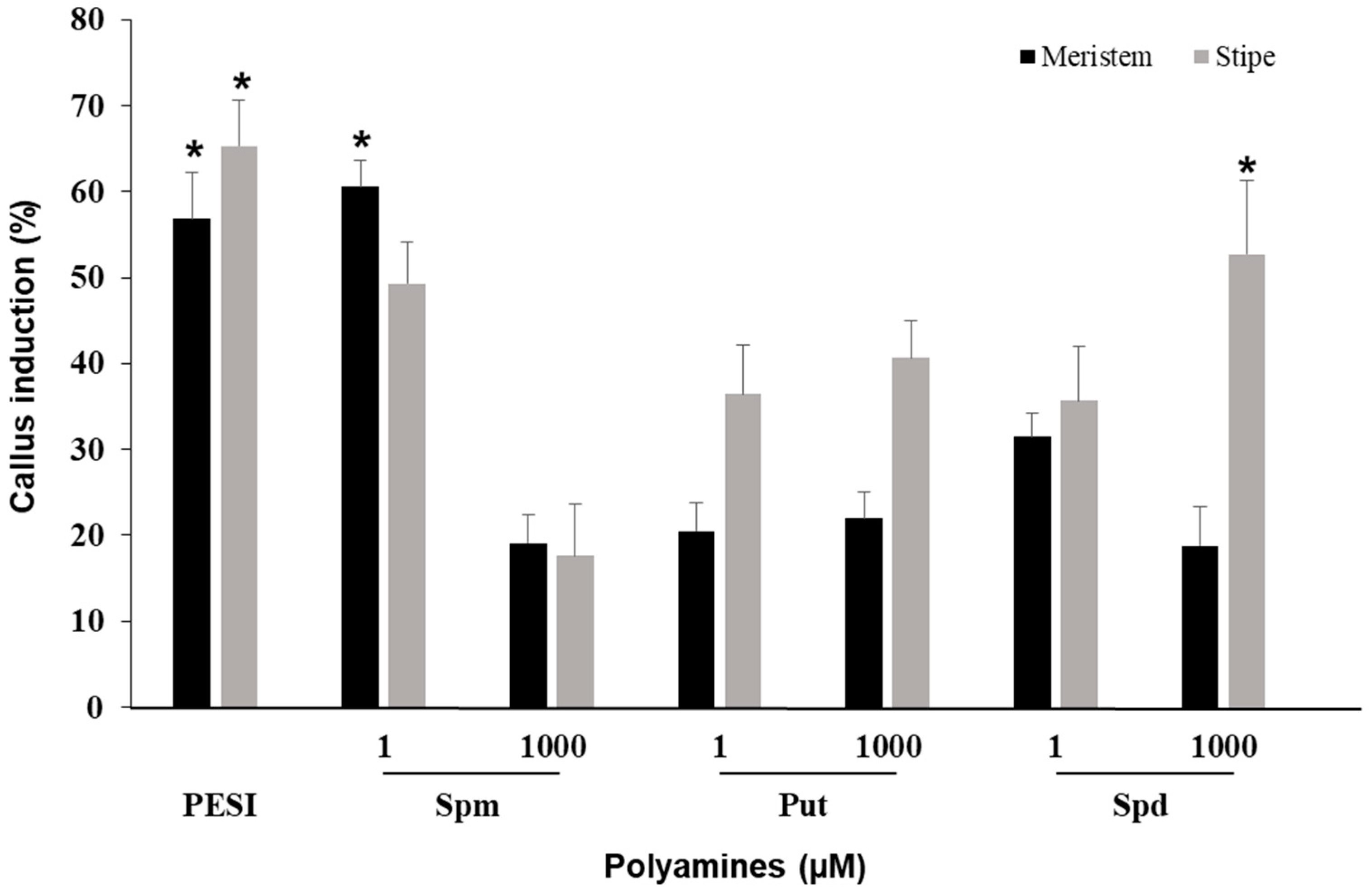
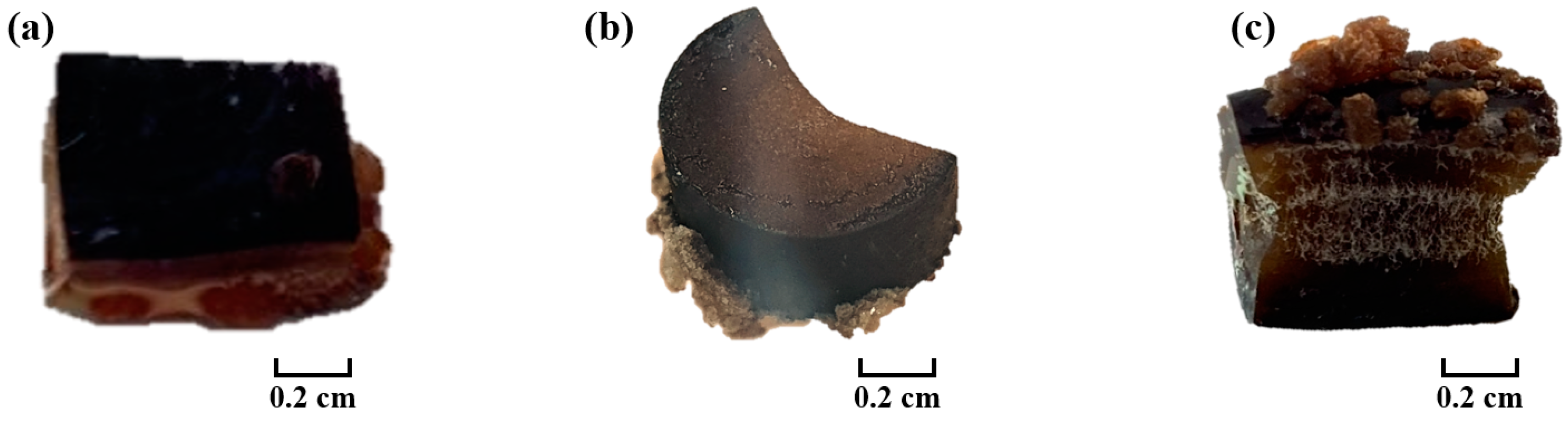
3.6. Effect of Polyamine
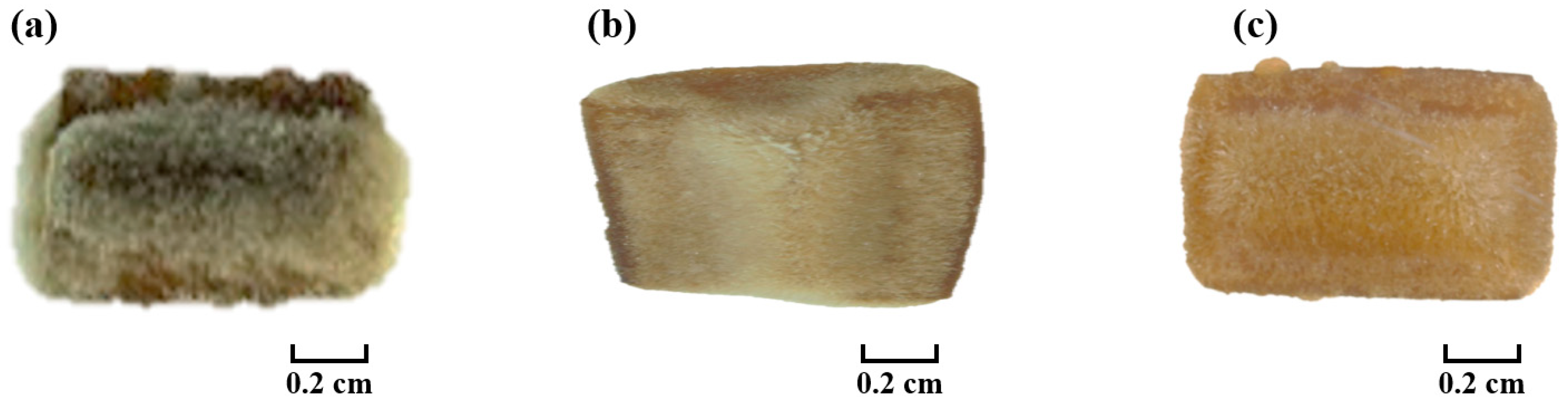
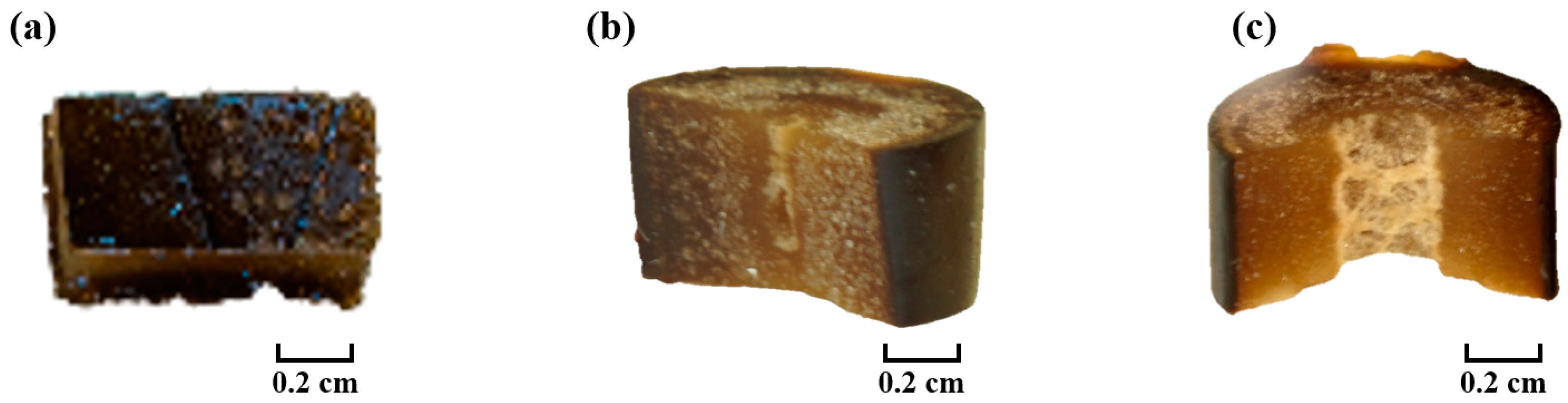
3.7. Effect of Plasma Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Stiger-Pouvreau, V.; Zubia, M. Macroalgal diversity for sustainable biotechnological development in French tropical overseas terri Stiger-Pouvreau, V.; Zubia, M. Macroalgal diversity for sustainable biotechnological development in French tropical overseas territories. Bot. Mar. 2020, 63, 17–41. [Google Scholar] [CrossRef]
- Sanches, P.F.; Pellizzari, F.; Horta, P.A. Multivariate analyses of Antarctic and sub-Antarctic seaweed distribution patterns: An evaluation of the role of the Antarctic Circumpolar Current. J. Sea Res. 2016, 110, 29–38. [Google Scholar] [CrossRef]
- Bedoux, G.; Bourgougnon, N. Bioactivity of secondary metabolites from macroalgae. In The Algae World. Cellular Origin, Life in Extreme Habitats and Astrobiology; Sahoo, D., Seckbach, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; Volume 26, pp. 391–401. [Google Scholar]
- Budzałek, G.; Śliwińska-Wilczewska, S.; Wiśniewska, K.; Wochna, A.; Bubak, I.; Latała, A.; Wiktor, J.M. Macroalgal defense against competitors and herbivores. Int. J. Mol. Sci. 2021, 22, 7865. [Google Scholar] [CrossRef] [PubMed]
- Tanna, B.; Mishra, A. Nutraceutical potential of seaweed polysaccharides: Structure, bioactivity, safety, and toxicity. Compr. Rev. Food Sci. Food Saf. 2019, 18, 817–831. [Google Scholar] [CrossRef] [PubMed]
- Biris-Dorhoi, E.S.; Michiu, D.; Pop, C.R.; Rotar, A.M.; Tofana, M.; Pop, O.L.; Socaci, S.A.; Farcas, A.C. Macroalgae—A sustainable source of chemical compounds with biological activities. Nutrients 2020, 12, 3085. [Google Scholar] [CrossRef] [PubMed]
- Mehdi, A.; Ali, H.; Mehdi, A. Seaweed proteins as a source of bioactive peptides. Curr. Pharm. Des. 2021, 27, 1342–1352. [Google Scholar]
- Meinita, M.D.N.; Harwanto, D.; Choi, J.-S. Seaweed exhibits therapeutic properties against chronic disease: An overview. Appl. Sci. 2022, 12, 2638. [Google Scholar] [CrossRef]
- Antony, T.; Chakraborty, K. Pharmacological properties of seaweeds against progressive lifestyle diseases. J. Aquat. Food Prod. Technol. 2019, 28, 1092–1104. [Google Scholar] [CrossRef]
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed nutraceuticals and their therapeutic role in disease prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Fortune Business Insights. Commercial Seaweed Market Size, Share & COVID-19 Impact Analysis, by Type (Red Seaweed, Brown Seaweed, and Green Seaweed), form (Flakes, Powder, and Liquid), End-Uses (Food & Beverages, Agricultural Fertilizer, Animal Feed Additives, Pharmaceutical, and Cosmetics & Personal Care), and Regional Forecast. 2021–2028. Available online: https://www.fortunebusinessinsights.com/industry-reports/commercial-seaweed-market-100077 (accessed on 26 March 2022).
- Obando, J.M.C.; dos Santos, T.C.; Martins, R.C.C.; Teixeira, V.L.; Barbarino, E.; Cavalcanti, D.N. Current and promising applications of seaweed culture in laboratory conditions. Aquaculture 2022, 560, 738596. [Google Scholar] [CrossRef]
- Kawai, H.; Motomura, T.; Okuda, K. Isolation and purification techniques for macroalgae. In Algal Culturing Techniques; Academic Press: Cambridge, MA, USA, 2005; Volume 133. [Google Scholar]
- Tirtawijaya, G.; Negara, B.F.S.P.; Lee, J.-H.; Cho, M.-G.; Kim, H.K.; Choi, Y.-S.; Lee, S.-H.; Choi, J.-S. The Influence of abiotic factors on the induction of seaweed callus. J. Mar. Sci. Eng. 2022, 10, 513. [Google Scholar] [CrossRef]
- Kang, J.W. Illustrated Encyclopedia of Fauna and Flora of Korea: Marine Algae; Samhwa Press: Seoul, Republic of Korea, 1968; pp. 147–148. [Google Scholar]
- Lee, Y.; Kang, S.A. Catalogue of the Seaweeds in Korea; Jeju National University Press: Jeju, Republic of Korea, 2001; pp. 107–108. [Google Scholar]
- Asanka Sanjeewa, K.K.; Fernando, I.P.S.; Kim, S.-Y.; Kim, W.-S.; Ahn, G.; Jee, Y.; Jeon, Y.-J. Ecklonia cava (Laminariales) and Sargassum horneri (Fucales) synergistically inhibit the lipopolysaccharide-induced inflammation via blocking NF-κB and MAPK pathways. Algae 2019, 34, 45–56. [Google Scholar] [CrossRef]
- Kang, C.; Jin, Y.B.; Lee, H.; Cha, M.; Sohn, E.T.; Moon, J. Brown alga Ecklonia cava attenuates type 1 diabetes by activating AMPK and Akt signaling pathways. Food Chem. Toxicol. 2010, 48, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Wijesekara, I.; Yoon, N.Y.; Kim, S.K. Phlorotannins from Ecklonia cava (Phaeophyceae): Biological activities and potential health benefits. Biofactors 2010, 36, 408–414. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Jeon, Y.J. Exploiting biological activities of brown seaweed Ecklonia cava for potential industrial applications: A review. Int. J. Food Sci. Nutr. 2012, 63, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.; Ahn, M.; Hyun, J.W.; Kim, S.H.; Moon, C. Antioxidant marine algae phlorotannins and radioprotection: A review of experimental evidence. Acta Histochem. 2014, 116, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Barde, S.R.; Sakhare, R.S.; Kanthale, S.B.; Chandak, P.G.; Jamkhande, P.G. Marine bioactive agents: A short review on new marine antidiabetic compounds. Algae 2015, 14, 15. [Google Scholar] [CrossRef]
- Sanjeewa, K.K.A.; Kim, E.A.; Son, K.T.; Jeon, Y.J. Bioactive properties and potentials cosmeceutical applications of phlorotannins isolated from brown seaweeds: A review. J. Photochem. Photobiol. B 2016, 162, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Chae, D.-H.; Kim, I.-S.; Kim, S.-K.; Song, Y.K.; Shim, W.J. Abundance and distribution characteristics of microplastics in surface seawaters of the Incheon/Kyeonggi coastal region. Arch. Environ. Contam. Toxicol. 2015, 69, 269–278. [Google Scholar] [CrossRef]
- Hurley, R.; Woodward, J.; Rothwell, J.J. Microplastic contamination of river beds significantly reduced by catchment-wide flooding. Nat. Geosci. 2018, 11, 251–257. [Google Scholar] [CrossRef]
- Kawashima, Y.; Tokuda, H. Callus formation in Ecklonia cava Kjellman (Laminariales, Phaeophyta). Hydrobiologia 1990, 204, 375–380. [Google Scholar] [CrossRef]
- Kumar, G.R.; Reddy, C.R.K.; Jha, B. Callus induction and thallus regeneration from callus of phycocolloid yielding seaweeds from the Indian coast. J. Appl. Phycol. 2007, 19, 15–25. [Google Scholar] [CrossRef]
- Mansoor, S.; Bashir, K.M.I.; Mohibbullah, M.; Meinita, M.D.N.; Khan, M.N.A.; Sohn, J.-H.; Choi, J.-S. Nutritional and health promoting perspectives of Monostroma spp. (Chlorophyta): A systematic review. J. Appl. Phycol. 2024; in press. [Google Scholar]
- Gordillo, F.J.; Dring, M.J.; Savidge, G. Nitrate and phosphate uptake characteristics of three species of brown algae cultured at low salinity. Mar. Ecol. Prog. Ser. 2002, 234, 111–118. [Google Scholar] [CrossRef]
- Nishihara, G.N.; Mori, Y.; Terada, R.; Noro, T. A simplified method to isolate and cultivate, Laurencia brongniartii J. Agardh (Rhodophyta, Ceramiales) from Kagoshima, Japan. Aquac. Sci. 2004, 52, 1–10. [Google Scholar]
- Steen, H. Effects of reduced salinity on reproduction and germling development in Sargassum muticum (Phaeophyceae, Fucales). Eur. J. Phycol. 2004, 39, 293–299. [Google Scholar] [CrossRef]
- Zou, D.H.; Gao, K.S. Comparative mechanisms of photosynthetic carbon acquisition in Hizikia fusiforme under submersed and emersed conditions. Acta Bot. Sin. 2004, 46, 1178–1185. [Google Scholar]
- Hayashi, L.; Yokoya, N.S.; Kikuchi, D.M.; Oliveira, E.C. Callus induction and micropropagation improved by colchicine and phytoregulators in Kappaphycus alvarezii (Rhodophyta, Solieriaceae). J. Appl. Phycol. 2008, 20, 653–659. [Google Scholar] [CrossRef]
- Kyaw, S.P.P.; Wai, M.K.; Nyunt, T.; Aye, M.M.; Soe-Htun, U. Effects of light intensity on the formation and growth of the secondary branches of Dictyota adnata zanardini grown in different salinity and temperature regimes. J. Myanmar Acad. Arts Sci. 2009, 7, 321–332. [Google Scholar]
- Xu, Z.; Zou, D.; Gao, K. Effects of elevated CO2 and phosphorus supply on growth, photosynthesis and nutrient uptake in the marine macroalga Gracilaria lemaneiformis (Rhodophyta). Bot. Mar. 2010, 53, 123–129. [Google Scholar] [CrossRef]
- Scherner, F.; Ventura, R.; Barufi, J.B.; Horta, P.A. Salinity critical threshold values for photosynthesis of two cosmopolitan seaweed species: Providing baselines for potential shifts on seaweed assemblages. Mar. Environ. Res. 2013, 91, 14–25. [Google Scholar] [CrossRef]
- Terada, R.; Inoue, S.; Nishihara, G.N. The effect of light and temperature on the growth and photosynthesis of Gracilariopsis chorda (Gracilariales, Rhodophtya) from geographically separated locations of Japan. J. Appl. Phycol. 2013, 25, 1863–1872. [Google Scholar] [CrossRef]
- Wei, X.; Shuai, L.; Lu, B.; Wang, S.; Chen, J.; Wang, G. Effects of temperature and irradiance on filament development of Grateloupia turuturu (Halymeniaceae, Rhodophyta). J. Appl. Phycol. 2013, 25, 1881–1886. [Google Scholar] [CrossRef]
- Mandal, S.K.; Ajay, G.; Monisha, N.; Malarvizhi, J.; Temkar, G.; Mantri, V.A. Differential response of varying temperature and salinity regimes on nutrient uptake of drifting fragments of Kappaphycus alvarezii: Implication on survival and growth. J. Appl. Phycol. 2015, 27, 1571–1581. [Google Scholar] [CrossRef]
- Torres, P.B.; Chow, F.; Santos, D.Y. Growth and photosynthetic pigments of Gracilariopsis tenuifrons (Rhodophyta, Gracilariaceae) under high light in vitro culture. J. Appl. Phycol. 2005, 27, 1243–1251. [Google Scholar] [CrossRef]
- Terada, R.; Vo, T.D.; Nishihara, G.N.; Shioya, K.; Shimada, S.; Kawaguchi, S. The effect of irradiance and temperature on the photosynthesis and growth of a cultivated red alga Kappaphycus alvarezii (Solieriaceae) from Vietnam, based on in situ and in vitro measurements. J. Appl. Phycol. 2016, 28, 457–467. [Google Scholar] [CrossRef]
- Endo, H.; Okumura, Y.; Sato, Y.; Agatsuma, Y. Interactive effects of nutrient availability, temperature, and irradiance on photosynthetic pigments and color of the brown alga Undaria pinnatifida. J. Appl. Phycol. 2017, 29, 1683–1693. [Google Scholar] [CrossRef]
- Han, T.; Qi, Z.; Huang, H.; Liao, X.; Zhang, W. Nitrogen uptake and growth responses of seedlings of the brown seaweed Sargassum hemiphyllum under controlled culture conditions. J. Appl. Phycol. 2018, 30, 507–515. [Google Scholar] [CrossRef]
- Yuniarti, L.S.; Sri, A.; Happy, N.; Muhammad, F. Concentration of liquid pes media on the growth and photosynthetic pigments of seaweeds Cotonii propagule (Kappaphycus alvarezii Doty) through tissue culture. Russ. J. Agric. Soc. Econ. Sci. 2018, 75, 133–144. [Google Scholar]
- Mo, V.T.; Cuong, L.K.; Tung, H.T.; Van Huynh, T.; Nghia, L.T.; Khanh, C.M.; Lam, N.N.; Nhut, D.T. Somatic embryogenesis and plantlet regeneration from the seaweed Kappaphycus striatus. Acta Physiol. Plant. 2020, 42, 104. [Google Scholar] [CrossRef]
- Wijayanto, A.; Widowati, I.; Winanto, T. Domestication of red seaweed (Gelidium latifolium) in different culture media. Indones. J. Mar. Sci. Ilmu Kelaut. 2020, 25, 39–44. [Google Scholar] [CrossRef]
- Aris, M.; Muchdar, F.; Labenua, R. Study of seaweed Kappaphycus alvarezii explants growth in the different salinity concentrations. J. Ilm. Perikan. Kelaut. 2021, 13, 97–105. [Google Scholar] [CrossRef]
- Öztaşkent, C.; Ak, İ. Effect of LED light sources on the growth and chemical composition of brown seaweed Treptacantha barbata. Aquac. Int. 2021, 29, 193–205. [Google Scholar] [CrossRef]
- Cai, Y.; Li, G.; Zou, D.; Hu, S.; Shi, X. Rising nutrient nitrogen reverses the impact of temperature on photosynthesis and respiration of a macroalga Caulerpa lentillifera (Ulvophyceae, Caulerpaceae). J. Appl. Phycol. 2021, 33, 1115–1123. [Google Scholar] [CrossRef]
- Nauer, F.; Borburema, H.D.; Yokoya, N.S.; Fujii, M.T. Effects of ocean acidification on growth, pigment contents and antioxidant potential of the subtropical Atlantic red alga Hypnea pseudomusciformis Nauer, Cassano & MC Oliveira (Gigartinales) in laboratory. Rev. Bras. Bot. 2021, 44, 69–77. [Google Scholar]
- Avila-Peltroche, J.; Won, B.Y.; Cho, T.O. An improved protocol for protoplast production, culture, and whole plant regeneration of the commercial brown seaweed Undaria pinnatifida. Algal Res. 2022, 67, 102851. [Google Scholar] [CrossRef]
- Asensi, A.; Gall, E.A.; Marie, D.; Billot, C.; Dion, P.; Kloareg, B. Clonal propagation of Laminaria digitata (Phaeophyceae) sporophytes through a diploid cell-filament suspension. J. Phycol. 2001, 37, 411–417. [Google Scholar] [CrossRef]
- Zhang, Q.S.; Qu, S.C.; Cong, Y.Z.; Luo, S.J.; Tang, X.X. High throughput culture and gametogenesis induction of Laminaria japonica gametophyte clones. J. Appl. Phycol. 2008, 20, 205–211. [Google Scholar] [CrossRef]
- Mussio, I.; Rusig, A.M. Morphogenetic responses from protoplasts and tissue culture of Laminaria digitata (Linnaeus) JV Lamouroux (Laminariales, Phaeophyta): Callus and thalloid-like structures regeneration. J. Appl. Phycol. 2009, 21, 255–264. [Google Scholar] [CrossRef]
- Gupta, V.; Bijo, A.J.; Kumar, M.; Reddy, C.R.K.; Jha, B. Detection of epigenetic variations in the protoplast-derived germlings of Ulva reticulata using methylation sensitive amplification polymorphism (MSAP). Mar. Biotechnol. 2012, 14, 692–700. [Google Scholar] [CrossRef]
- Luhan, M.R.J.; Mateo, J.P. Clonal production of Kappaphycus alvarezii (Doty) Doty in vitro. J. Appl. Phycol. 2017, 29, 2339–2344. [Google Scholar] [CrossRef]
- Reddy, C.R.K.; Jha, B.; Fujita, Y.; Ohno, M. Seaweed micropropagation techniques and their potentials: An overview. J. Appl. Phycol. 2007, 20, 159–167. [Google Scholar]
- Baweja, P.; Sahoo, D.; García-Jiménez, P.; Robaina, R.R. Review: Seaweed tissue culture as applied to biotechnology: Problems, achievements and prospects. Phycol. Res. 2009, 57, 45–58. [Google Scholar] [CrossRef]
- Kumar, L.R.G.; Paul, P.T.; Anas, K.K.; Tejpal, C.S.; Chatterjee, N.S.; Anupama, T.K.; Mathew, S.; Ravishankar, C.N. Phlorotannins-bioactivity and extraction perspectives. J. Appl. Phycol. 2022, 34, 2173–2185. [Google Scholar] [CrossRef] [PubMed]
- Tatewaki, M. Formation of a crustaceous sporophyte with unilocular sporangia in Scytosiphon lomentaria. Phycologia 1966, 6, 62–66. [Google Scholar] [CrossRef]
- Berges, J.A.; Franklin, D.J.; Harrison, P.J. Evolution of an artificial seawater medium: Improvements in enriched seawater, artificial water over the past two decades. J. Phycol. 2001, 37, 1138–1145. [Google Scholar] [CrossRef]
- Provasoli, L.; McLaughlin, J.J.A.; Droop, M.R. The development of artificial media for marine algae. Arch. Mikrobiol. 1957, 25, 392–428. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, G.M.; Genovese, G.; Morabito, M.; Culoso, F.; De Masi, F. Sexual and asexual reproduction in a freshwater population of Bangia atropurpurea (Bangiales, Rhodophyta) from eastern Sicily (Italy). Phycologia 2001, 40, 88–96. [Google Scholar] [CrossRef]
- Bian, J.Y.; Guo, X.Y.; Lee, D.H.; Sun, X.R.; Liu, L.S.; Shao, K.; Kwon, T. Non-thermal plasma enhances rice seed germination, seedling development, and root growth under low-temperature stress. Appl. Biol. Chem. 2024, 67, 2. [Google Scholar] [CrossRef]
- Anderson, R.L.; Bishop, W.E.; Campbell, R.L. A review of the environmental and mammalian toxicology of nitrilotriacetic acid. CRC Crit. Rev. Toxicol. 1985, 15, 1–102. [Google Scholar] [CrossRef]
- El-Bahr, M.K.; Abd EL-Hamid, A.; Matter, M.A.; Shaltout, A.; Bekheet, S.A.; El-Ashry, A.A. In vitro conservation of embryogenic cultures of date palm using osmotic mediated growth agents. J. Genet. Eng. Biotechnol. 2016, 14, 363–370. [Google Scholar] [CrossRef]
- Chen, G.-Q.; Chen, F. Growing phototrophic cells without light. Biotechnol. Lett. 2006, 28, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Engin, I.K.; Cekmecelioglu, D.; Yücel, A.M. Heterotrophic growth and oil production from Micractinium sp. ME05 using molasses. J. Appl. Phycol. 2018, 30, 3483–3492. [Google Scholar] [CrossRef]
- Afshari, R.T.; Angoshtari, R.; Kalantari, S. Effects of light and different plant growth regulators on induction of callus growth in rapeseed (Brassica napus L.) genotypes. Plant Omics 2011, 4, 60–67. [Google Scholar]
- Bashir, K.M.I.; Mansoor, S.; Kim, N.R. Effect of organic carbon sources and environmental factors on cell growth and lipid content of Pavlova lutheri. Ann. Microbiol. 2019, 69, 353–368. [Google Scholar] [CrossRef]
- Gaspar, T.; Keveks, C.; Penel, C.; Greppin, H.; Reid, D.M.; Thorpe, T.A. Plant hormones and plant growth regulators in plant tissue culture. In Vitro Cell. Dev. Biol. Plant 1996, 32, 272–289. [Google Scholar] [CrossRef]
- Phillips, G.C.; Garda, M. Plant tissue culture media and practices: An overview. Plant 2019, 55, 242–257. [Google Scholar] [CrossRef]
- Martinez, M.E.; Jorquera, L.; Poirrier, P.; Díaz, K.; Chamy, R. Effect of the carbon source and plant growth regulators (PGRs) in the induction and maintenance of an in vitro callus culture of Taraxacum officinale (L.) Weber Ex F.H. Wigg. Agronomy 2021, 11, 1181. [Google Scholar] [CrossRef]
- Coenen, C.; Lomax, T.L. Auxin-cytokinin interactions in higher plants: Old problems and new tools. Trends Plant Sci. 1997, 2, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Praveena, C.; Veeresham, C. Multiple shoot regeneration and effect of sugars on growth and nitidine accumulation in shoot cultures of Toddalia asiatica. Pharmacogn. Mag. 2014, 10, S480. [Google Scholar]
- Tognetti, J.A.; Pontis, H.G.; Martínez-Noël, G.M.A. Sucrose signaling in plants: A world yet to be explored. Plant Signal Behav. 2013, 8, e23316. [Google Scholar]
- Chen, D.; Shao, Q.; Yin, L.; Younis, A.; Zheng, B. Polyamine function in plants: Metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019, 9, 1945. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Hu, Q.; Pu, Q.; Chen, M.; Zhu, X.; Gao, C.; Cao, Y. Spermidine enhanced free polyamine levels and expression of polyamine biosynthesis enzyme gene in rice spikelets under heat tolerance before heading. Sci. Rep. 2020, 10, 8976. [Google Scholar] [CrossRef] [PubMed]
- Eren, B.; Türkoğlu, A.; Haliloğlu, K.; Demirel, F.; Nowosad, K.; Özkan, G.; Niedbała, G.; Pour-Aboughadareh, A.; Bujak, H.; Bocianowski, J. Investigation of the influence of polyamines on mature embryo culture and DNA methylation of wheat (Triticum aestivum L.) using the machine learning algorithm method. Plants 2023, 12, 3261. [Google Scholar] [CrossRef] [PubMed]
- Alcázar, R.; Tiburcio, A.F. Plant polyamines in stress and development: An emerging area of research in plant sciences. Front. Plant Sci. 2014, 5, 319. [Google Scholar] [CrossRef] [PubMed]
- Quinet, M.; Ndayiragije, A.; Lefèvre, I.; Lambillotte, B.; Dupont-Gillain, C.C.; Lutts, S. Putrescine differently influences the effect of salt stress on polyamine metabolism and ethylene synthesis in rice cultivars differing in salt resistance. J. Exp. Bot. 2010, 61, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.H. Change in free polyamine contents and expression profiles of two polyamine biosynthetic genes in citrus embryogenic callus under abiotic stresses. Biotechnol. Biotechnol. Equip. 2009, 23, 1289–1293. [Google Scholar] [CrossRef]
- Wi, S.J.; Kim, W.T.; Park, K.Y. Overexpression of carnation S-adenosylmethionine decarboxylase gene generates a broad-spectrum tolerance to abiotic stresses in transgenic tobacco plants. Plant Cell Rep. 2006, 25, 1111–1121. [Google Scholar] [CrossRef]

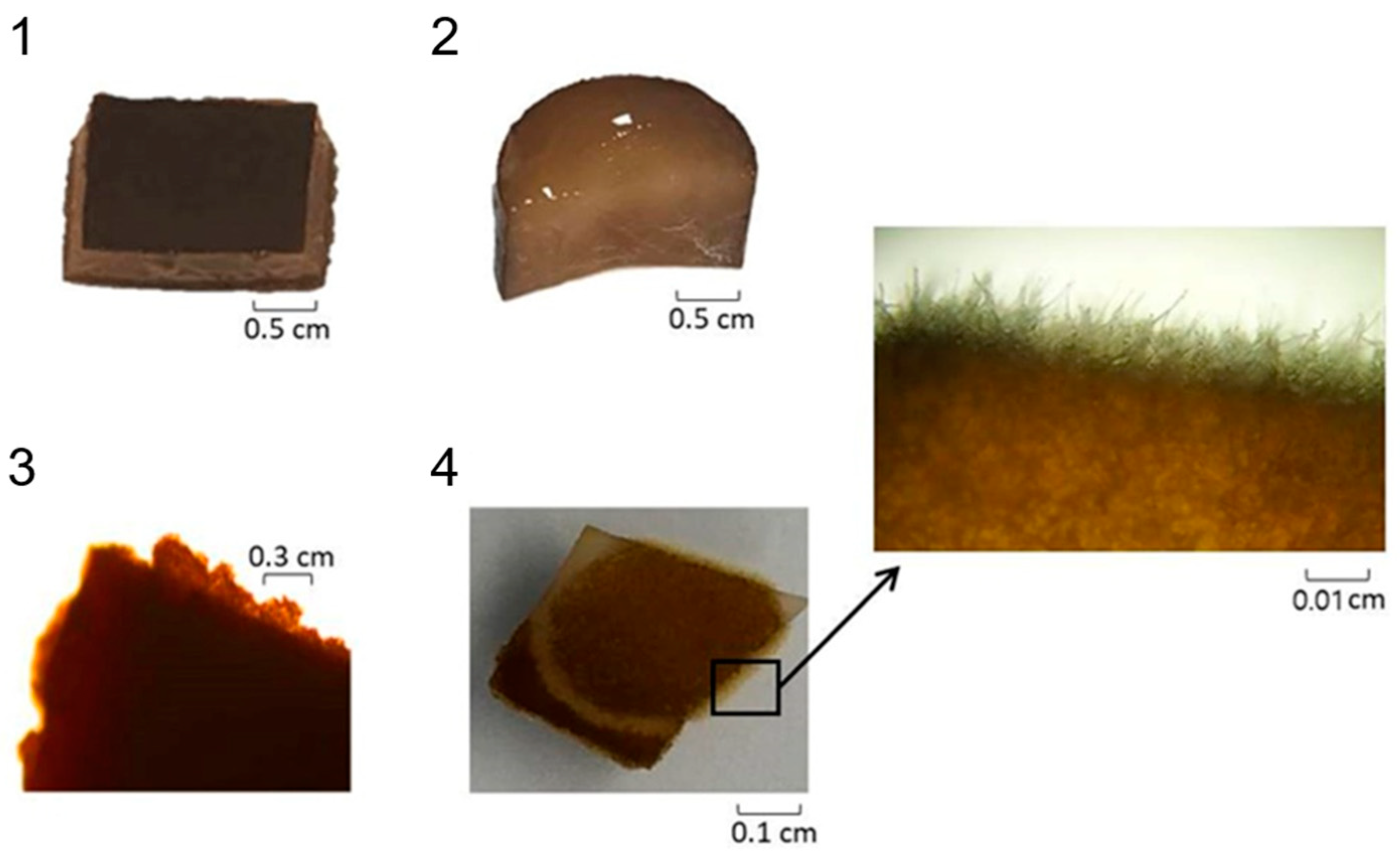
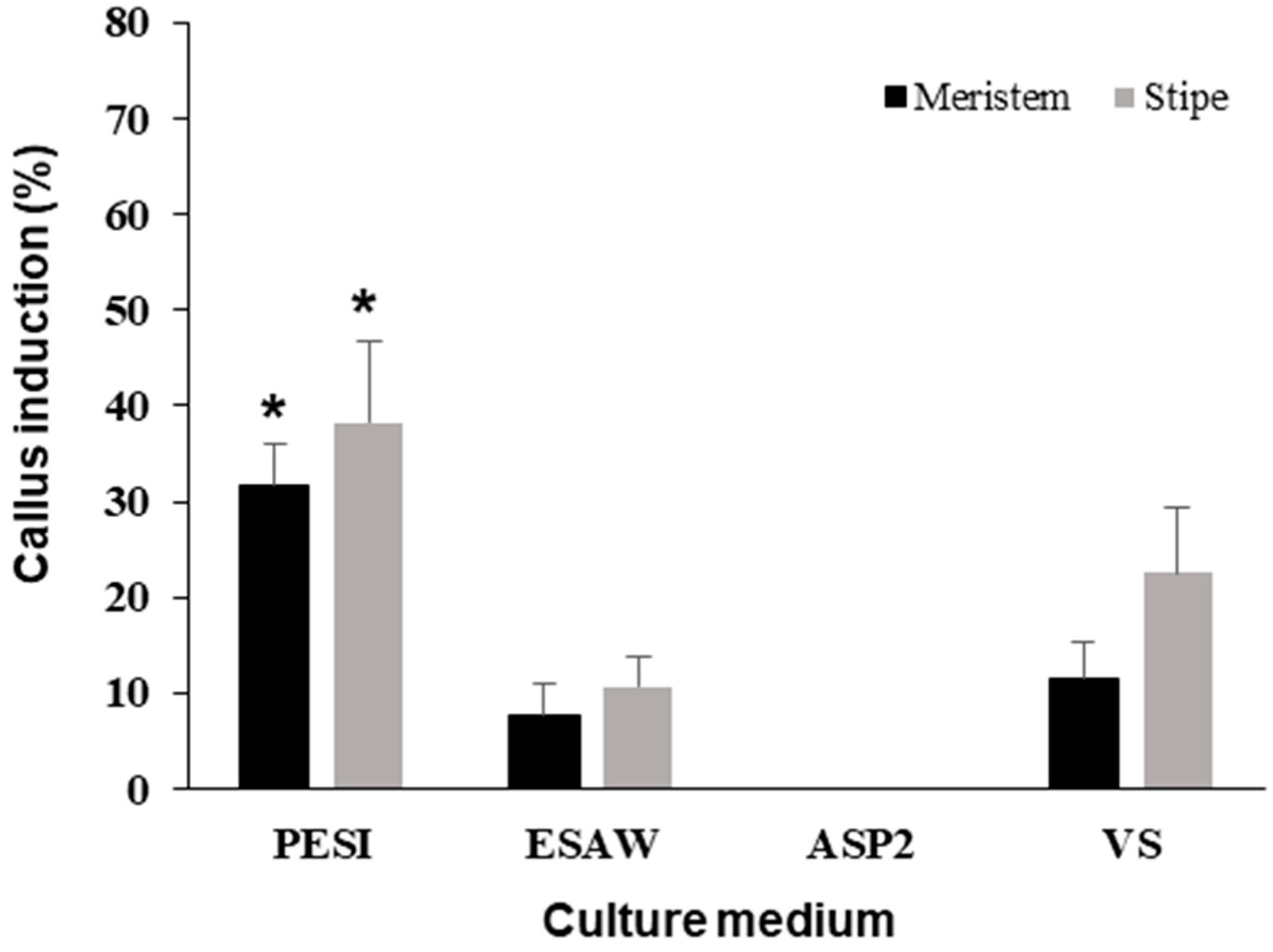
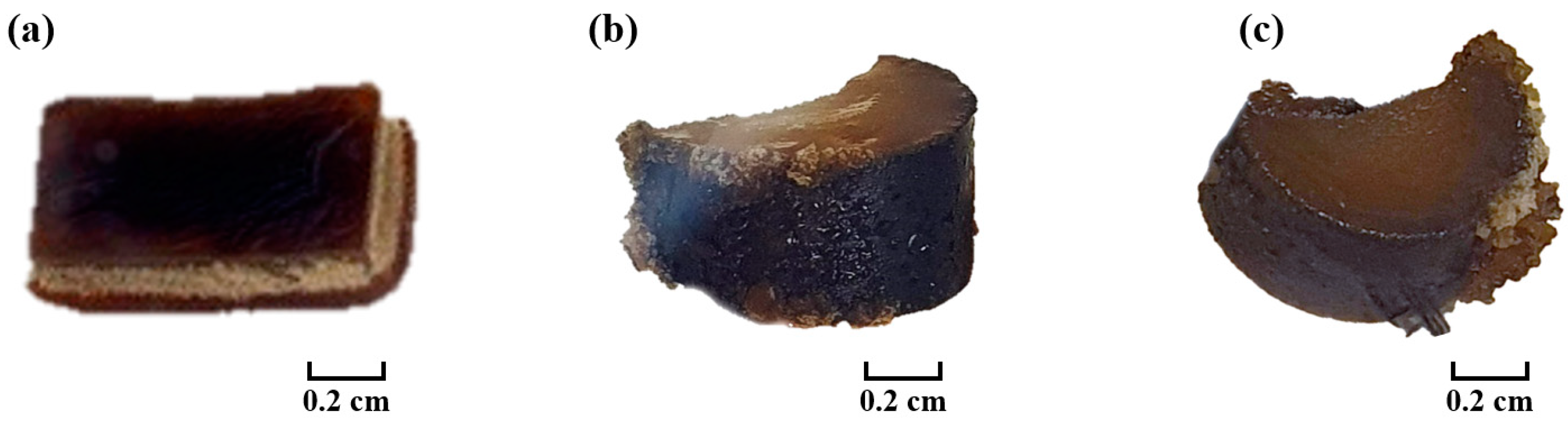
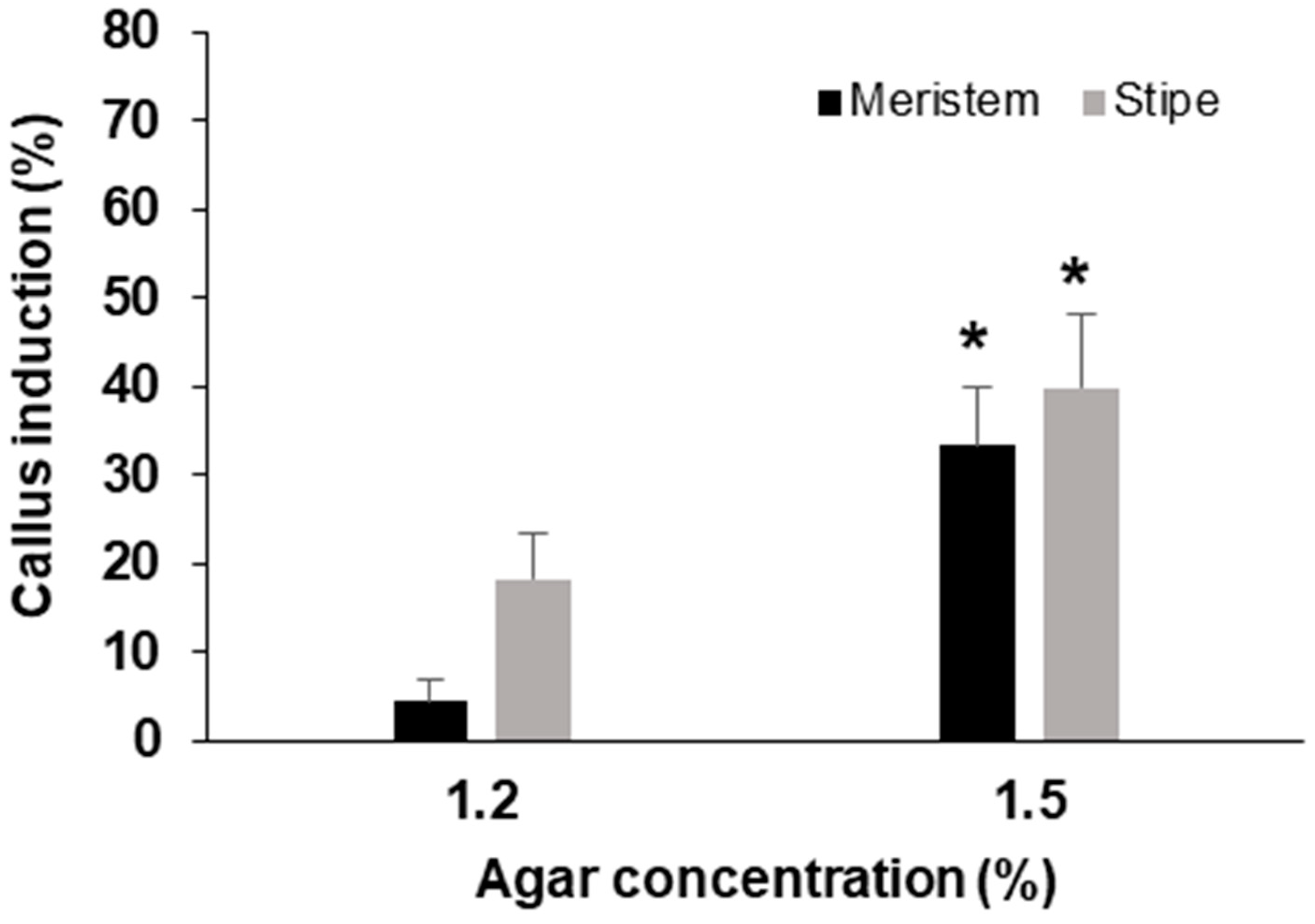


| Parameter | Experimental Conditions |
|---|---|
| Effect of culture medium |
|
| Effect of agar concentration |
|
| Effect of photoperiod and temperature |
|
| Effect of growth regulator |
|
| Effect of carbon source |
|
| Effect of polyamine |
|
| Effect of plasma treatment |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-H.; Bashir, K.M.I.; Tirtawijaya, G.; Negara, B.F.S.P.; Choi, J.-S. Establishment of Effective Callus Induction in the Economically Important Brown Seaweed Ecklonia cava. Appl. Sci. 2024, 14, 3480. https://doi.org/10.3390/app14083480
Lee J-H, Bashir KMI, Tirtawijaya G, Negara BFSP, Choi J-S. Establishment of Effective Callus Induction in the Economically Important Brown Seaweed Ecklonia cava. Applied Sciences. 2024; 14(8):3480. https://doi.org/10.3390/app14083480
Chicago/Turabian StyleLee, Jin-Hwa, Khawaja Muhammad Imran Bashir, Gabriel Tirtawijaya, Bertoka Fajar Surya Perwira Negara, and Jae-Suk Choi. 2024. "Establishment of Effective Callus Induction in the Economically Important Brown Seaweed Ecklonia cava" Applied Sciences 14, no. 8: 3480. https://doi.org/10.3390/app14083480








